Abstract
The production of lithium (Li) increased by 256% in recent years due to unprecedented demands from technological industries. Intensive harvesting poses serious impacts on the sustainability of Li production. Herein, we address the global Li cycle and predict the peak production to reach 740000 million tons in 2041. Global Li accumulation in water bodies is mapped, and the consequences on human health of a wide range (<0.06–>20 mg L−1) of Li concentrations in drinking water are explored. The implications to human health of Li exposure remains unresolved and needs further investigation. There are still no recommendations on safe limits of Li in drinking water for humankind. In conclusion, there is an emergency call to health governing bodies, environmental protection agencies and scientific communities for urgent efforts on sustainable production of Li and identify their thresholds levels in drinking water to minimize the emerging consequences of Li on humans.
Similar content being viewed by others
Introduction
Since the discovery of lithium (Li) in 1970, it has been prescribed as a therapeutic drug for bipolar diseases, protection against suicide, mania, and short-term mortality1. More recently, Li has been used in various industries, including construction, glass and ceramics, lubricating greases, and electronic appliances2. A sharp rise in demand since 2010 for rechargeable Li-ion batteries (LiBs) has remarkably increased Li production by about 100,000 tons per year, tripling the usage3,4. We predict that Li production will reach its peak in 2041, and then wind down to the end of this century when the Li resource is depleted. Furthermore, the estimated 700 million road vehicles in 2050, alongside the minuscule recycling rate (3–5%) of LiBs will drastically elevate the global Li usage and likely lead to increased Li accumulations in aquatic and terrestrial environments, including the food chain5. Currently, there exists limited knowledge and an absence of conclusive evidence on the consequences of Li concentrations in natural water environments and in drinking water.
Recently, anthropogenic activities (like waste material of LiBs, lubricating grease, and pharmaceuticals etc.) triggered Li concentration in surface and drinking water which varied greatly depending upon the geography and remains a graveyard threat to the environment and public health. For example, current studies documented that Li level in groundwater is 300 µg L−1 and 500 µg L−1 in Vehari, Pakistan and in South East Ireland, respectively6,7. Lindsey et al. reported <1–1700 µg L−1 and <1–396 µg L−1 Li in public supply wells and domestic supply wells of United States, respectively8. Consequently, elevated range of Li (0.057–5460 µg L−1) was detected in 132 brands of drinking water bottled across 28 countries9. However, health governing bodies have not yet reported regulatory standards or recommendations for Li levels in drinking water.
Li has a double-edged sword role in human health as Liaugaudaite et al. reported the lower suicide rate at high Li concentrations (0.48–35.53 µg L−1) in drinking water of Lithuania10. Consequently, Harari et al. conducted a study on 178 pregnant women of Argentina (drinking water having 5–1660 µg Li L−1) and found that elevated Li in drinking water disrupts calcium homeostasis during pregnancy11. Given the recent ambiguities in published literature, there is a dire need to unveil the accumulation of Li in drinking water sources for its beneficial or harmful association with human health.
Recently, Li has drawn the attention of the public and the scientific community due to higher production and consumption3 and its possible effects on human health12,13,14. Although an extensive body of data is available on Li accumulation in the food chain15,16,17, few studies documented the potential effects of chronic exposure to Li through water intake11,15. Our systematic perspective presents the current snapshot of global geographical distribution of Li in water bodies, geochemical cycles and peak production curve suggesting the sustainable application of Li in modern tech-industries. Furthermore, we also highlighted the double edge sword role of Li in water bodies to human health and challenges linked with Li to health governing bodies.
Biogeochemical distribution of Li in the environment
Li is delivered to the terrestrial system from various natural processes (brine, leaching from granitic rocks, and weathering), and each of which represents a small source relative to weathering. Weathering plays a key role in the biogeochemical cycle of Li as it leads to a major release of Li from rocks, which upon chemical processes dissolves in rivers and by physical transport processes eventually resides in sediments of rivers and oceans. Rocks possess uneven Li distributions with a general range of about 1–60 ppm18. Three major groups of Li minerals with high commercial values are silicates, micas and phosphates. Climate change might affect weathering processes but further efforts are required to understand the combined effects of climatic factors, types of rocks and weathering on Li fluxes and distributions. A quantitative summary of the global biogeochemical Li cycle as estimated for current conditions with both anthropogenic and natural fluxes is illustrated in Fig. 1.
