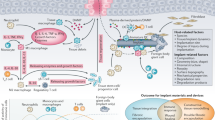
Abstract
Despite advances in orthopaedic materials, the development of drug-eluting bone and joint implants that can sustain the delivery of the drug and maintain the necessary mechanical strength to withstand loading has remained elusive. Here, we demonstrate that modifying the eccentricity of drug clusters and the percolation threshold in ultra-high molecular weight polyethylene (UHMWPE) results in maximized drug elution and the retention of mechanical strength. The optimized UHMWPE eluted antibiotic at a higher concentration for longer than the clinical gold standard antibiotic-eluting bone cement, while retaining the mechanical and wear properties of clinically used UHMWPE joint prostheses. Treatment of lapine knees infected with Staphylococcus aureus with the antibiotic-eluting UHMWPE led to complete bacterial eradication and the absence of detectable systemic effects. We argue that the antibiotic-eluting UHMWPE joint implant is a promising candidate for clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
National Hospital Discharge Survey 777–782 (Centers for Disease Control and Prevention/National Center for Health Statistics, 2010).
Paxton, E. W., Inacio, M., Slipchenko, T. & Fithian, D. C. The Kaiser Permanente National Total Joint Replacement Registry. Perm. J. 12, 12–16 (2008).
Hip and Knee Arthroplasty: Annual Report 2015 (Australian Orthopaedic Association National Joint Replacement Registry, 2015).
Kurtz, S. M., Lau, E., Watson, H., Schmier, J. K. & Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplasty 27, 61–65 (2012).
Kubista, B. et al. Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int. Orthop. 36, 65–71 (2012).
Lentino, J. R. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. 36, 1157–1161 (2003).
Segawa, H., Tsukuyama, D. T., Kyle, R. F., Becker, D. A. & Gustilo, R. B. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J. Bone Joint Surg. Am. 81, 1434–1445 (1999).
Spellberg, B. & Lipsky, B. A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 54, 393–407 (2012).
Pioletti, D. P. et al. Orthopedic implant used as drug delivery system: clinical situation and state of research. Curr. Drug Deliv. 5, 59–63 (2008).
Duncan, C. P. & Masri, B. A. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr. Course Lect. 44, 305–313 (1995).
Radin, S., Campbell, J. T., Ducheyne, P. & Cuckler, J. M. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 18, 777–782 (1997).
Lin, S. S. et al. Development of a biodegradable antibiotic delivery system. Clin. Orthop. Relat. R. 362, 240–250 (1999).
Burnett, R. S., Kelly, M., Hanssen, A. D. & Barrack, R. L. Technique and timing of two-stage exchange for infection in TKA. Clin. Orthop. Relat. R. 464, 164–178 (2007).
Jung, J., Schmid, N. V., Kelm, J., Schmitt, E. & Anagnostakos, K. Complications after spacer implantation in the treatment of hip joint infections. Int. J. Med. Sci. 6, 265–273 (2009).
Kühn, K.-D. Bone Cements: Up-to-Date Comparison of Physical and Chemical Properties of Commercial Materials 89–93 (Springer, 2000).
Lee, C. in The Well-Cemented Total Hip Arthroplasty: Theory and Practice (eds Breusch, S. & Malchau, H. ) 60–66 (Springer, 2005).
Cho, C. H. et al. Elasto-plastic contact analysis of fatigue wear behaviour of UHMWPE tibial components. Japan. J. Clin. Biomech. 23, 373–379 (2002).
Korhonen, R. K., Kostinen, A., Konttinen, Y. T., Santavirta, S. S. & Lapallainen, R. The effect of geometry and abduction angle on the stresses in cemented UHMWPE acetabular cups – finite element simulations and experimental tests. BioMed. Eng. Online 4, 32 (2005).
Lilikakis A & Sutcliffe, M. P. F. The effect of vancomycin addition to the compression strength of antibiotic-loaded bone cements. Int. Orthop. 33, 815–819 (2009).
Meyer, J., Piller, G., Spiegel, C. A., Hetzel, S. & Squire, M. Vacuum-mixing significantly changes antibiotic elution characteristics of commercially available antibiotic-impregnated bone cements. J. Bone Joint Surg. Am. 93, 2049–2056 (2011).
