Abstract
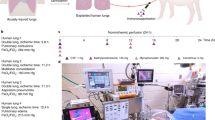
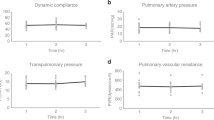
The shortage of transplantable donor organs has profound consequences, especially for patients with end-stage lung disease, for which transplantation remains the only definitive treatment. Although advances in ex vivo lung perfusion have enabled the evaluation and reconditioning of marginally unacceptable donor lungs, clinical use of the technique is limited to ~6 h. Extending the duration of extracorporeal organ support from hours to days would enable longer recovery and recipient-specific manipulations of the donor lung, with the goal of expanding the donor organ pool and improving long-term outcomes. By using a clinically relevant swine model, here we report the development of a cross-circulation platform wherein recipient support enabled 36 h of normothermic perfusion that maintained healthy lungs and allowed for the recovery of injured lungs. Extended support enabled multiscale therapeutic interventions in all extracorporeal lungs. Lungs exceeded transplantation criteria, and recipients tolerated cross-circulation with no significant changes in physiologic parameters throughout 36 h of support. Our findings suggest that cross-circulation should enable extended support and interventions in extracorporeal organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rabe, K. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555 (2007).
Kochanek, K. D., Murphy, S. L., Xu, J. & Arias, E. Mortality in the United States, 2013. NCHS Data Brief 178, 1–8 (2014).
Valapour, M. et al. OPTN/SRTR 2013 annual data report: lung. Am. J. Transplant. 15, 1–28 (2015).
Ware, L. B. et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 360, 619–620 (2002).
Pomfret, E. et al. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons’ State-of-the-Art Winter Symposium. Am. J. Transplant. 8, 745–752 (2008).
Klein, A. et al. Organ donation and utilization in the United States, 1999–2008. Am. J. Transplant. 10, 973–986 (2010).
Javidfar, J. & Bacchetta, M. Bridge to lung transplantation with extracorporeal membrane oxygenation support. Curr. Opin. Org. Transplant. 17, 496–502 (2012).
Fuehner, T. et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am. J. Respir. Crit. Care Med. 185, 763–768 (2012).
Bittle, G. J. et al. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J. Heart Lung Transplant. 32, 760–768 (2013).
Van Raemdonck, D. et al. Lung donor selection and management. Proc. Am. Thorac. Soc. 6, 28–38 (2009).
Elgharably, H., Shafii, A. E. & Mason, D. P. Expanding the donor pool: donation after cardiac death. Thorac. Surg. Clin. 25, 35–46 (2015).
Cypel, M. & Keshavjee, S. Extending the donor pool: rehabilitation of poor organs. Thorac. Surg. Clin. 25, 27–33 (2015).
Rosen, C. et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat. Med. 21, 869–879 (2015).
Wagner, D. E. et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18, 895–911 (2013).
Petersen, T. H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Ott, H. C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933 (2010).
Wobma, H. & Vunjak-Novakovic, G. Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng. Part B Rev. 22, 101–113 (2016).
Cooper, D. K. et al. Clinical lung xenotransplantation—what donor genetic modifications may be necessary? Xenotransplantation 19, 144–158 (2012).
Laird, C., Burdorf, L. & Pierson, R. N. III Lung xenotransplantation: a review. Curr. Opin. Org. Transplant. 21, 272–278 (2016).
Popov, A.-F. et al. Ex vivo lung perfusion–state of the art in lung donor pool expansion. Med. Sci. Monit. Basic Res. 21, 9–14 (2015).
Mohite, P. et al. Utilization of the Organ Care System Lung for the assessment of lungs from a donor after cardiac death (DCD) before bilateral transplantation. Perfusion 30, 427–430 (2015).
Cypel, M. et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 364, 1431–1440 (2011).
Zych, B. et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J. Heart Lung Transplant. 31, 274–281 (2012).
Wallinder, A. et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J. Thorac. Cardiovasc. Surg. 144, 1222–1228 (2012).
Warnecke, G. et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet 380, 1851–1858 (2012).
