Abstract
The Centers for Disease Control and Prevention announced in January 2023 a potential connection between administration of the Pfizer novel coronavirus disease-2019 (COVID-19) bivalent vaccine booster and ischemic stroke (IS). A retrospective cohort study was conducted to compare the hazard of IS in patients aged 65 years and over administered the Pfizer bivalent booster versus those administered the Pfizer/Moderna monovalent or Moderna bivalent boosters. De-identified patient electronic health data were collected from TriNetX, a cloud-based analytics platform that includes data from over 90 million unique patients in the United States. Patients aged 65 years and over at the time of administration of a Pfizer bivalent, Moderna bivalent, or Pfizer/Moderna monovalent booster were included for analysis. Cohorts were propensity-score matched. The hazard ratios (HR) and 95% confidence intervals (CI) for IS between matched cohorts at 1–21 and 22–42 days after booster administration were calculated. There was reduced hazard of IS in the Pfizer bivalent cohort compared to the monovalent cohort at both timepoints: 1–21 days after vaccination (HR: 0.54, 95% CI: 0.47–0.62), and 22–42 days after vaccination (HR: 0.62, 95% CI: 0.54–0.72) (n = 79,036 patients per cohort). There was reduced hazard of IS in the Pfizer bivalent cohort compared to the Moderna bivalent cohort at 1–21 days after vaccination (HR: 0.75, 95% CI: 0.58–0.96) (n = 26,962 patients per cohort). This analysis provides no evidence that the Pfizer bivalent vaccine is associated with increased hazard of IS compared to the monovalent or Moderna bivalent vaccines.
Similar content being viewed by others
Introduction
In January 2023, the Centers for Disease Control and Prevention (CDC) announced that their Vaccine Safety Datalink met the threshold to investigate the risk of ischemic stroke (IS) within three weeks of administration of the Pfizer/BioNTech novel coronavirus disease-2019 (COVID-19) bivalent vaccine (Pfizer bivalent booster)1. No such concern was raised in the CDC’s statement for the Moderna COVID-19 bivalent vaccine (Moderna bivalent booster). The earlier monovalent Moderna and Pfizer vaccines display no increased risk for IS in the general American population2; however, COVID-19 infection itself appears to be a risk factor for IS in patients ages 65 years and over3. This CDC announcement was therefore unanticipated, and since then both the Food and Drug Administration and European Medicines Agency have reported no increased IS risk for the Pfizer bivalent vaccine in their respective databases4,5. A recent analysis from the French National Health Data System has come to similar conclusions6. In response to these inconsistent findings and the wide use of COVID-19 bivalent vaccines in older adults in the United States, we set out to examine the comparative hazard of IS in patients ages 65 years and over who were administered the Pfizer bivalent booster, Moderna bivalent booster, or Pfizer/Moderna monovalent booster.
Results
The study population of patients aged 65 years and over at the time of booster administration on or before August 27, 2023, included 110,667 who received the Pfizer bivalent booster, 26,962 who received the Moderna bivalent booster, and 96,156 who received a monovalent booster. Most monovalent vaccine booster doses were administered between August 2021 and February 2022, while most bivalent vaccine booster doses were administered between September 2022 and May 2023. The Pfizer bivalent booster cohort did not differ significantly from the monovalent cohort at baseline in demographics and had a significantly higher prevalence of pre-existing medical conditions, including COVID-19 (Table 1). After matching for the primary analysis, the two cohorts were balanced, and there were 79,036 patients in each cohort (Table 1). Details on the propensity-score matching results between the Pfizer bivalent and Moderna bivalent cohorts are available in Supplementary Table 1; for this analysis, there were 26,962 patients in each cohort after matching.
There was reduced hazard of an IS encounter diagnosis in the Pfizer bivalent cohort compared to the matched monovalent cohort at 1–21- and 22–42-days post-administration: Hazard Ratio (HR) = 0.54, 95% Confidence Interval (CI) (0.47–0.62), and HR = 0.62, 95% CI (0.54–0.72), respectively (Fig. 1). There was reduced hazard of an IS encounter diagnosis in the Pfizer bivalent cohort compared to the matched Moderna bivalent cohort at 1-21 days: HR = 0.75, 95% CI (0.58–0.96), and no difference was observed at 22–42 days post-administration: HR = 0.99, 95% CI (0.78–1.28) (Fig. 1). There was no difference in the hazard of first-time IS encounter diagnoses between the Pfizer bivalent cohort and the matched monovalent cohort at either timepoint: HR = 1.07, 95% CI (0.69–1.67), and HR = 1.25, 95% CI (0.84, 1.86), respectively (Fig. 1).