Atmospheric deposition via sea salt aerosols, gaseous and particulate emissions from volcanoes, and dust arising from wind erosion are the major fluxes of Li in air. Lithium deposition by precipitation happens via water suspensions of Li containing aeolian dust present in the atmosphere and by rain water containing dissolved Li. Li concentrations have been reported in a range of 17–41 mg kg−1 in aeolian dusts, with the concentrations varying among dust sources19. Globally, aeolian dusts are the largest natural flux source of Li in the atmosphere accounting for 38.4 × 109 g year−1 20. Variations in Li depositions due to precipitation have seasonal and local influences, including the human activities. So far, Li is known to diffuse into certain materials (lithium hydroxide, lithium nitride, lithium hydroxide monohydrate, lithium oxide, and lithium carbonate) and reacts with atmospheric air by forming several reaction products. However, the impact of Li on atmospheric deposition rates via reaction of atmospheric carbon and nitrogen are still unknown.
Due to demand and volatile residues, coal is a primary source of Li in the global cycle. The average Li concentration in global coals is about 12 mg kg−1 21, however, concentrations vary widely in industrialized regions, with upto 17 mg kg−1 in Russia22 and 165 mg kg−1 in China23. The impacts of coal combustion are considerable at the global scale, with the mobilization of 550 × 103 tons of Li in 201920 when coal production was 8,129 × 1012 g in 201924. Given the total estimated Li deposition of up to 143 × 109 g year−1 from the atmosphere through different weathering rate might be greater than the other atmospheric deposition20. Thus, particular attention should be given to the range of particles present near emission sites.
Li tends to accumulate in water systems due to its high solubility and mobility characteristics. The residence time of Li is quite long in oceans with an order of about 1000 years for Li to thoroughly mix in an ocean25. Gaillardet et al.26 estimated the global flux of Li transported as the dissolved load in rivers as 69 × 109 g Li year−1, based on their estimation of the average dissolved Li contents in rivers of 1.84 µg L−1 and river discharge of 3.74 × 104 km year−1 27. Additionally, Kavanagh et al.7 assessed Li concentration in rivers which came out in a range of about 0.01–0.05 mg L−1 in dissolved river water with an average of 0.02 mg L−1. So, by referring to both values, ~75 × 103 tons Li year−1 is added by terrestrial rivers to oceans in dissolved form. Apart from this, about 4–21 × 103 tons Li year−1 may reach the oceans by submarine groundwater discharge in coastal areas28. Lithium fluxes from hydrothermal vents (3–15 × 109 mol year−1) and rivers with annual fluxes of 8–16 × 109 mol year−1 are the top sources of Li inputs to oceans (Fig. 1)29.
There was a Li concentration of 0.007 µg L−1 in an East Antarctica ice core30, and a < 0.04 µg L−1 Li concentration in Guadeloupe (Lesser Antilles) France31. In contrast, there were Li concentrations of 0.87 µg L−1 in the Yellow River, Longman Station in China and Tibet32 and about 2.62 µg L−1 in the Yangtze River Basin (snowmelt)33. In 2010, one study reported that the sum of the leading anthropogenic inputs (19–38%) to rivers (ranging from 82 to 95 × 109 g year−1) exceeded the analyzed natural transport of dissolved Li to the sea via rivers (69 × 109 g year−1)20. The simplest explanation for this is that most of the anthropogenic input to freshwaters is precipitated rapidly. A researcher proposed that as little as 10% of the Li in rivers may be transported to the open ocean. The increase in soluble Li in river waters may alter the global mean seawater Li concentration over a long time period34. This perturbation is likely to increase in the coming years.
For instance, each year about 1 billion cell phones are produced, with 1–3 g of Li per phone20 battery. Electric car batteries contain 12 kg of Li20. The number of Li batteries used in technological industries is huge, but the contribution of this emerging potential source of Li to water bodies is unknown. Similarly, the USGS (2020) indicates that ≤1% of the global annual production of Li is used in therapeutic drugs3, amounting to about 0.8 × 109 g year−1 globally, and the roughly estimated contributions of Li drugs to wastewater is ~1% of the annual transport of Li in world rivers20, but it needs further investigation.
Sustainable approaches to handle the peak Li production curve
Total global Li mine reserves are about 210 million tons3, and the reserves from other sources, such as geothermal brines, continental brines, oilfield brines, and hectorite, are about 78 million tons3. We fit a Gaussian distribution to published production data of Li from 1989 to 202035 to estimate that Li production could reach a peak value of 741000 MT in the year 2041. This result encourages an aggressive effort for sustainable management of Li resources. Additionally, we found that Li production increased by 13% per year, and a 256% increase in Li production occurred between 2010 and 20214. To account for the resources available for extraction, we used the value of 21 × 106 MT as the area under the curve and modeled its production under the scenario in Fig. 2.
Production of electric vehicles and their batteries is the sector with the most significant Li use and growth, and batteries are the primary product using ~50% of annual Li resources36. Electric cars used 15 kWh LiBs and the required reserves for LIBs are about 6 × 106 MT. Therefore, accounting for the rest of the Li products and LiBs, the needed resources by 2050 may be around 12 × 106 MT or just over 50% of the current resources and close to our estimated peak value in 2054 (Fig. 2). The recycling of Li ion batteries alongside Li in glass and ceramics (14%), lubricating grease (4%), and air treatment (1%) presents critical challenges37.
