Abstract
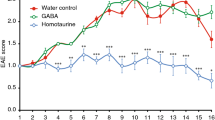
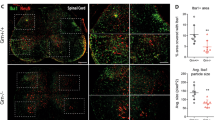
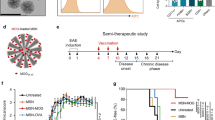
Interleukin-4 (IL-4) suppresses the development of multiple sclerosis in a murine model of experimental autoimmune encephalomyelitis (EAE). Here, we show that, in mice with EAE, the accumulation and persistence in the lymph nodes and spleen of a systemically administered serum albumin (SA)–IL-4 fusion protein leads to higher efficacy in preventing disease development than the administration of wild-type IL-4 or of the clinically approved drug fingolimod. We also show that the SA–IL-4 fusion protein prevents immune-cell infiltration in the spinal cord, decreases integrin expression in antigen-specific CD4+ T cells, increases the number of granulocyte-like myeloid-derived suppressor cells (and their expression of programmed-death-ligand-1) in spinal cord-draining lymph nodes and decreases the number of T helper 17 cells, a pathogenic cell population in EAE. In mice with chronic EAE, SA–IL-4 inhibits immune-cell infiltration into the spinal cord and completely abrogates immune responses to myelin antigen in the spleen. The SA–IL-4 fusion protein may be prophylactically and therapeutically advantageous in the treatment of multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request.
Change history
22 October 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41551-020-00649-x
References
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Ellwardt, E., Walsh, J. T., Kipnis, J. & Zipp, F. Understanding the role of T cells in CNS homeostasis. Trends Immunol. 37, 154–165 (2016).
Hofstetter, H. H. et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237, 123–130 (2005).
Chun, J. & Hartung, H. P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Rice, G. P., Hartung, H. P. & Calabresi, P. A. Anti-α4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64, 1336–1342 (2005).
Cooney, L. A., Towery, K., Endres, J. & Fox, D. A. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J. Immunol. 187, 4440–4450 (2011).
Gadani, S. P., Cronk, J. C., Norris, G. T. & Kipnis, J. IL-4 in the brain: a cytokine to remember. J. Immunol. 189, 4213–4219 (2012).
Racke, M. K. et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 180, 1961–1966 (1994).
Butti, E. et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 15, 504–515 (2008).
Vogelaar, C. F. et al. Fast direct neuronal signaling via the IL-4 receptor as therapeutic target in neuroinflammation. Sci. Transl. Med. 10, eaao2304 (2018).
van Zwam, M. et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J. Pathol. 217, 543–551 (2009).
Dennis, M. S. et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J. Biol. Chem. 277, 35035–35043 (2002).
Liao, W. et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 9, 1288–1296 (2008).
Nilsen, J. et al. Human and mouse albumin bind their respective neonatal Fc receptors differently. Sci. Rep. 8, 14648 (2018).
Andersen, J. T. et al. Single-chain variable fragment albumin fusions bind the neonatal Fc receptor (FcRn) in a species-dependent manner: implications for in vivo half-life evaluation of albumin fusion therapeutics. J. Biol. Chem. 288, 24277–24285 (2013).
Andrews, R., Rosa, L., Daines, M. & Khurana Hershey, G. Reconstitution of a functional human type II IL-4/IL-13 receptor in mouse B cells: demonstration of species specificity. J. Immunol. 166, 1716–1722 (2001).
Pierson, E. R., Stromnes, I. M. & Goverman, J. M. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J. Immunol. 192, 929–939 (2014).
Rothhammer, V. et al. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 208, 2465–2476 (2011).
Sasaki, K. et al. IL-4 suppresses very late antigen-4 expression which is required for therapeutic Th1 T-cell trafficking into tumors. J. Immunother. 32, 793–802 (2009).
Ioannou, M. et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J. Immunol. 188, 1136–1146 (2012).
Guenova, E. et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc. Natl Acad. Sci. USA 112, 2163–2168 (2015).
Lotfi, N. et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front. Immunol. 10, 1265 (2019).
Luna, G. et al. Infection risks among patients with multiple sclerosis treated with fingolimod, Natalizumab, Rituximab, and injectable therapies. JAMA Neurol. 77, 184–191 (2019).
De Angelis, F., John, N. A. & Brownlee, W. J. Disease-modifying therapies for multiple sclerosis. BMJ 363, k4674 (2018).