Comparison of ischemic stroke hazard in the 1–21- and 22–42-day time-windows that followed from the day of vaccine administration between propensity-score matched Pfizer bivalent and monovalent cohorts (all strokes, first-time [“new”] strokes), and between propensity-score matched Pfizer bivalent and Moderna bivalent cohorts (all strokes).
Discussion
We observed a reduced hazard of IS encounter diagnosis in the Pfizer bivalent cohort compared to the monovalent cohort, perhaps due to bivalent boosters providing stronger protection against severe COVID-19 infection and hospitalization than their monovalent counterparts7,8. Additionally, severe COVID-19 infection increases the risk of IS9, and the Omicron strain (dominant in 2022 when bivalent vaccines were distributed) produces less severe disease than the Delta strain (dominant in 2021 when monovalent vaccines were distributed)10, so perhaps this explains why patients administered the Pfizer bivalent booster displayed reduced hazard of an IS encounter diagnosis. There was also significantly reduced hazard of IS encounter diagnosis in the Pfizer bivalent versus Moderna bivalent cohorts, but only at the 1–21 day period. Additionally, there was no significant difference in the hazard of first-time IS encounter diagnosis between patients in the Pfizer bivalent and monovalent cohorts. Patients with prior stroke are at high risk for subsequent stroke due to inflammatory and vascular factors11 that severe COVID-19 may further exacerbate, whereas patients without prior stroke lack these factors.
There are case reports of IS being associated with vaccine-induced immune thrombotic thrombocytopenia12, so this may explain why some patients in our cohorts experienced IS after vaccination. Considering the high incidence of IS in the general population (as the fifth leading cause of death in the United States) and the high prevalence of IS risk factors in both cohorts (older age, dyslipidemia, hypertension, type II diabetes mellitus, overweight/obesity, and cerebrovascular disease amongst others)13, it is likely that a greater etiology of these strokes are the classic risk factors that were going to cause them regardless of COVID-19 vaccination. The CDC warned of a potentially increased risk of IS within three weeks of administration of Pfizer bivalent boosters1; this may be a reflection of the higher prevalence of pre-existing medical conditions that are IS risk factors among patients who received the Pfizer bivalent booster compared to those who received the monovalent booster (Table 1).
Limitations of this study include the use of the TriNetX platform, which is not a random sampling of the entire United States population over the age of 65 years; therefore, the generalizability of these results needs to be tested in other cohorts. Both Pfizer and Moderna bivalent vaccines were approved in August 2022; however, monovalent vaccines were approved earlier. While patients in the Pfizer bivalent cohort and the monovalent cohort were followed for the same length of time, the dominant SARS-Cov-2 variants that patients in these two cohorts encountered were different, which may confound the results. Additionally, this study does not include a complete sample of those who were vaccinated in the population of interest because many vaccines were administered outside of the healthcare organizations (HCOs) that report data to the TriNetX platform. In this study, patients were followed for up to six weeks; future studies should examine longer-term associations between COVID-19 vaccination and IS. In summary, our analysis provides no evidence that American patients ages 65 years and over have an increased risk of IS after Pfizer bivalent booster administration; patients and healthcare providers should not be dissuaded from receiving or administering this booster vaccine.
Methods
Data collection, study population, variables, and outcomes
We used the TriNetX platform to access aggregated, de-identified electronic health records (EHR) of over 90 million patients from 56 HCOs across all 50 American states, covering diverse geographic, age, race, and ethnic groups (United States Collaborative Network)5. The MetroHealth System, Cleveland Ohio, Institutional Review Board (IRB) has determined that research using the de-identified and aggregated data from TriNetX as described in this study is not Human Subject Research and therefore IRB review was not required. We have previously used the TriNetX platform to study risk factors and outcomes of COVID-19 infection and vaccination14,15,16.