Extraction of Li for the technological industries invariably comes with an environmental cost such as air pollution, encroachment on indigenous lands, destruction and fragmentation of wildlife habitats, and depletion and contamination of water resources. Learning from this perspective, future studies are warranted to perform more detailed analysis on the management of Li resources and production that provide additional insights such as ecological and carbon footprints of Li. We call on policymakers, joined by leaders in business and civil society, to develop comprehensive and creative solutions that mindfully address the coming global environmental Li crisis. Some strategies entail the invention and application of new technologies by reducing the amount of virgin Li that must be mined (e.g., recycling and remanufacturing), while others entail battery technologies that do not require Li (e.g., zinc-iron or solid-state technologies).
Li status quo in water bodies
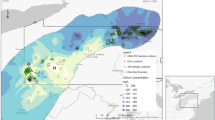
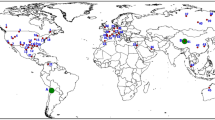
The concentration of Li is highly variable depending on its geographical location38,39, for example the North Atlantic Sea has 220 µg L−1, the Pacific Ocean has 1173 µg L−1, the Indian ocean has 160 µg L−1 and the North Sea has 100 µg L−1 Li40. Most of the Li studies focused on surface waters, e.g., rivers and lakes. About 51% of the studies are related to surface water environmental monitoring projects followed by about 25% in groundwater and 24% in drinking water (Fig. 3). Additionally, Li concentrations in surface waters have been reported to range between 0.01 and 17.5 mg L−1. A location in Chile, Salar de Aguas Calientes, was reported to have an exceptionally large Li concentration of 1500 mg L−1 41 due to high Li mining intensity.
The total concentration of Li in sea water of Ireland was 230,000 to 250,000 megatons (Mt)36. Steinmetz42 reported 10,000 µg L−1 Li in Andean plateau brine water, while 2.2 µg L−1 Li was detected in the Amazon river43. Qi et al. reported 240 to 260 µg L−1 in sea water, 580 to 660 µg L−1 in brine water, and 110–450 µg L−1 in groundwater of Laizhou Bay, China44. Li content in the water of Chongqing city, China varies from 0.14 to 110 µg L−1 45. High mobility due to anthropogenic activities in modern tech industries, urban areas, and geogenic sources (leaching from weathering of minerals and silicate rocks) might be a strong reason for Li contamination in riverine systems. Li in water fluctuates with natural and anthropogenic activities, thus Li determination, quantification, and risk assessment are essential for a sustainable and healthy society.
Groundwater quality has declined over the past decades, Li concentrations in groundwater are generally higher than surface waters with ranges between <0.01 and 14,000 µg L−1 (Fig. 4). Khalid et al.6 reported a 300 µg L−1 Li concentration in groundwater of the Vehari district in Pakistan, but the reason for this high concentration remains unknown. Groundwater Li concentrations range from 16.5 to 1790 µg L−1 in China46, and 1.40–12.2 µg L−1 in Romania, with an average value of 4.56 µg L−1 (Fig. 4)47. This variation in groundwater Li concentration might be due to improper dumping of Li containing appliances with subsequent impacts on the underground system. Thus, analyzing the groundwater chemistry and preventing groundwater pollution from Li are keys to sustainable environments and healthy human lives. Current approaches to protect drinking water may not keep up with increasing human based Li inputs to the environment (Fig. 3). It is well established that drinking water contamination with Li is the main route of Li exposure to human beings, and Li concentrations in drinking waters differ from country to country. Although drinking water guidelines are not commonly reported, the USA drinking-water-only threshold of 60 µg L−1 and the Eurasian Economic Union limit of 30 µg L−1 provide some guidelines8,48.
Data sets were collected for 17 countries from published reports. The categorization (<0.06– >20 mg L−1) used is based of the recommendation by the United States Geological Survey (USGS). Three types of datasets (Li level in water (<0.06, 0.07-3, 3.1-10, 10.1-20, and >20), Sample type (drinking water, surface water, and groundwater), and Li reserves in the world) are presented. Surface water includes lakes, rivers, ponds, and glaciers. Different colors inside the icons highlight the different Li levels in water, various icons show the sample type, and color inside the map denotes the Li reserves in the world.
