Abstract
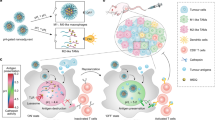
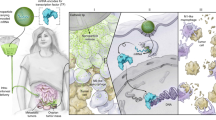
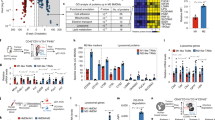
Tumour-associated macrophages are abundant in many cancers, and often display an immune-suppressive M2-like phenotype that fosters tumour growth and promotes resistance to therapy. Yet, macrophages are highly plastic and can also acquire an anti-tumorigenic M1-like phenotype. Here, we show that R848, an agonist of the toll-like receptors TLR7 and TLR8 identified in a morphometric-based screen, is a potent driver of the M1 phenotype in vitro and that R848-loaded β-cyclodextrin nanoparticles (CDNP-R848) lead to efficient drug delivery to tumour-associated macrophages in vivo. As a monotherapy, the administration of CDNP-R848 in multiple tumour models in mice altered the functional orientation of the tumour immune microenvironment towards an M1 phenotype, leading to controlled tumour growth and protecting the animals against tumour rechallenge. When used in combination with the immune checkpoint inhibitor anti-PD-1, we observed improved immunotherapy response rates, including in a tumour model resistant to anti-PD-1 therapy alone. Our findings demonstrate the ability of rationally engineered drug–nanoparticle combinations to efficiently modulate tumour-associated macrophages for cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Pittet, M. J., Nahrendorf, M. & Swirski, F. K. The journey from stem cell to macrophage. Ann. NY Acad. Sci. 1319, 1–18 (2014).
De Palma, M. & Lewis, C. E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23, 277–286 (2013).
Mantovani, A. & Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 212, 435–445 (2015).
Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472 (2015).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Romano, E. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA 112, 6140–6145 (2015).
Arlauckas, S. P. et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 9, eaal3604 (2017).
Steidl, C. et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 362, 875–885 (2010).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Bronte, V. & Murray, P. J. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat. Med. 21, 117–119 (2015).
Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Cuccarese, M. F. et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 8, 14293 (2017).
Cook, R. S. et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J. Clin. Invest. 123, 3231–3242 (2013).
Weissleder, R., Nahrendorf, M. & Pittet, M. J. Imaging macrophages with nanoparticles. Nat. Mater. 13, 125–138 (2014).
Miller, M. A., Arlauckas, S. P. & Weissleder, R. Prediction of anti-cancer nanotherapy efficacy by imaging. Nanotheranostics 1, 296–312 (2017).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 (2015).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Miller, M. A. et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017).
Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998).
Davis, M. E. & Brewster, M. E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004).
Wang, N. X. & Recum, H. A. V. Affinity‐based drug delivery. Macromol. Biosci. 11, 321–332 (2011).
Webber, M. J. & Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 6600–6620 (2017).
Mealy, J. E., Rodell, C. B. & Burdick, J. A. Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host–guest mediated retention. J. Mater. Chem. B 3, 8010–8019 (2015).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Jablonski, K. A. et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 10, e0145342 (2015).
McWhorter, F. Y., Wang, T., Nguyen, P., Chung, T. & Liu, W. F. Modulation of macrophage phenotype by cell shape. Proc. Natl Acad. Sci. USA 110, 17253–17258 (2013).
Rostam, H. M., Reynolds, P. M., Alexander, M. R., Gadegaard, N. & Ghaemmaghami, A. M. Image based machine learning for identification of macrophage subsets. Sci. Rep. 7, 3521 (2017).
Marklein, R. A., Lam, J., Guvendiren, M., Sung, K. E. & Bauer, S. R. Functionally-relevant morphological profiling: a tool to assess cellular heterogeneity. Trends Biotechnol. 36, 105–118 (2017).
Phillip, J. M. et al. Biophysical and biomolecular determination of cellular age in humans. Nat. Biomed. Eng. 1, s41551-017 (2017).
Caicedo, J. C. et al. Data-analysis strategies for image-based cell profiling. Nat. Methods 14, 849–863 (2017).
Bray, M. A. et al. Cell painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 11, 1757 (2016).
Chi, H. et al. Anti-tumor activity of toll-like receptor 7 agonists. Front. Pharmacol. 8, 304 (2017).
Liu, J. et al. A five-amino-acid motif in the undefined region of the TLR8 ectodomain is required for species-specific ligand recognition. Mol. Immunol. 47, 1083–1090 (2010).
Zhang, J. & Ma, P. X. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug Deliv. Rev. 65, 1215–1233 (2013).
Rodell, C. B., Mealy, J. E. & Burdick, J. A. Supramolecular guest–host interactions for the preparation of biomedical materials. Bioconjug. Chem. 26, 2279–2289 (2015).
Behzadi, S. et al. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46, 4218–4244 (2017).
He, C., Hu, Y., Yin, L., Tang, C. & Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31, 3657–3666 (2010).
Dondossola, E. et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 1, 0007 (2017).
Thurber, G. M. et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 4, 1504 (2013).