TriNetX data are collected from participating HCOs, primarily from EHR systems comprised of structured demographics, diagnoses, procedures, and medications but also from facts extracted from clinical documents using natural language processing17. TriNetX completes intensive data preprocessing to minimize missing values. The platform also maps data to a clinical model with consistent semantic meanings so that the data can be queried consistently regardless of the underlying data source. All covariates are either binary, categorical, or continuous. Missing sex values are represented as “Unknown Sex,” while missing data for race and ethnicity are represented as “Unknown Race” and “Unknown Ethnicity,” respectively. The data available in TriNetX go back decades, depending on the HCO, and are frequently updated (80% of data providers update their data in 1, 2, or 4-week intervals)18. For this study, the EHR data were queried and analyzed on October 8, 2023.
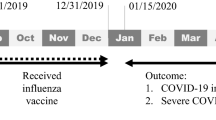
The primary analysis compared the hazard of IS in patients aged 65 years and over after Pfizer bivalent booster versus monovalent booster; the secondary analysis compared the hazard of IS in patients aged 65 years and over after Pfizer bivalent booster versus Moderna bivalent booster (Fig. 2). The exposure of interest was vaccination by either the Pfizer bivalent booster (“Pfizer bivalent” cohort), Moderna bivalent booster (“Moderna bivalent” cohort), or Pfizer/Moderna monovalent booster (“monovalent” cohort) prior to August 27, 2023, to ensure sufficient time for follow-up at 21 and 42 days (Fig. 2). Patients in the monovalent cohort were included beginning in August 2021, while those in the Pfizer and Moderna bivalent cohorts were included beginning in September 2022, as these time periods represent when the cohorts began receiving booster vaccines in TriNetX. Cohorts were matched by demographics (age, sex, race, ethnicity), COVID-19 infection, medical conditions that are risk factors for both IS and severe COVID-19 infection19,20, and adverse socioeconomic determinants of health (Table 1). Self-reported race and ethnicity data in TriNetX come from the clinical EHR systems of the contributing HCOs. TriNetX maps race and ethnicity data from its contributing HCOs to the following categories: (1) Race: Asian, American Indian or Alaskan Native, Black or African American, Native Hawaiian or Other, White, Unknown Race; and (2) Ethnicity: Hispanic or Latino, Not Hispanic or Latino, Unknown Ethnicity. The outcome of interest was an encounter diagnosis for IS in TriNetX at either 1–21 days or 22–42 days after booster administration (Fig. 2). Details of clinical codes for covariates, exposures, and outcomes are described in Supplementary Table 2.
Statistical analysis
To compare the hazard of IS between the Pfizer bivalent and monovalent cohorts, as well as the Pfizer bivalent and Moderna bivalent cohorts, the cohorts were propensity-score matched (1:1 matching by nearest neighbor greedy matching algorithm with a caliper of 0.25 standard deviations) for the variables enumerated above. Kaplan–Meier survival analysis was used to estimate the probability of IS at 1–21 days or 22–42 days after booster administration. The Kaplan–Meier analysis estimates the probability of an outcome at a respective time interval (daily time interval in this analysis). To account for the patients who exited the cohort during the analysis period, and therefore should not be included in the analysis, censoring was applied. Patients are censored when the last data point in the patient’s record is within the time interval of interest, or if the outcome of interest occurs after the index event but before the start of the time window21. The Cox proportional hazard assumption was tested using Schoenfeld residuals22. The TriNetX platform calculates HR and associated 95% CI using the R survival package v3.2-3. For generating HR, TriNetX sets robust=FALSE using the R survival package, which is a limitation of the TriNetX platform since it does not consider potential clustering of patients within HCOs or specific geolocations. All statistical tests were conducted in October 2023 within the TriNetX Analytics platform with significance set at p-value < 0.05 (two-sided). A sub-analysis was conducted to compare the hazard of first-time IS between the Pfizer bivalent cohort and monovalent cohort, but not between the Pfizer bivalent cohort and Moderna bivalent cohort due to limited sample size.
Data availability
A subscription to TriNetX Analytics is required to query the aggregated, de-identified patient data analyzed in this study. All relevant data are available from the authors.
Code availability
The custom code used to generate hazard ratios in this study is proprietary to TriNetX Analytics. A subscription to the TriNetX platform is required for description of this code, which can be found in the TriNetX Help Center once the subscription is obtained.