Krachler and Shotyk9 analyzed 132 brands of bottled water from 28 countries and discovered Li concentrations to range from 0.057 to 5460 µg L−1. The highest concentration of Li (5460 µg L−1) was in bottled water from France, while the bottled water from Germany had concentrations ranging from 1300 to 1400 µg L−1. Li concentrations in drinkng waters of various regions are presented in Fig. 4. For example, the ranges of Li concentrations in drinking water of Texas, USA (2.8–219 µg L−1) and Samos, Greece (0.1–121 µg L−1) are alarming as there are no mining in these areas49,50. However, some geographical regions of Chile, Argentina and Austria have reported some extremely large drinking water Li concentrations of above 1000 µg L−1 38,51. Although, due to the absence of a regulatory threshold, it is unclear which Li concentrations are beneficial or harmful to people. Thus, our perspective is to highlight the urgent need to uncover the reasons for varying levels of Li in drinking water and to establish a threshold Li level in drinking water to avoid major sources of human disorders. It is also worth noting that drinking water analysis in highly mined areas, especially Australia and Chile, are greatly needed at this time because earlier drinking water analysis in these regions was done 10 years ago. Furthermore, researchers and health regulatory bodies must provide in-depth Li risk assessments in various aquatic environments.
Li is a double-edged sword
Lithium is not considered an essential element for biota; however, a recent study suggests that Li may have beneficial neurological and anti-aging effects in humans52. Indeed, studies performed in various countries including Austria51,53, United Kingdom54, Greece50, Italy55, United States49 and Japan56,57 repeatedly documented the reverse correlation between Li concentrations in drinking waters and the risk of suicide. Some researchers reported that, the anti-suicidal effects of low Li content may be explained by the long-term exposure to Li through drinking water. For twelve drinking water samples in Chile (1–207 µg L−1 Li), König et al.58 found lower suicide rates at the higher Li concentrations. Liaugaudaite et al.10 reported Li concentration in nine samples of drinking water ranging 0.48–35.53 µg L−1 (Fig. 4), that lower suicide rates in men occurred at higher Li concentrations. However, Kozaka et al.59 reported that there was no association between Li concentrations of 0.2 to 12.3 µg L−1 and suicide mortality rates in Miyazaki Prefecture Japan. Resolving the apparent contradictions on the impact of Li concentrations on humans is necessary to advance our knowledge of human disorders.
Although Li might be a useful element for the human body, its toxicological action at high levels must not be neglected (Fig. 5). Adverse side effects of Li can be seen at therapeutic dose levels, resulting in a narrow therapeutic index60. Lithium treatment in humans is mainly associated with an increased risk of reduced urinary concentrating ability, hypothyroidism, hyperparathyroidism, and weight gain60,61. These adverse effects of Li are associated with serum concentrations ranging from 3.5 mg L−1 to >17.4 mg L−1. Nephrotoxicity is the most common adverse effect of Li therapy62, affecting 30–40% of patients taking Li-containing drugs. Lithium nephrotoxicity manifests as an impaired ability of renal ducts to concentrate urine due to Li induced changes leading to a syndrome called “nephrogenic diabetes insipidus or NDI”63 (Fig. 5). Other outcomes associated with Li overdoses include neurological and psychiatric effects (tremor, muscle hyperirritability, muscle incoordination, blackout spells, epileptic seizures, slurred speech, coma, psychosomatic retardation, increased thirst, anorexia), cardio-vascular effects (cardiac arrhythmia, hypertension, circulatory collapse), and gastrointestinal effects (nausea, vomiting, abdominal pain)60 (Table 1)62. Schullehner et al.64 reported that a Li concentration in drinking water ranging from 0.6–30.7 µg L−1 significantly increased the incidence of schizophrenia spectrum disorder and schizophrenia in Denmark, furthermore no association was found in bipolar incidence rates.
Illustration of (a) Lithiumeter depicting the acute and chronic toxicity categories for various Li concentration ranges in the human body (b) Drinking water containing Li and associated consequences on human body organs, and (c) Impact of Li on functions linked with the human brain arrow highlight that Li impact on macro organs to micro organs of the human body. “↑” highlights the increase and “↓” show the decrease of disease levels.
High Li levels in waterbodies and tap water due to pollution from Li batteries, caused toxicity to human cardiomyocytes12. Indeed, Li significantly repressed cell capability and proliferation of human cardiomyocytes and promoted cell apoptosis. These effects were attributed to the regulation of glycogen synthase kinase 3 beta (GSK3β), an enzyme in humans that is encoded by the GSK3β gene12. Abnormal regulation and expression of GSK3β are associated with mood disorders, bipolar disorder and depression, and schizophrenia12. In Hetao Plain China, Li concentrations measured at fifty four groundwater sampling sites ranged from 16.5 to 1790 µg L−1 and had 37.4% non-carcinogenic risk to human46. Harari et al.65 observed 5.0–1660 µg L−1 Li in drinking water of Argentina and found that Li intake via drinking water may adversely affect thyroid function during pregnancy, as Li harms thyroid homeostasis at the pituitary level, directly in thyroid follicles and through the disruption of thyroid hormone transport.
