Abstract
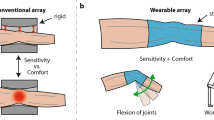
Densely packed resonant structures used for magnetic resonance imaging (MRI), such as nuclear magnetic resonance phased array detectors, suffer from resonant inductive coupling, which restricts the coil design to fixed geometries, imposes performance limitations and narrows the scope of MRI experiments to motionless subjects. Here, we report the design of high-impedance detectors, and the fabrication and performance of a wearable detector array for MRI of the hand, that cloak themselves from electrodynamic interactions with neighbouring elements. We experimentally verified that the detectors do not suffer from the signal-to-noise degradation mechanisms typically observed with the use of traditional low-impedance elements. The detectors are adaptive and can accommodate movement, providing access to the imaging of soft-tissue biomechanics with unprecedented flexibility. The design of the wearable detector glove exemplifies the potential of high-impedance detectors in enabling a wide range of applications that are not well suited to traditional coil designs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
29 August 2018
Owing to a technical error, this Article was originally published with an incorrect published online date of ‘4 May 2018’; it should have been ‘7 May 2018’. This has now been corrected.
References
Bloch, F., Nicodemus, D. & Staub, H. A. Quantitative determination of the magnetic moment of the neutron in units of the proton moment. Phys. Rev. 74, 1025 (1948).
Lauterbur, P. C. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242, 190–191 (1973).
Mansfield, P. & Grannell, P. K. NMR ‘diffraction’ in solids? J. Phys. C Solid State 6, L422–L426 (1973).
Roemer, P. B. et al. The NMR phased array. Magn. Reson. Med. 16, 192–225 (1990).
Wang, J., Reykowski, A. & Dickas, J. Calculation of the signal-to-noise ratio for simple surface coils and arrays of coils. IEEE Trans. Biomed. Eng. 42, 908–917 (1995).
Ocali, O. & Atalar, E. Ultimate intrinsic signal-to-noise ratio in MRI. Magn. Reson. Med. 39, 462–473 (1998).
Schnell, W., Renz, W., Vester, M. & Ermert, H. Ultimate signal-to-noise ratio of surface and body antennas for magnetic resonance imaging. IEEE Trans. Antenn. Propag. 48, 418–428 (2000).
Ohliger, M. A., Grant, A. K. & Sodickson, D. K. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn. Reson. Med. 50, 1018–1030 (2003).
Wiesinger, F., Boesiger, P. & Pruessmann, K. P. Electrodynamics and ultimate SNR in parallel MR imaging. Magn. Reson. Med. 52, 376–390 (2004).
Lattanzi, R. et al. Performance evaluation of a 32-element head array with respect to the ultimate intrinsic SNR. NMR Biomed. 23, 142–151 (2010).
Lattanzi, R. & Sodickson, D. K. Ideal current patterns yielding optimal signal-to-noise ratio and specific absorption rate in magnetic resonance imaging: computational methods and physical insights. Magn. Reson. Med. 68, 286–304 (2012).
Wiggins, G. C. et al. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn. Reson. Med. 62, 754–762 (2009).
Schmitt, M. et al. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn. Reson. Med. 59, 1431–1439 (2008).
Fujita, H., Zheng, T., Yang, X., Finnerty, M. J. & Handa, S. RF surface receive array coils: the art of an LC circuit. J. Magn. Reson. Imaging 38, 12–25 (2013).
Kurs, A. et al. Wireless power transfer via strongly coupled magnetic resonances. Science 317, 83–86 (2007).
Tierney, B. & Grbic, A. Planar shielded-loop resonators for wireless non-radiative power transfer. In IEEE Antennas and Propagation Soc. Int. Symp. 842–843 (IEEE, 2014).
Gonord, P., Kan, S. & Leroy-Willig, A. Parallel-plate split-conductor surface coil: analysis and design. Magn. Reson. Med. 6, 353–358 (1988).
Serfaty, S., Haziza, N., Darrasse, L. & Kan, S. Multi-turn split-conductor transmission-line resonators. Magn. Reson. Med. 38, 687–689 (1997).
Frass-Kriegl, R. et al. Multi-turn multi-gap transmission line resonators: concept, design and first implementation at 4.7T and 7T. J. Magn. Reson. 273, 65–72 (2016).
Corea, J. R. et al. Screen-printed flexible MRI receive coils. Nat. Commun. 7, 10839 (2016).
