Abstract
Alterations in the kynurenine pathway of tryptophan metabolism have been implicated in the pathophysiology of schizophrenia. Here, we performed an in-depth analysis of all metabolites of the kynurenine pathway, i.e., tryptophan (TRY), kynurenic acid (KYNA), L-kynurenine (KYN), 3-hydroxykynurenine (3-HK), anthranylic acid (ANA), 3-hydroxyanthranylic acid (3-HANA), xanthurenic acid (XA) and quinolinic acid (QUINA), in postmortem samples of the dorsolateral prefrontal cortex (DLPFC, Brodmann area 46, 9) of individuals affected by schizophrenia and non-schizophrenic controls. The analysis was carried out in the gray and white matter. Levels of KYN, 3-HK, ANA, and 3-HANA were significantly increased in both the gray and white matter of the DLPFC of individuals affected by schizophrenia, whereas levels of TRY, KYNA, and QUINA were increased exclusively in the white matter and remained unchanged in the gray matter. These increases in kynurenine metabolites did not correlate with age, sex, duration of the disease, and duration and type of antipsychotic medication. These findings suggest that the two major branches of the kynurenine pathway, i.e., the transamination of KYN into KYNA, and hydroxylation of KYN into 3-HK are activated in the white matter of individuals affected by schizophrenia, perhaps as a result of neuroinflammation, and support the evidence that abnormalities of the white matter are consistenly associated with schizophrenia.
Similar content being viewed by others
Introduction
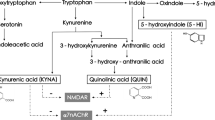
The efficacy of current antipsychotics is limited in improving negative and cognitive symptoms of schizophrenia1,2. Type-2 and −4 muscarinic receptors and type-1 trace amine receptors are examples of targets for new therapeutic agents that showed efficacy in clinical studies3,4. To our knowledge, there are no drugs that slow the progression of schizophrenia, which is driven by neuroinflammation and loss of gray and white matter occurring since the early, preclinical phase of the disease5. The kynurenine pathway of tryptophan metabolism6,7,8 is a biochemical trait d’union between neuroinflammation and abnormalities in excitatory neurotransmission, and has been implicated in the pathophysiology of schizophrenia8,9,10,11,12. The first step of the pathway is the opening of the pyrrole moiety of L-tryptophan to yield formyl-kynurenine, which is spontaneously converted into L-kynurenine (KYN). The reaction is catalyzed by type-1 and −2 indoleamine 2,3-dioxygenase (IDO1 and −2) and tryptophan 2,3-dioxygenase (TDO). IDO is induced by interferon-γ, interleukin-1β, and other proinflammatory cytokines in many organs, including the CNS13,14,15,16,17,18. KYN is hydroxylated into 3-hydroxykynurenine (3-HK) by kynurenine monooxygenase (KMO), or, alternatively, is transaminated into kynurenic acid (KYNA) by kynurenine aminotransferase (KAT). 3-HK is sequentially transformed into 3-hydroxyanthranilic acid (3-HANA) and quinolinic acid (QUINA), or, alternatively, is transminated by KAT into xanthurenic acid (XA)19,20. Some kynurenine metabolites interact with membrane receptors and modulate neurotransmission in the CNS. KYNA is an antagonist at the glycine site of N-methyl-D-aspartate (NMDA) receptors21, but can also block other glutamate receptor subtypes at high concentrations22. In addition, KYNA antagonizes homopentameric neuronal nicotinic receptors containing the α7 subunit23, and interacts with GPR35 an HCAR3 G-protein coupled receptors24,25. In contrast, QUINA binds to the GluN2 subunits of NMDA receptors acting as an orthosteric agonist26,27. Xanthurenic acid modulates the function of mGlu2 metabotropic glutamate receptors, whereas cinnabarinic acid, a by-product of the kynurenine pathway formed by the condensation of two molecules of 3-HANA, is a weak orthosteric agonist of mGlu4 receptors28,29,30,31.
CSF and brain KYNA levels are consistently increased in individuals affected by schizophrenia32,33, owing to a reduced activity of KMO34. The increase in KYNA:QUINA ratio, which is expected to restrain the endogenous activation of NMDA receptors, is consistent with the hypoglutamatergic hypothesis of schizophrenia35,36,37,38. We found a reduction in the levels of cinnabarinic acid in the prefrontal cortex of individuals affected by schizophrenia31. This reduction might be also linked to the pathophysiology of schizophrenia because cinnabarinic acid displayed potent antipsychotic-like activity in animal models31. However, the connection between the kynurenine pathway and schizophrenia may not be restricted to KYNA and QUINA.
Whether changes in other kynurenine metabolites occur in brain tissue of individuals affected by schizophrenia is less clear. Here, we aim to address the existing gaps in the literature pertaining to the comprehensive analysis of kynurenine pathway metabolites in postmortem samples of the dorsolateral prefrontal cortex (Broadmann area 46, 9) of individuals affected by schizophrenia and non-schizophrenic controls. By performing a simultaneous measurement of all metabolites of the kynurenine pathway in optically dissected gray and white matter, our research not only contributes to a deeper understanding of the neurobiological underpinnings of schizophrenia, but may also pave the way to novel therapeutic strategies.