As far as we know, still there are no recommendations on safe life limits of Li in drinking water from the World Health Organization (WHO) or other health governing bodies. The WHO should provide such recommendations and guidance on the direct intake of drinking water, especially in high mining areas. Furthermore, although Li toxicity might be especially risky to children, and so far there are no studies on children’s health due to Li exposure in drinking water.
Li as an open challenge for scientific and health governing bodies
The estimations of daily intake (DI) of oral Li are very diverse, ranging from 100 to 1000 mg day−1 66 depending on its availability in the environment and in food products. The recommended dietary allowance (RDA) for Li intake is 1.0 mg day−1 for a 70 kg adult39,67. Recently, a range (0.033–0.080 mg day−1) of DI levels from municipal drinking water has also been reported62. Furthermore, Li concentrations in drinking waters are expected to increase drastically as Li inputs into all of the environmental compartments increase with advancements in technological industries. We urge the need to formalize existing traditional and gray literature knowledge of Li in waters and expand research partnerships with all stack holders to conduct research on sustainable uses of Li.
Lithium in drinking water is a major source of overall Li intake into the human body. For example, in Chile, Li-rich saline can contain up to 1500 mg L−1 Li, and although total Li intake may reach 10 mg day−1, the adverse effects on the local population remains unknown. The Tolerable Daily Intake (TDI) of Li is reported to be 0.02 mg kg−1 day−1, which is equivalent to 1.40 mg day−1 for a 70 kg adult68, but these findings are outdated. There is a dire need to update the TDI values of Li because the production of Li has increased significantly (256%) in recent years. The estimated daily intake of Li in Germany and France is 0.2 and 0.5 mg day−1 respectively (ANSES, 2011)69. According to the Oregon Health Authority, the chronic oral exposure to high doses of Li is 140 mg day−1 70.
Lindsay et al.8 reported a 60 µg L−1 of Li in drinking water, while the proposed health based screening level of Li in drinking water was 10 µg L−1. Consequently, these values were calculated by using Environmental Protection Agencies (EPA) chronic exposure dose of 20 µg kg−1 day−1. Additionally, the Eurasian Economic Union has fixed a limit of 30 µg L−1 for Li in drinking water48. The above mentioned recommendations are based on regional data, and there are no WHO recommendations on safe levels of Li in drinking water. The scientific community is not in agreement on Li toxicity levels in the human body, as mixed reports have been provided on suicide rates at high Li exposure. Thus, Li is an open challenge to health governing bodies and scientists, and Li recommendations are needed for human health outcomes. Environmental protection agencies must also address growing Li sources that threaten groundwater quality.
Conclusion: future-road map
We present a global life cycle assessment of Li resources, which includes Li discharges into various environmental compartments. A minor amount of Li is delivered to the terrestrial system via natural processes, while sources such as mining activity, coal combustion and aerial deposition are the major sources to the environmental Li cycle. We estimate that Li production can reach its peak (741000 MT) in 2054 based on an average annual growth of 13%. The 256% increase in Li production between 2010 and 2021 is a clarion call for sustainable management of Li resources. Our estimation of the Li peak provides guidance to consumer agencies on planning future Li extractions that minimize environmental and human health consequences. The Li peak timing can be effectively delayed by enhancing Li recycling and adopting sustainable approaches for Li applications in consumer industries. Much of the data on Li in drinking water systems was obtained >10 years ago, and the scientific community is in dire need for new data on Li distributions due to recent increases in industrial uses of Li.
A low recycling rate of Li will ultimately give rise to Li in our water bodies through anthropogenic resources. Concerning human health, contradictory findings about suicide mortality and other diseases have been reported due to human exposure to Li through drinking water. Currently, there are no regulatory standards from health organization agencies on the safe Li level in drinking water. Thus, we conclude that sustainable applications of Li and proper recycling are the best approaches to safely handle and utilize Li resources. Moreover, we provide a future road map (Fig. 6), with practical suggestions for sustainable management and a way forward to reduce Li risks that arise from drinking water.
-
Sustainable actions are needed to recycle and properly handle Li containing wastes to meet the balance between demand and supply.
-
International policy frameworks and collaborations need to adopt holistic approaches considering human and environmental health cost, and expanded inclusion of supply-demand relationship for sustainable execution of Li resources.
-
Role of Li as a mood stabilizer and neuron transmitter have been documented, however the specific effect of Li on brain functioning of males and females is unknown. So, additional gender-based studies should be performed to identify the mechanistic roles of Li in human males and females.
-
The data presented in our review is based on available published resources, and safe limits of Li in drinking water and other sources are still unknown and need to be confirmed by the health governing bodies.