References
CDC. COVID-19 Vaccination. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/bivalent-boosters.html (2020).
Klein, N. P. et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326, 1390–1399 (2021).
Yang, Q., Tong, X., George, M. G., Chang, A. & Merritt, R. K. COVID-19 and risk of acute ischemic stroke among medicare beneficiaries aged 65 years or older. Neurology 98, e778 (2022).
Erman, M. U.S. CDC still looking at potential stroke risk from Pfizer bivalent COVID shot. Reuters (2023).
Reuters. EU drug regulator has not seen signal of possible Pfizer COVID shot stroke link. Reuters (2023).
Jabagi, M.-J. et al. Stroke, myocardial infarction, and pulmonary embolism after bivalent booster. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2302134 (2023).
Lin, D.-Y. et al. Effectiveness of bivalent boosters against severe omicron infection. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2215471 (2023).
Collier, A. Y. et al. Immunogenicity of BA.5 Bivalent mRNA vaccine boosters. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2213948 (2023).
Sagris, D. et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 28, 3826–3836 (2021).
Antonelli, M., Pujol, J. C., Spector, T. D., Ourselin, S. & Steves, C. J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 399, 2263–2264 (2022).
Hankey, G. J. Secondary stroke prevention. Lancet Neurol. 13, 178–194 (2014).
Kolahchi, Z., Khanmirzaei, M. & Mowla, A. Acute ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 439, 120327 (2022).
Guzik, A. & Bushnell, C. Stroke epidemiology and risk factor management. Contin. Lifelong Learn. Neurol. 23, 15–39 (2017).
Wang, L., Davis, P. B., Kaelber, D. C., Volkow, N. D. & Xu, R. Comparison of mRNA-1273 and BNT162b2 vaccines on breakthrough SARS-CoV-2 infections, hospitalizations, and death during the delta-predominant period. JAMA 327, 678–680 (2022).
Wang, L. et al. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the omicron and delta variants in children younger than 5 years in the US. JAMA Pediatr. 176, 811–813 (2022).
Wang, W., Kaelber, D. C., Xu, R., & Berger, N. A. Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Mortality in Vaccinated Patients With Cancer in the US Between December 2020 and November 2021. JAMA Oncol. 8, 1027–1034 (2022).
Where do TriNetX data come from? TriNetX Help Center https://support.trinetx.com/hc/en-us/articles/360004265733-Where-do-TriNetX-data-come-from (2021).
What is the range of dates covered by TriNetX data? TriNetX Help Center https://support.trinetx.com/hc/en-us/articles/360004241114-What-is-the-range-of-dates-covered-by-TriNetX-data (2018).
Boehme, A. K., Esenwa, C. & Elkind, M. S. V. Stroke risk factors, genetics, and prevention. Circ. Res. 120, 472–495 (2017).
Gao, Y. et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 76, 428–455 (2021).
In outcomes, when are patients censored in Kaplan-Meier Analysis? TriNetX Help Center https://support.trinetx.com/hc/en-us/articles/360003074853-In-outcomes-when-are-patients-censored-in-Kaplan-Meier-Analysis (2018).
Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 69, 239–241 (1982).
Acknowledgements
We acknowledge support from the National Institute on Aging (Grant no. RF1AG076649), National Institute on Alcohol Abuse and Alcoholism (Grant no. R01AA029831), and the Clinical and Translational Science Collaborative (CTSC) of Cleveland (Grant no. 1UL1TR002548) as well as the CTSC of Northern Ohio which is funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Science Award (Grant no. UM1TR004528). The funders have no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
R.X. conceived, designed, and supervised the study and contributed to manuscript preparation. M.P.G. designed and conducted the study and drafted the manuscript. P.B.D. critically contributed to study design, result interpretation, and manuscript preparation. D.C.K. provided access to the TriNetX database and reviewed the final manuscript. We confirm the originality of content. M.P.G. has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
M.P.G., P.B.D., D.C.K., and R.X. have no competing interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gorenflo, M.P., Davis, P.B., Kaelber, D.C. et al. Ischemic stroke after COVID-19 bivalent vaccine administration in patients aged 65 years and older in the United States. npj Vaccines 8, 180 (2023). https://doi.org/10.1038/s41541-023-00777-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00777-w