Vasanawala, A. et al. Development and clinical implementation of very light weight and highly flexible AIR technology arrays. In Proc. Intl. Soc. Magn. Reson. Med. 0755 (ISMRM, 2017).
Stormont, R. et al. Reimagining Flexible Coil Technology 69–71 (SIGNA, 2017).
Stengaard, A. Planar quadrature coil design using shielded-loop resonators. J. Magn. Reson. 125, 84–91 (1997).
Sodickson, D. K. & Manning, W. J. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn. Reson. Med. 38, 591–603 (1997).
Pruessmann, K., Weiger, M., Scheidegger, M. B. & Boesiger, P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 42, 952–962 (1999).
Griswold, M. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 47, 1202–1210 (2002).
Larkman, D. J. et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 13, 313–317 (2001).
Setsompop, K. et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 67, 1210–1224 (2012).
Schnall, M. D., Harihara Subramanian, V., Leigh, J. S. & Chance, B. A new double-tuned probed for concurrent 1H and 31P NMR. J. Magn. Reson. 65, 122–129 (1985).
Avdievich, N. I. & Hetherington, H. P. 4 T Actively detuneable double-tuned 1H/31P head volume coil and four-channel 31P phased array for human brain spectroscopy. J. Magn. Reson. 186, 341–346 (2007).
Brown, R. et al. Design of a nested eight-channel sodium and four-channel proton coil for 7T knee imaging. Magn. Reson. Med. 70, 259–268 (2013).
Shajan, G. et al. Three-layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla. Magn. Reson. Med. 75, 906–916 (2016).
Kriegl, R. et al. Novel inductive decoupling technique for flexible transceiver arrays of monolithic transmission line resonators. Magn. Reson. Med. 73, 1669–1681 (2015).
Hosseinezhadian, S. et al. A flexible transceiver array for 7 T cardiac MRI: first imaging experiments. In Proc. Eur. Soc. Magnetic Resonance in Medicine and Biology 315 (ESMRMB, 2017).
Keil, B. et al. A 64-channel 3T array coil for accelerated brain MRI. Magn. Reson. Med. 70, 248–258 (2013).
Noeske, R., Seifert, F., Rhein, K. H. & Rinneberg, H. Human cardiac imaging at 3T using phased array coils. Magn. Reson. Med. 44, 978–982 (2000).
Chung, S., Kim, D., Breton, E. & Axel, L. Rapid B1 + mapping using a preconditioning RF pulse with TurboFLASH readout. Magn. Reson. Med. 64, 439–446 (2010).
Kellman, P. & McVeigh, E. R. Image reconstruction in SNR units: a general method for SNR measurement. Magn. Reson. Med. 54, 1439–1447 (2005).
Winkelmann, S., Schaeffter, T., Koehler, T., Eggers, H. & Doessel, O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans. Med. Imaging 26, 68–76 (2007).
Acknowledgements
This work was performed under the rubric of the Center for Advanced Imaging Innovation and Research (www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183). We thank R. Brown for critically reading the manuscript, M. Vester for many valuable discussions and Z. Yu for help during the experiments.
Author information
Authors and Affiliations
Contributions
B.Z. built the coils and interfaces. B.Z. and M.A.C. designed the experiments and collected the data. B.Z., D.K.S. and M.A.C. analysed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
NYU has filed provisional patent applications directed to this technology.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and video captions.
Supplementary Video 1
Magnetic resonance imaging of joint kinematics while playing piano.
Supplementary Video 2
Magnetic resonance imaging of joint kinematics when grabbing objects.
Rights and permissions
About this article
Cite this article
Zhang, B., Sodickson, D.K. & Cloos, M.A. A high-impedance detector-array glove for magnetic resonance imaging of the hand. Nat Biomed Eng 2, 570–577 (2018). https://doi.org/10.1038/s41551-018-0233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0233-y
This article is cited by
-
Citizen science approach to assessing patient perception of MRI with flexible radiofrequency coils
Scientific Reports (2024)
-
Simulation-based evaluation of SAR and flip angle homogeneity for five transmit head arrays at 14 T
Magnetic Resonance Materials in Physics, Biology and Medicine (2023)
-
A flexible MRI coil based on a cable conductor and applied to knee imaging
Scientific Reports (2022)
-
Soft wearable devices for deep-tissue sensing
Nature Reviews Materials (2022)
-
Stretchable self-tuning MRI receive coils based on liquid metal technology (LiquiTune)
Scientific Reports (2021)