-
Site specific assessments of Li effects on terrestrial biota should be performed, especially in mining areas with high Li concentrations in drinking water.
-
Sustainable actions are needed to recycle and properly handle LiBs and Li containing wastes, especially with the recent large increases (256%) in Li consumption and production.
-
Replacement of Li with Na or other metals/materials should be considered for the future point of research to minimize the dependence on Li.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Draaisma, D. Lithium: the gripping history of a psychiatric success story. Nature 572, 584–586 (2019).
Zhang, Y. et al. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 128, 141–152 (2018).
Summaries, M. C. Mineral Commodity Summaries; USGS Unnumbered Series (US Geological Survey, 2021).
USGS. Mineral Commodity Summaries. https://www.usgs.gov/centers/national-minerals-information-center/lithium-statistics-and-information (2022).
Ghandi, A. & Paltsev, S. Global CO2 impacts of light-duty electric vehicles. Transp. Res. Part D: Transp. Environ. 87, 102524 (2020).
Khalid, S. et al. Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ. Sci. Poll. Res. 27, 39852–39864 (2020).
Kavanagh, L., Keohane, J., Cleary, J., Garcia Cabellos, G. & Lloyd, A. Lithium in the natural waters of the South East of Ireland. Intern. J. Environ. Res. Public Health 14, 561 (2017).
Lindsey, B. D., Belitz, K., Cravotta, C. A. III, Toccalino, P. L. & Dubrovsky, N. M. Lithium in groundwater used for drinking-water supply in the United States. Sci. Total Environ. 767, 144691 (2021).
Krachler, M. & Shotyk, W. Trace and ultratrace metals in bottled waters: survey of sources worldwide and comparison with refillable metal bottles. Sci. Total Envir. 407, 1089–1096 (2009).
Liaugaudaite, V., Mickuviene, N., Raskauskiene, N., Naginiene, R. & Sher, L. Lithium levels in the public drinking water supply and risk of suicide: a pilot study. J. Trace Elem. Med. Biol. 43, 197–201 (2017).
Harari, F., Åkesson, A., Casimiro, E., Lu, Y. & Vahter, M. Exposure to lithium through drinking water and calcium homeostasis during pregnancy: a longitudinal study. Environ. Res. 147, 1–7 (2016).
Shen, J. et al. The toxicity of lithium to human cardiomyocytes. Environ. Sci. Eur. 32, 1–12 (2020).
Eyre-Watt, B. et al. The association between lithium in drinking water and neuropsychiatric outcomes: a systematic review and meta-analysis from across 2678 regions containing 113 million people. Aust. N.Z. J. Psychiatry 55, 139–152 (2021).
Shakoor, N. et al. Environment relevant concentrations of lithium influence soybean development via metabolic reprogramming. J. Hazard. Mater. 441, 129898 (2023).
Voica, C., Roba, C. & Iordache, A. Lithium levels in food from the Romanian market by inductively coupled Plasma–Mass Spectrometry (ICP-MS): a pilot study. Anal. Lett. 54, 242–254 (2021).
Figueroa, L. et al. Environmental lithium exposure in the North of Chile—I. Natural water sources. Biol. Trace Elem. Res.149, 280–290 (2012).
Figueroa, L. T. et al. Environmental lithium exposure in the north of Chile-II. Natural food sources. Biol. Trace Elem. Res. 151, 122–131 (2013).
Schulz, K. J., DeYoung, J. H., Seal, R. R. & Bradley, D. C. Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply (Geological Survey, 2018).
Liu, X.-M., Rudnick, R. L., McDonough, W. F. & Cummings, M. L. Influence of chemical weathering on the composition of the continental crust: Insights from Li and Nd isotopes in bauxite profiles developed on Columbia River Basalts. Geochim. Cosmochim. Acta 115, 73–91 (2013).
Schlesinger, W. H., Klein, E. M., Wang, Z. & Vengosh, A. Global Biogeochemical Cycle of Lithium (Wiley Online Library, 2021).
Ketris, M. á & Yudovich, Y. E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Intern. J. Coal Geol. 78, 135–148 (2009).
Tewalt, S. J. et al. Chemical Analyses in the World Coal Quality Inventory. Report No. 2331–1258 (US Geological Survey, 2010).
Qin, S., Zhao, C., Li, Y. & Zhang, Y. Review of coal as a promising source of lithium. Intern. J. Oil, Gas. Coal Tech. 9, 215–229 (2015).
BP. Statistical Review of World Energy 2020 69th edn (BP, 2020).
Li, G. & West, A. J. Evolution of Cenozoic seawater lithium isotopes: coupling of global denudation regime and shifting seawater sinks. Earth Planet. Sci. Lett. 401, 284–293 (2014).
Gaillardet, J., Viers, J. & Dupré, B. Trace elements in river waters. Treat. Geochem 5, 605 (2003).
