Abstract
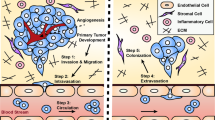
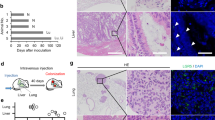
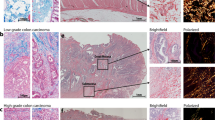
Metastatic disease remains the primary cause of mortality in cancer patients. Yet the number of available in vitro models to study metastasis is limited by challenges in the recapitulation of the metastatic microenvironment in vitro, and by difficulties in maintaining colonized-tissue specificity in the expansion and maintenance of metastatic cells. Here, we show that decellularized scaffolds that retain tissue-specific extracellular-matrix components and bound signalling molecules enable, when seeded with colorectal cancer cells, the spontaneous formation of three-dimensional cell colonies that histologically, molecularly and phenotypically resemble in vivo metastases. Lung and liver metastases obtained by culturing colorectal cancer cells on, respectively, lung and liver decellularized scaffolds retained their tissue-specific tropism when injected in mice. We also found that the engineered metastases contained signet ring cells, which has not previously been observed ex vivo. A culture system with tissue-specific decellularized scaffolds represents a simple and powerful approach for the study of organ-specific cancer metastases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fidler, I. J. The organ microenvironment and cancer metastasis. Differentiation 70, 498–505 (2002).
Katt, M. E., Placone, A. L., Wong, A. D., Xu, Z. S. & Searson, P. C. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 4, 12 (2016).
Higashiyama, M. et al. Differences in chemosensitivity between primary and paired metastatic lung cancer tissues: in vitro analysis based on the collagen gel droplet embedded culture drug test (CD-DST). J. Thorac. Dis. 4, 40–47 (2012).
Bandyopadhyay, A. et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 66, 6714–6721 (2006).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Baptista, P. M. et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53, 604–617 (2011).
Uygun, B. et al. Organ re-engineering through develpment of a transplantatble recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Soto-Gutierrez, A. et al. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant. 19, 815–822 (2010).
Petersen, T. H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Wang, Y. et al. Lineage restriction of hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 53, 293–305 (2011).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Flatmark, K., Maelandsmo, G. M., Martinsen, M., Rasmussen, H. & Fodstad, O. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur. J. Cancer 40, 1593–1598 (2004).
Vinci, M. et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 10, 29 (2012).
Schelter, F. et al. Tumor cell-derived Timp-1 is necessary for maintaining metastasis-promoting Met-signaling via inhibition of Adam-10. Clin. Exp. Metastas. 28, 793–802 (2011).
Weidle, U. H., Birzele, F. & Kruger, A. Molecular targets and pathways involved in liver metastasis of colorectal cancer. Clin. Exp. Metastas. 32, 623–635 (2015).
Yao, K. et al. In vitro hypoxia-conditioned colon cancer cell lines derived from HCT116 and HT29 exhibit altered apoptosis susceptibility and a more angiogenic profile in vivo. Br. J. Cancer 93, 1356–1363 (2005).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Ring, B. Z. & Ross, D. T. Predicting the sites of metastases. Genome Biol. 6, 241 (2005).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Ahmed, D. et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2, e71 (2013).
Uronis, J. M. et al. Histological and molecular evaluation of patient-derived colorectal cancer explants. PLoS ONE 7, e38422 (2012).
Khanna, C. & Hunter, K. Modeling metastasis in vivo. Carcinogenesis 26, 513–523 (2005).
Fidler, I. J. et al. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastas. Rev. 13, 209–222 (1994).
Helling, T. S. & Martin, M. Cause of death from liver metastases in colorectal cancer. Ann. Surg. Oncol. 21, 501–506 (2014).
Klaas, M. et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 6, 27398 (2016).
Acknowledgements
The authors thank Microscopy Service Laboratory, Animal Studies Core, Genomics Core Facility, and Animal Histopathology Core Facility at the University of North Carolina at Chapel Hill. This work was supported by the University Cancer Research Fund from the University of North Carolina and by R21 CA182322 from the National Institutes of Health/National Cancer Institute. This work was also supported by a generous gift from Mr and Mrs E. Barkley. L.Z. was supported by two funds from the National Natural Science Foundation of China (no. 81372424 and no. 81071831) and the Foundation Research Project of Jiangsu Province (no. BK20131131).
Author information
Authors and Affiliations
Contributions
X.T., M.E.W., K.C.R., H.P.F., S.K.Y., J.A.C., H.L. and L.Z. performed the experiments. X.T., M.E.W., H.P.F. and L.E.H. analysed data. A.D.H., S.B.W., E.L.W. and D.S.H. provided essential protocols and technical support. X.T., M.E.W., K.C.R., T.Z. and A.Z.W. designed the experiments. X.T., M.E.W., K.C.R., H.P.F. and A.Z.W. wrote the manuscript. X.T., A.D.H., K.C.R., J.E.T., J.M.C., T.Z., L.M.R. and A.Z.W. edited the manuscript. A.Z.W. directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Tian, X., Werner, M.E., Roche, K.C. et al. Organ-specific metastases obtained by culturing colorectal cancer cells on tissue-specific decellularized scaffolds. Nat Biomed Eng 2, 443–452 (2018). https://doi.org/10.1038/s41551-018-0231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0231-0
This article is cited by
-
Scaffold-based 3D cell culture models in cancer research
Journal of Biomedical Science (2024)
-
Proteomic analysis of decellularized mice liver and kidney extracellular matrices
Journal of Biological Engineering (2024)
-
Glycolysis-related lncRNA TMEM105 upregulates LDHA to facilitate breast cancer liver metastasis via sponging miR-1208
Cell Death & Disease (2023)
-
Decellularized normal and cancer tissues as tools for cancer research
Cancer Gene Therapy (2022)
-
Mast Cells Tryptase Promotes Intestinal Fibrosis in Natural Decellularized Intestinal Scaffolds
Tissue Engineering and Regenerative Medicine (2022)