Eschbach, J. Jr, Hutchings, R., Meston, B., Burnell, J. & Scribner, B. A technique for repetitive and long-term human cross circulation. ASAIO J. 10, 280–284 (1964).
Burnell, J. et al. Observations on cross circulation in man. Am. J. Med. 38, 832–841 (1965).
Burnell, J. et al. Acute hepatic coma treated by cross-circulation or exchange transfusion. N. Engl. J. Med. 276, 935–943 (1967).
Saxena, P. et al. Procurement of lungs for transplantation following donation after circulatory death: the Alfred technique. J. Surg. Res. 192, 642–646 (2014).
Sundaresan, S., Trachiotis, G. D., Aoe, M., Patterson, G. A. & Cooper, J. D. Donor lung procurement: assessment and operative technique. Ann. Thorac. Surg. 56, 1409–1413 (1993).
Sanchez, P. G., Bittle, G. J., Burdorf, L., Pierson, R. N. & Griffith, B. P. State of art: clinical ex vivo lung perfusion: rationale, current status, and future directions. J. Heart Lung Transplant. 31, 339–348 (2012).
Roman, M. et al. Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J. Heart Lung Transplant. 34, 978–987 (2015).
Lee, J. W., Fang, X., Gupta, N., Serikov, V. & Matthay, M. A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl Acad. Sci. USA 106, 16357–16362 (2009).
Cypel, M. et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci. Transl. Med. 1, 4ra9 (2009).
Inci, I. et al. Reconditioning of an injured lung graft with intrabronchial surfactant instillation in an ex vivo lung perfusion system followed by transplantation. J. Surg. Res. 184, 1143–1149 (2013).
Veevers-Lowe, J., Ball, S. G., Shuttleworth, A. & Kielty, C. M. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J. Cell Sci. 124, 1288–1300 (2011).
O’Neill, J. D. et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 96, 1046–1056 (2013).
Petersen, T. H., Calle, E. A., Colehour, M. B. & Niklason, L. E. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195, 222–231 (2011).
Yeung, J. C. et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir. Med. 5, 119–124 (2016).
Steen, S. et al. Transplantation of lungs from non–heart-beating donors after functional assessment ex vivo. Ann. Thorac. Surg. 76, 244–252 (2003).
Cypel, M. et al. Technique for prolonged normothermic ex vivo lung perfusion. J. Heart Lung Transplant. 27, 1319–1325 (2008).
Kitsiouli, E., Nakos, G. & Lekka, M. E. Phospholipase A 2 subclasses in acute respiratory distress syndrome. Biochim. Biophys. Acta 1792, 941–953 (2009).
Maxey, T. S. et al. Tumor necrosis factor-α from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 127, 541–547 (2004).
Zhao, M. et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L1018–L1026 (2006).
Eppinger, M. J., Jones, M. L., Deeb, G. M., Bolling, S. F. & Ward, P. A. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J. Surg. Res. 58, 713–718 (1995).
Kim, J., O’Neill, J. D., Dorrello, N. V., Bacchetta, M. & Vunjak-Novakovic, G. Targeted delivery of liquid microvolumes into the lung. Proc. Natl Acad. Sci. USA 112, 11530–11535 (2015).
Loer, S. A., Scheeren, T. W. & Tarnow, J. How much oxygen does the human lung consume? Anesthesiology 86, 532–537 (1997).
Wagner, D. E. et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35, 3281–3297 (2014).
Hogan, B. L. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Sachs, D. H. et al. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation 22, 559–567 (1976).
Voyta, J. C., Via, D. P., Butterfield, C. E. & Zetter, B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J. Cell Biol. 99, 2034–2040 (1984).
Islam, M. N., Gusarova, G. A., Monma, E., Das, S. R. & Bhattacharya, J. F-actin scaffold stabilizes lamellar bodies during surfactant secretion. Am. J. Physiol. Lung Cell Mol. Physiol. 306, L50–L57 (2014).
Rampersad, S. N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 12, 12347–12360 (2012).