Thomes, B., Murray, P. & Bouchier-Hayes, D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J. Bone Joint Surg. Br. 84, 758–760 (2002).
Xie, Z. et al. Gentamicin-loaded borate bioactive glass eradicates osteomyelitis due to Escherichia coli in a rabbit model. Antimicrob. Agents Ch. 57, 3293–3298 (2013).
Fan, J. B., Huang, C., Jiang, L. & Wang, S. Nanoporous microspheres: from controllable synthesis to healthcare applications. J. Mater. Chem. B. 2013, 2222–2235 (2013).
Bawa, R., Siegel, R., Marasca, B., Karel, M. & Langer, R. S. An explanation for the controlled release of macromolecules from polymers. J. Control. Release 1, 259–267 (1985).
Guo, Q. H., Guo, S. & Wang, Z. M. Estimation of 5-fluorouracil-loaded ethylene-vinyl acetate stent coating based on percolation threshold. Int. J. Pharm. 333, 95–102 (2007).
Yi, Y. B. & Sastry, A. M. Analytical approximation of the percolation threshold for overlapping ellipsoids of revolution. Proc. R. Soc. Lond. A 460, 2353–2380 (2004).
Plumlee, K. P. & Schwartz, C. J. Development of porous UHMWPE morphologies for fixation of gel-based materials. J. Appl. Poly. Sci. 114, 2555–2563 (2009).
Amin, T. J., Lamping, J. W., Hendriks, K. J. & McIff, T. E. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J. Bone Joint Surg. Am. 94, 1946–1951 (2012).
Collier J. P. et al. Comparison of cross-linked polyethylene materials for orthopaedic applications. Clin. Orthop. Relat. R. 414, 289–304 (2003).
Ertl, P., Rohde, B. & Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 43, 3714–3717 (2000).
Laverty, G., Alkawareek, M. Y. & Gilmore, B. F. The in vitro susceptibility of biofilm forming medical device related pathogens to conventional antibiotics. Dataset Papers Sci. 2014, 250694 (2014).
Goldman, M., Gronsky, R. & Pruitt, L. The influence of sterilization technique and ageing on the structure and morphology of medical-grade ultra-high molecular weight polyethylene. J. Mater. Sci.-Mater. M. 9, 207–212 (1998).
Kurtz, A. & Patel, J. D. in UHMWPE Biomaterials Handbook 3rd edn (ed. Kurtz, S. M. ) 57–71 (Elsevier, 2016).
Besemer D. C. et al. Evidence of vitamin E grafting to polyethylene. In Orthopaedic Research Society 2013 Annual Meeting Poster 1055 (Orthopaedic Research Society, 2013).
Cui, Q., Mihalko, W. M., Shields, J. S., Ries, M. & Saleh, K. J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Joint Surg. Am. 89, 871–882 (2007).
Mellor, J. A., Kingdom, J., Cafferkey, M. & Keane, C. T. Vancomycin toxicity: a prospective study. J. Antimicrob. Chemoth. 15, 773–780 (1985).
Glaudemas, A. W. J. M., Galli, F., Pacilio, M. & Signore, A. Leukocyte and bacteria imaging in prosthetic joint infection. Eur. Cell. Mater. 25, 61–77 (2013).
Rose, W. E. & Poppens, P. T. Impact of biofilm on the in vitro activity of vancomycin alone and in combination with tigecycline and rifampicin against Staphylococcus aureus. J. Antimicrob. Chemoth. 63, 485–488 (2008).
Yee, Y. C., Kisslinger, B., Yu, V. L & Jin, D. J. A mechanism of rifamycin inhibition and resistance in Pseudomonas aeruginosa. J. Antimicrob. Chemoth. 38, 133–137 (1996).
Bradley, J. S. & Scheld, W. M. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin. Infect. Dis. 24 (Suppl. 2), S213–S221 (1997).
Oral, E. et al. A surface cross-linked UHMWPE stabilized by vitamin E with low wear and high fatigue strength. Biomaterials 31, 7051–7060 (2010).
Lauderdale, K. J., Malone, C. L., Boles, B. R., Morcuende, J. & Horswill, A. R. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 28, 55–61 (2010).