Berner, E. K. & Berner, R. A. Global Environment: Water, Air and Geochemical Cycles (Princeton University Press, 2012).
Mayfield, K. K. et al. Groundwater discharge impacts marine isotope budgets of Li, Mg, Ca, Sr, and Ba. Nat. commun. 12, 1–9 (2021).
Hathorne, E. C. & James, R. H. Temporal record of lithium in seawater: a tracer for silicate weathering? Earth Planet. Sci. Lett. 246, 393–406 (2006).
Siggaard-Andersen, M.-L. et al. Soluble and insoluble lithium dust in the EPICA DomeC ice core—Implications for changes of the East Antarctic dust provenance during the recent glacial–interglacial transition. Earth Planet. Sci. Lett. 258, 32–43 (2007).
Clergue, C. et al. Influence of atmospheric deposits and secondary minerals on Li isotopes budget in a highly weathered catchment, Guadeloupe (Lesser Antilles). Chem. Geol. 414, 28–41 (2015).
Gou, L.-F. et al. Li isotopes in the middle Yellow River: seasonal variability, sources and fractionation. Geochim. Cosmochim. Acta 248, 88–108 (2019).
Ma, T. et al. Lithium isotope compositions of the Yangtze River headwaters: Weathering in high-relief catchments. Geochim. Cosmochim. Acta 280, 46–65 (2020).
Wimpenny, J. et al. The behaviour of Li and Mg isotopes during primary phase dissolution and secondary mineral formation in basalt. Geochim. Cosmochim. Acta 74, 5259–5279 (2010).
Cordell, D. & White, S. Peak phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3, 2027–2049 (2011).
Kavanagh, L., Keohane, J., Garcia Cabellos, G., Lloyd, A. & Cleary, J. Global lithium sources—industrial use and future in the electric vehicle industry: a review. Resources 7, 57 (2018).
Bolan, N. et al. From mine to mind and mobiles–Lithium contamination and its risk management. Environ. Pollut. 290, 118067 (2021).
Concha, G. et al. High-level exposure to Lithium, Boron, Cesium, and Arsenic via drinking water in the Andes of Northern Argentina. Environ. Sci. Technol. 44, 6875–6880 (2010).
Schrauzer, G. N. Lithium: occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 21, 14–21 (2002).
Choubey, P. K., Kim, M.-s, Srivastava, R. R., Lee, J.-c & Lee, J.-Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 89, 119–137 (2016).
Aral, H. & Vecchio-Sadus, A. Toxicity of lithium to humans and the environment-a literature review. Ecotoxicol. Environ. Saf. 70, 349–356 (2008).
Steinmetz, R. Lithium-and boron-bearing brines in the Central Andes: exploring hydrofacies on the eastern Puna plateau between 23 and 23 30′ S. Miner. Depos. 52, 35–50 (2017).
Dellinger, M. et al. Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes. Geochim. Cosmochim. Acta 164, 71–93 (2015).
Qi, H., Ma, C., He, Z., Hu, X. & Gao, L. Lithium and its isotopes as tracers of groundwater salinization: a study in the southern coastal plain of Laizhou Bay, China. Sci. Total Environ. 650, 878–890 (2019).
Liu, Y., Yuan, Y. & Luo, K. Regional distribution of longevity population and elements in drinking water in Jiangjin District, Chongqing City, China. Biol. Trace Elem. Res. 184, 287–299 (2018).
Chen, L., Ma, T., Wang, Y. & Zheng, J. Health risks associated with multiple metal (loid) s in groundwater: a case study at Hetao Plain, northern China. Environ. l Poll. 263, 114562 (2020).
Török, A. I. et al. Assessment of Lithium, macro-and microelements in water, soil and plant samples from Karst areas in Romania. Materials 14, 4002 (2021).
EAEU. EAEU Technical Regulation on Safety of Packaged Potable Water Including Natural Mineral Water (TR EAEU 044/2017), as Translated by the United States Department of Agriculture, Foreign Agricultural Service, Global Agricultural Information (No. Network Report Number RS1752). https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=EAEU%20Technical%20Regulation%20on%20Packaged%20Water_Moscow_Russian%20Federation_10-30-2017.pdf (2017).
Blüml, V. et al. Lithium in the public water supply and suicide mortality in Texas. J. Psychiatr. Res. 47, 407–411 (2013).
Giotakos, O., Nisianakis, P., Tsouvelas, G. & Giakalou, V.-V. Lithium in the public water supply and suicide mortality in Greece. Biol. Trace Elem. Res. 156, 376–379 (2013).
Kapusta, N. D. et al. Lithium in drinking water and suicide mortality. Br. J. Psych. 198, 346–350 (2011).
Martone, G. Nutritional lithium. J. Clin. Psychiatry Neurosci. 1, 3–4 (2018).
