Abstract
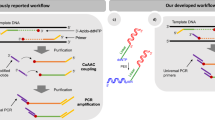
As the catalogue of sequenced genomes and metagenomes continues to grow, massively parallel approaches for the comprehensive and functional analysis of gene products and regulatory elements are becoming increasingly valuable. Current strategies to synthesize or clone complex libraries of DNA sequences are limited by the length of the DNA targets, throughput and cost. Here, we show that long-adapter single-strand oligonucleotide (LASSO) probes can capture and clone thousands of kilobase DNA fragments in a single reaction. As proof of principle, we simultaneously cloned over 3,000 bacterial open reading frames (ORFs) from Escherichia coli genomic DNA (spanning 400- to 5,000-bp targets). Targets were enriched up to a median of around 60-fold compared with non-targeted genomic regions. At a cutoff of three times the median non-target reads per kilobase of genetic element per million reads, around 75% of the targeted ORFs were successfully captured. We also show that LASSO probes can clone human ORFs from complementary DNA, and an ORF library from a human-microbiome sample. LASSO probes could be used for the preparation of long-read sequencing libraries and for massively multiplexed cloning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Van Dijk, E. L., Auger, H., Jaszczyszyn, Y. & Thermes, C. Ten years of next-generation sequencing technology. Trends Genet. 30, 418–426 (2014).
Nilsson, M., Dahl, F., Larsson, C., Gullberg, M. & Stenberg, J. Analyzing genes using closing and replicating circles. Trends Biotechnol. 24, 83–88 (2006).
Dahl, F. et al. Multigene amplification and massively parallel sequencing for cancer mutation discovery. Proc. Natl Acad. Sci. USA 104, 9387–9392 (2007).
Turner, E. H., Lee, C., Ng, S. B., Nickerson, D. A. & Shendure, J. Massively parallel exon capture and library-free resequencing across 16 genomes. Nat. Methods 6, 315–316 (2009).
Nilsson, M. et al. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science 265, 2085–2088 (1994).
Landegren, U. et al. Molecular tools for a molecular medicine: analyzing genes, transcripts and proteins using padlock and proximity probes. J. Mol. Recognit. 17, 194–197 (2004).
Hagerman, P. J. Flexibility of DNA. Annu. Rev. Biophys. Biophys. Chem. 17, 265–286 (1988).
Krishnakumar, S. et al. A comprehensive assay for targeted multiplex amplification of human DNA sequences. Proc. Natl Acad. Sci. USA 105, 9296–9301 (2008).
Shen, P. et al. Multiplex target capture with double-stranded DNA probes. Genome Med. 5, 50 (2013).
Shen, P. et al. High-quality DNA sequence capture of 524 disease candidate genes. Proc. Natl Acad. Sci. USA 108, 6549–6554 (2011).
Kosuri, S. & Church, G. M. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods 11, 499–507 (2014).
Tewhey, R. et al. Direct identification of hundreds of expression-modulating variants using a multiplexed reporter assay. Cell 165, 1519–1529 (2016).
Boeke, J. D. et al. The genome project—write. Science 353, 126–127 (2016).
Bolger, A. M. et al. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Acknowledgements
This work was supported in part by the Shriners Hospitals for Children (B.P. and L.T.), a Prostate Cancer Foundation Young Investigator award (H.B.L.), and National Institutes of Health Grants R01EB012521 (B.P.), K01DK087770 (B.P.) and 1U24AI118633 (H.B.L.).
Author information
Authors and Affiliations
Contributions
L.T., H.B.L. and B.P. conceived and designed the study. L.T., V.S., Y.Y., D.G., P.S. and N.S. performed the experiments, and analysed and interpreted the data. L.T., H.B.L. and B.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application on the technology has been filed (PCT/US2016/035919). The authors declare no other competing financial interests.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and references. (PDF 3963 kb)
Supplementary dataset
Supplementary sequence data. (XLSX 2241 kb)
Rights and permissions
About this article
Cite this article
Tosi, L., Sridhara, V., Yang, Y. et al. Long-adapter single-strand oligonucleotide probes for the massively multiplexed cloning of kilobase genome regions. Nat Biomed Eng 1, 0092 (2017). https://doi.org/10.1038/s41551-017-0092
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0092
This article is cited by
-
Unbiased discovery of autoantibodies associated with severe COVID-19 via genome-scale self-assembled DNA-barcoded protein libraries
Nature Biomedical Engineering (2022)
-
Quantitative assessment of LASSO probe assembly and long-read multiplexed cloning
BMC Biotechnology (2019)
-
Genetic engineering: Lassoing genomic libraries
Nature Biomedical Engineering (2017)