Mohan, J. F. et al. Imaging the emergence and natural progression of spontaneous autoimmune diabetes. Proc. Natl Acad. Sci. USA 114, E7776–E7785 (2017).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661 (2007).
Pockros, P. J. et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J. Hepatol. 47, 174–182 (2007).
Lynn, G. M. et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 33, 1201 (2015).
Engel, A. L., Holt, G. E. & Lu, H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert Rev. Clin. Pharmacol. 4, 275–289 (2011).
Tang, J., Shalabi, A. & Hubbard-Lucey, V. M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 29, 84–91 (2017).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010).
Lin, E. Y., Nguyen, A. V., Russell, R. G. & Pollard, J. W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740 (2001).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Guerriero, J. L. et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543, 428–432 (2017).
Andón, F. T. et al. Targeting tumor associated macrophages: The new challenge for nanomedicine. Semin. Immunol. 34, 103–113 (2017).
Gaglia, J. L. et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc. Natl Acad. Sci. USA 112, 2139–2144 (2015).
Zhang, Y., Chan, J. W., Moretti, A. & Uhrich, K. E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control Release 219, 355–368 (2015).
Kaittanis, C. et al. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat. Commun. 5, 3384 (2014).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Singh, M. et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J. Immunol. 193, 4722–4731 (2014).
Mauldin, I. S. et al. Topical treatment of melanoma metastases with imiquimod, plus administration of a cancer vaccine, promotes immune signatures in the metastases. Cancer Immunol. Immunother. 65, 1201–1212 (2016).
Huang, S. J. et al. Imiquimod enhances IFN-γ production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J. Invest. Dermatol. 129, 2676–2685 (2009).
Sabado, R. L. et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high risk melanoma. Cancer Immunol. Res. 3, 278–287 (2015).
Cubillos-Ruiz, J. R. et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015).
Savage, P. et al. A phase I clinical trial of imiquimod, an oral interferon inducer, administered daily. Br. J. Cancer 74, 1482 (1996).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Kamaly, N., Yameen, B., Wu, J. & Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016).
Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H. & Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J. Visual. Exp. 76, e50323 (2013).
Reinhardt, R. L., Hong, S., Kang, S. J., Wang, Z. E. & Locksley, R. M. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J. Immunol. 177, 1618–1627 (2006).
Jones, T. R. et al. Scoring diverse cellular morphologies in image-based screens with iterative feedback and machine learning. Proc. Natl Acad. Sci. USA 106, 1826–1831 (2009).
Higuti, I. H. et al. Colorimetric determination of α and β-cyclodextrins and studies on optimization of CGTase production from B. firmus using factorial designs. Braz. Arch. Biol. Technol. 47, 837–841 (2004).
Lai, P., Xu, X. & Wang, L. V. Dependence of optical scattering from Intralipid in gelatin-gel based tissue-mimicking phantoms on mixing temperature and time. J. Biomed. Opt. 19, 035002 (2014).
Acknowledgements
This work was supported in part by grants from the US National Institutes of Health (NIH T32CA079443; R01CA206890; U01CA206997; R01HL131495). We thank H. Im, A. Magnuson and M. Miller for assistance with some of the experiments and G. Wojtkiewicz and M. Prytyskach for technical help. Our special thanks go to C. Benoist and D. Mathis for critical review of the data, helpful suggestions and general discussions. The anti-PD1 antibody was a kind gift from G. J. Freeman.
Author information
Authors and Affiliations
Contributions
R.W. and C.B.R. conceived and designed the CDNP–drug conjugate. C.B.R., S.P.A., M.F.C., C.S.G., R.L., M.S.A. and R.H.K. performed the experiments and data analysis. C.B.R., S.P.A., M.J.P. and R.W. wrote the manuscript. All authors contributed feedback on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
C.B.R. and R.W. are listed on a patent filed by Partners Healthcare. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables, and video caption
Supplementary Video 1
Rapid uptake of CDNPs by tumour-associated macrophages in vivo.
Rights and permissions
About this article
Cite this article
Rodell, C.B., Arlauckas, S.P., Cuccarese, M.F. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2, 578–588 (2018). https://doi.org/10.1038/s41551-018-0236-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0236-8
This article is cited by
-
Induction of therapeutic immunity and cancer eradication through biofunctionalized liposome-like nanovesicles derived from irradiated-cancer cells
Journal of Nanobiotechnology (2024)
-
Exploring the next generation of antibody–drug conjugates
Nature Reviews Clinical Oncology (2024)
-
Harnessing innate immune pathways for therapeutic advancement in cancer
Signal Transduction and Targeted Therapy (2024)
-
Combined immunochemotherapy achieving targeted co-delivery of chlorogenic acid and doxorubicin by sialic acid-modified liposomes enhances anti-cancer efficacy
Drug Delivery and Translational Research (2024)
-
The Streptococcus virulence protein PepO triggers anti-tumor immune responses by reprograming tumor-associated macrophages in a mouse triple negative breast cancer model
Cell & Bioscience (2023)