Isefuku, S., Joyner, C. J. & Simpson, A. H. Toxic effect of rifampicin on human osteoblast-like cells. J. Orthop. Res. 19, 950–954 (2010).
Moses, M. A., Brem, H. & Langer, R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell 4, 337–341 (2003).
Ambrose, C. G. et al. Effective treatment of osteomyelitis with biodegradable microspheres in a rabbit model. Clin. Orthop. Relat. R. 421, 203–299 (2004).
Surdam, J. W., Licini, D. J., Baynes, N. T. & Arce, B. R. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J. Arthroplasty 30, 325–329 (2015).
Meyer, F. et al. Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J. Orthop. Res. 30, 864–871 (2012).
Bohner, M. in Injectable Biomaterials: Science and Applications (ed. Vernon, B. ) 24–39 ( Woodhead, 2011).
Hua, X. et al. Experimental validation of finite element modelling of a modular metal-on-polyethylene total hip replacement. Proc. Inst. Mech. Eng. H 228, 682–692 (2014).
Neut, D. et al. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J. Antimicrob. Chemoth. 47, 885–891 (2001).
van de Belt, H. et al. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 22, 1607–1611 (2001).
Antoci, V. et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J. Orthop. Res. 25, 858–866 (2007).
Stewart, S. et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep. J. Bone Joint Surg. Am. 94, 1406–1415 (2012).
Castaneda, P., McLaren, A., Tavaziva, G. & Overstreet, D. Biofilm antimicrobial susceptibility increases with antimicrobial exposure time. Clin. Orthop. Relat. R. 474, 1659–1664 (2016).
Xiong, Y. Q. et al. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob. Agents Ch. 49, 380–387 (2005).
Kadurugamuw, J. L. et al. Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrob. Agents Ch. 47, 3130–3137 (2003).
Zhang, Q. et al. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science 333, 1764–1767 (2011).
Gullberg, E. et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 7, e1002158 (2011).
Pardo-Alonso, S., Vicente, J., Solorzano, E., Rodriguez-Perez, M. A. & Lehmhus, D. Geometrical tortuosity 3D calculations in infiltrated aluminium cellular materials. Proc. Mater. Sci. 4, 145–150 (2014).
Ortez, J. H. in Manual of Antimicrobial Susceptibility Testing (ed. Coyle M. B. ) 39–52 (American Society for Microbiology, 2005).
Acknowledgements
We are grateful to G. Wojkiewicz and B. Tricot from the Center for Systems Biology, Massachusetts General Hospital (MGH) for their assistance with the bioluminescence imaging. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNN), which is supported by the National Science Foundation (NSF) under NSF award no. ECS-0335765. The CNS is part of Harvard University. This study was supported in part by the Harris Orthopedics Lab Sundry Fund, MGH Orthopedics Departmental Fund, US National Institutes of Health grants P01-HL120839 and P41-EB015903.
Author information
Authors and Affiliations
Contributions
V.J.S., D.A.B, E.O., O.K.M., H.R., A.A.F., H.M., S.J.J.K. and S.H.Y. designed the experiments. V.J.S., D.A.B. and S.J.J.K. performed the experiments. All authors were involved in the analyses and interpretation of the data. V.J.S., D.A.B., S.J.J.K., E.O. and O.K.M. wrote the paper, with the help of the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary discussion, figures and tables. (PDF 1736 kb)
Rights and permissions
About this article
Cite this article
Suhardi, V., Bichara, D., Kwok, S. et al. A fully functional drug-eluting joint implant. Nat Biomed Eng 1, 0080 (2017). https://doi.org/10.1038/s41551-017-0080
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0080
This article is cited by
-
Synergistic use of anti-inflammatory ketorolac and gentamicin to target staphylococcal biofilms
Journal of Translational Medicine (2024)
-
Self-promoted electroactive biomimetic mineralized scaffolds for bacteria-infected bone regeneration
Nature Communications (2023)
-
Ibuprofen-loaded UHMWPE for orthopedics applications: preliminary evaluation of mechanical and biological properties
Polymer Bulletin (2023)
-
Investigation of the possibility of deposition of thin silicone films on the surface of quartz glasses by laser radiation from the gas phase
Optical and Quantum Electronics (2023)
-
Interfacial friction at action: Interactions, regulation, and applications
Friction (2023)