Francis, R. & Lo, C. Ex vivo method for high resolution imaging of cilia motility in rodent airway epithelia. J. Vis. Exp. 78, e50343 (2013).
Gibson-Corley, K. N., Olivier, A. K. & Meyerholz, D. K. Principles for valid histopathologic scoring in research. Vet. Pathol. 50, 1007–1015 (2013).
Reece, T. B. et al. Adenosine A 2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J. Thorac. Cardiovasc. Surg. 129, 1137–1143 (2005).
Mulloy, D. P. et al. Ex vivo rehabilitation of non–heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J. Thorac. Cardiovasc. Surg. 144, 1208–1216 (2012).
Gimble, J. M., Katz, A. J. & Bunnell, B. A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100, 1249–1260 (2007).
Huang, S. X. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2014).
Acknowledgements
The authors thank J. Sonett and A. Griesemer for discussions of the experimental design; D. Sachs for providing research swine; S. Halligan for administrative and logistical support; the Institute of Comparative Medicine veterinary staff, including K. Fragoso and A. Rivas, for their support with the animal studies; S. Ma for live cell imaging; B. Lee and H. Wobma for manuscript review; K. Brown and L. Cohen-Gould for TEM imaging; the Herbert Irving Comprehensive Cancer Center Molecular Pathology Shared Resources, including T. Wu, D. Sun and R. Chen for help with the analytics; M. Cheerharan, M. P. Salna and A. Taubman for experimental assistance; V. Dorrello for valuable discussions; J. Bernhard and J. Ng for providing mesenchymal cells; J. Bhattacharya for providing experimental reagents. The authors gratefully acknowledge funding support from the National Institutes of Health (grants HL134760, EB002520 and HL007854), the Richard Bartlett Foundation and the Mikati Foundation.
Author information
Authors and Affiliations
Contributions
J.D.O., B.A.G., J.K., S.C., D.Q., K.F., A.R. and M.B. performed the experiments. S.X.L.H. provided the human lung stem cells and experimental reagents. Y.-W.C. performed the live imaging. C.M. performed the blinded pathologic assessment. J.D.O., B.A.G., H.-W.S., M.B. and G.V.-N. co-analysed the data. J.D.O., B.A.G., H-W.S., M.B. and G.V.-N co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary text, figures and tables. (PDF 8336 kb)
Supplementary Video 1
Time-lapse footage (1 frame per minute) of the extracorporeal lung, recipient vitals (top right panel), and perfusion data showing the trans-pulmonary pressure gradient (bottom right panel), from cannulation through 36 hours of cross-circulation. (MOV 25221 kb)
Supplementary Video 2
Bronchoscopic video obtained on an ischemic recovered lung. Video obtained at the start of cross-circulation (left panel; demonstrating pulmonary edema), and video obtained at the conclusion of 36 hours of extracorporeal support (right panel; demonstrating intact bronchial microvasculature and the absence of airway edema and secretions). (MP4 10158 kb)
Supplementary Video 3
Real-time transpleural video surveillance of near-infrared (NIR)-labelled cells delivered into the extracorporeal lung. The transpleural imaging system allows the lung to be ventilated while imaging. (MOV 2302 kb)
Supplementary Video 4
Live cilia imaging demonstrating coordinated ciliary beating via the movement of fluorescent microbeads (diameter, 0.2 µm). Airway specimen obtained after 36 hours of cross-circulation support. (MOV 26628 kb)
Rights and permissions
About this article
Cite this article
O’Neill, J., Guenthart, B., Kim, J. et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng 1, 0037 (2017). https://doi.org/10.1038/s41551-017-0037
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0037
This article is cited by
-
Novel Strategies for Optimization of the Pre-transplant Donor Lung
Current Pulmonology Reports (2024)
-
Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches
Drug Delivery and Translational Research (2023)
-
Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs
Nature Medicine (2020)
-
Regeneration of severely damaged lungs using an interventional cross-circulation platform
Nature Communications (2019)
-
Organ preservation: from the past to the future
Acta Pharmacologica Sinica (2018)