Comi, G. et al. Efficacy of fingolimod and interferon beta-1b on cognitive, MRI, and clinical outcomes in relapsing-remitting multiple sclerosis: an 18-month, open-label, rater-blinded, randomised, multicentre study (the GOLDEN study). J. Neurol. 264, 2436–2449 (2017).
Sanford, M. & Lyseng-Williamson, K. A. Subcutaneous recombinant interferon-β-1a (Rebif®): a review of its use in the treatment of relapsing multiple sclerosis. Drugs 71, 1865–1891 (2011).
Barun, B. & Bar-Or, A. Treatment of multiple sclerosis with anti-CD20 antibodies. Clin. Immunol. 142, 31–37 (2012).
Taupin, P. Antibodies against CD20 (rituximab) for treating multiple sclerosis: US20100233121. Expert Opin. Ther. Pat. 21, 111–114 (2011).
Apolloni, E. et al. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J. Immunol. 165, 6723–6730 (2000).
Crook, K. R. & Liu, P. Role of myeloid-derived suppressor cells in autoimmune disease. World J. Immunol. 4, 26–33 (2014).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Lee, P. W. et al. IL-23R-activated STAT3/STAT4 is essential for Th1/Th17-mediated CNS autoimmunity. JCI Insight 2, e91663 (2017).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Wang, Y. et al. In vivo albumin labeling and lymphatic imaging. Proc. Natl Acad. Sci. USA 112, 208–213 (2015).
Mirzaei, S. et al. Sentinel lymph node detection with large human serum albumin colloid particles in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 30, 874–878 (2003).
Yao, Z., Dai, W., Perry, J., Brechbiel, M. W. & Sung, C. Effect of albumin fusion on the biodistribution of interleukin-2. Cancer Immunol. Immunother. 53, 404–410 (2004).
Fan, Y. Y. et al. Human FcRn tissue expression profile and half-life in PBMCs. Biomolecules 9, 373 (2019).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4, 360–370 (2004).
Pyzik, M. et al. Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury. Proc. Natl Acad. Sci. USA 114, E2862–E2871 (2017).
Pyzik, M. et al. The neonatal Fc receptor (FcRn): a misnomer? Front. Immunol. 10, 1540 (2019).
Hashem, L., Swedrowska, M. & Vllasaliu, D. Intestinal uptake and transport of albumin nanoparticles: potential for oral delivery. Nanomedicine 13, 1255–1265 (2018).
Salou, M., Nicol, B., Garcia, A. & Laplaud, D.-A. Involvement of CD8+ T cells in multiple sclerosis. Front. Immunol. 6, 604–604 (2015).
Acknowledgements
We thank the Human Tissue Resource Center of the University of Chicago for histological analysis. We thank the Integrated Light microscopy Core facility and Cytometry and Antibody Technology core facility. We thank S. Gomes for experimental support. We thank T. Sano (University of Illinois at Chicago) for experimental advice and helpful discussions. This work was supported by the University of Chicago.
Author information
Authors and Affiliations
Contributions
A.I., J.I. and J.A.H. designed the project. A.I., J.I., E.Y., E.A.W., A.C.T., K.K., M.N., A.S., A.M., E.B., A.T.A., P.H., L.M. and J.W.R. performed the experiments. M.N. and A.S. performed the blinded EAE clinical score measurements. A.I. read the blinded histology. A.I., J.I., E.A.W., A.M., E.B., P.H., M.A.S. and J.A.H. analysed the data. J.I., A.I. and J.A.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
J.I., A.I, K.K., A.M. and J.A.H. are inventors on International Patent application PCT/US20/19668. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Ishihara, A., Ishihara, J., Watkins, E.A. et al. Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis. Nat Biomed Eng 5, 387–398 (2021). https://doi.org/10.1038/s41551-020-00627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00627-3
This article is cited by
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice
Nature Biomedical Engineering (2024)
-
Strategies to therapeutically modulate cytokine action
Nature Reviews Drug Discovery (2023)
-
CARs: a new approach for the treatment of autoimmune diseases
Science China Life Sciences (2023)
-
The circular RNA circINPP4B acts as a sponge of miR-30a to regulate Th17 cell differentiation during progression of experimental autoimmune encephalomyelitis
Cellular & Molecular Immunology (2021)