Helbich, M., Leitner, M. & Kapusta, N. D. Lithium in drinking water and suicide mortality: interplay with lithium prescriptions. Br. J. Psychiatry 207, 64–71 (2015).
Kabacs, N., Memon, A., Obinwa, T., Stochl, J. & Perez, J. Lithium in drinking water and suicide rates across the East of England. Br. J. Psychiatry 198, 406–407 (2011).
Pompili, M. et al. Relationships of local lithium concentrations in drinking water to regional suicide rates in Italy. World J. Biol. Psychiatry 16, 567–574 (2015).
Ishii, N. et al. Low risk of male suicide and lithium in drinking water. J. Clin. Psychiatry 76, 10893 (2015).
Shiotsuki, I. et al. Trace lithium is inversely associated with male suicide after adjustment of climatic factors. J. Affect Disord. 189, 282–286 (2016).
König, D. et al. Impact of natural lithium ressources on suicide mortality in Chile 2000–2009: a geographical analysis. Neuropsychiatrie 31, 70–76 (2017).
Kozaka, N., Takeuchi, S., Ishii, N., Terao, T. & Kuroda, Y. Association between lithium in tap water and suicide mortality rates in Miyazaki Prefecture. Environ. Health Prev. Med. 25, 1–7 (2020).
Gitlin, M. Lithium side effects and toxicity: prevalence and management strategies. Intern. J. Bipolar Disord. 4, 1–10 (2016).
Guideline, I. H. Guideline for elemental impurities. Q3D. Current Step 4, 1–83 (2014).
EPA, U. S. Provisional Peer-Reviewed Toxicity Values for Lithium (U.S. Environmental Protection Agency, 2008).
Rej, S. et al. Molecular mechanisms in lithium-associated renal disease: a systematic review. Intern. Urolo. Nephr. 48, 1843–1853 (2016).
Schullehner, J. et al. Lithium in drinking water associated with adverse mental health effects. Schizophr. Res. 210, 313–315 (2019).
Harari, F., Bottai, M., Casimiro, E., Palm, B. & Vahter, M. Exposure to lithium and cesium through drinking water and thyroid function during pregnancy: a prospective cohort study. Thyroid 25, 1199–1208 (2015).
Szklarska, D. & Rzymski, P. Is Lithium a micronutrient? From biological activity and epidemiological observation to food fortification. Biol. Trace Elem. Res. 189, 18–27 (2019).
Marshall, T. M. Lithium as a nutrient. J. Am. Physicians Surg. 20, 104–109 (2015).
U.S.E.P. US EPA region III. (U.S.E.P. Agency, 2007).
Shakoor, N. et al. Reimagining safe lithium applications in the living environment and its impacts on human, animal, and plant system. Environ. Sci. Ecotech. https://doi.org/10.1016/j.ese.2023.100252 (2023).
Authority, O. H. Health Effects Information, Lithium (Healthy Waters Program, Office of Environmental Public Health, 2011).
Liaugaudaite, V., Naginiene, R., Raskauskiene, N., Mickuviene, N., Bunevicius, A. & Sher, L. Relationship between lithium levels in drinking water and suicide rates: A nationwide study in Lithuania. Arch Suicide Res. 25, 340–352 (2021).
Sugawara, N., Yasui-Furukori, N., Ishii, N., Iwata, N. & Terao, T. Lithium in tap water and suicide mortality in Japan. Int. J. Environ. Res. Public Health 10, 6044–6048 (2013).
Broberg, K., Concha, G., Engström, K., Lindvall, M., Grandér, M. & Vahter, M. Lithium in drinking water and thyroid function. Environ. Health Perspect. 119, 827–830 (2011).
Acknowledgements
This work was funded by National Key Research and Development Program of China, (2018YFA0606500 and 2017YFA0604300). Authors are truly grateful to “Xiao Li” Song from JOYO Technology Co., LTD for help with figure preparation.
Author information
Authors and Affiliations
Contributions
M.A. contributed to the idea and concept, analyzed/interpreted the literature studies, wrote the original and revised manuscript, and provided his insights during revision. M.Z. and N.S. proposed the idea and concept, collected relevant studies, and wrote the original manuscript. M.A.A. and I.A. collected relevant studies, wrote the original and revised manuscript. M.A.A., R.X.S.T., A., M.T., R.H., M.X., and R.Y. proposed the idea and concept, wrote the original and revised manuscript, provided his advice during revision, and polished the final version. All authors contributed to the interpretation and discussion of reviewed studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adeel, M., Zain, M., Shakoor, N. et al. Global navigation of Lithium in water bodies and emerging human health crisis. npj Clean Water 6, 33 (2023). https://doi.org/10.1038/s41545-023-00238-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00238-w