Results
We measured levels of kynurenine metabolites in the gray and white matter dissected from the DLPFC of individuals affected by schizophrenia (SCZ, 14 males and 7 females, age: 25–61 years; mean ± SEM = 49 ± 2.3) and non-schizophrenic controls (CTRL, 20 males and 4 females, age: 36–63 years; mean ± SEM = 55 ± 1.5). There was no statistical difference between the ages of controls and individuals affected by schizophrenia (Mann-Whitney Rank Sum Test). Information on drug treatment was available for 15 subjects of the CTRL group and 16 subjects of the SCZ group. Individuals affected by SCZ had been treated with either first- or second-generation antipsychotics (n = 2 or 3 and 9, respectively), in some cases combined with valproate or lithium. Duration of antipsychotic medication ranged between 1 and 30 years. Subjects of both groups had also been treated with other types of drugs, such as drugs for cardiovascular disorders, anti-diabetic drugs, and opioid analgesics (Tables 1 and 2). Neuropathological analysis showed signs of amyloid and tau pathology, and cerebrovascular pathology in some samples of both groups. One individual of the SCZ group was also affected by multiple sclerosis (Tables 1 and 2). All available data provided by the biobank relative to controls and subjects affected by schizophrenia, and neuropathological data of brain samples are shown in Tables 1 and 2.
As the rate of degradation of kynurenine metabolites in human brain tissue is unknown, it was important to examine the possible correlation between levels of all kynurenine metabolites and the postmortem interval (PMI), i.e., the time elapsed between death and tissue removal. PMI ranged from 4.75 and 23.18 h in samples of both groups. No significant correlation was found between any kynurenine metabolite and PMI in both the gray and white matter of the DLPFC (Fig. 1S and ref. 31). Covariance analysis confirmed the lack of correlation between PMI, and all kynurenine metabolites in CTRL and SCZ samples with the exception of KYNA levels, which were inversily correlated to PMI in the gray matter (Pearson coefficient = −0.33, p = 0.035).
Changes in the levels of kynurenine metabolites in the DLPFC gray matter of individuals affected by schizophrenia
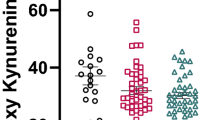
Schematic representation of the kynurenine pathway is shown in the Fig. 1. Levels of KYN, 3-HK, ANA, and 3-HANA were significantly increased in the DLPFC gray matter of individuals affected by SCZ with respect to non-schizophrenic CTRL, whereas levels of TRY, KYNA, QUINA, and XA were unchanged (Figs. 2 and 2S). The increase in KYN, 3-HK, and 3-HANA was also statistically significant when the analysis was restricted to males of the SCZ and CTRL groups (Fig. 3). The analysis of female subjects was biased by the low number of samples in both groups (Fig. 3). We measured the ratio between TRY and KYN (a low ratio is indicative of the activation of the kynurenine pathway), and the ratio between KYNA and QUINA (a higher ratio is consistently observed in individuals affected by SCZ) (see Introduction and references therein). There was no significant difference between CTRL and SCZ in both the TRY:KYN and KYN:QUINA ratios (Fig. 4). However, the TRY:KYN ratio was reduced by 36%, and the KYNA:QUINA ratio increased by 55% in the gray matter of the SCZ group (Fig. 4).
*p < 0.05 (Student’s t test) vs. control subjects. The control group is composed of 20 males and 4 females whereas the group of patients affected by schizophrenia is composed of 14 males and 7 females. Grubbs’s test was performed once and one sample for each group was excluded as an outlier, when the case.
Increased levels of all kynurenine metabolites in the DLPFC white matter of individuals affected by schizophrenia
Levels of TRY and all kynurenine metabolites, with the exception of XA, were significantly increased in the DLPFC white matter of the SCZ group, and the increase in KYN, 3-HK, 3-HANA, and QUINA remained statistically significant when the analysis was restricted to males of both groups (Fig. 3).
Similarly to what observed in the gray matter, there was no significant difference between CTRL and SCZ in both the TRY:KYN and KYN:QUINA ratios (Fig. 4). However, the TRY:KYN ratio was reduced by 33%, and the KYNA:QUINA ratio was increased by 34% in the white matter of the SCZ group (Fig. 4).
Correlation analysis between levels of kynurenine metabolites in the gray or white matter and age or duration of antipsychotic medication
There was no significant correlation between levels of TRY or kynurenine metabolites in the gray or white matter and age in either the CTRL or the SCZ group (Fig. 3S). In addition, in the SCZ group, there was no correlation between levels of TRY or any kynurenine metabolite and the duration (Fig. 5) and type of antipsychotic medication (Fig. 6). Covariance analysis confirmed the lack of correlation between age and kynurenine metabolites. There was also no correlation between sex and kynurenine metabolites in the gray matter. In contrast levels of 3-HANA (Pearson coefficient = 0.326, p = 0.028), ANA (Pearson coefficient = 0.401, p = 0.07), and KYNA (Pearson coefficient = 0.421, p = 0.004) in the white matter showed a significant correlation with sex, being greater in females.
Discussion
The kynurenine pathway meets the requirement to play a key role in the pathophysiology of psychotic disorders because it regulates energy metabolism (in the form of nicotinamide adenine dinucleotide), the immune system, and excitatory neurotransmission39. As KYNA is the only known NMDA receptor antagonist, it was postulated that an increased production of KYNA might be causally related to schizophrenia. It is consistent with this hypothesis that experimentally induced increases in CNS KYNA levels causes a schizophrenia-like phenotype in experimental animals40, and KYNA levels are increased in the postmortem brain and CSF of individuals affected by schizophrenia41,42,43. However, how the other kynurenine metabolites behave in the brain of individuals affected by schizophrenia is less clear. Robert Schwarcz and his associates found that the activity of KMO and 3-hydroxyanthranilic acid dioxygenase (3-HAO, the enzyme that transforms 3-HANA into QUINA) was reduced in the prefrontal cortex (Brodmann areas 9 and 10) of individuals affected by schizophrenia, whereas activities of kynureninase, kynurenine aminotransferase II, and quinolinic acid phosphoribosyltransferase were unchanged. This was associated with an increase in KYNA levels and suggested an increased KYNA:QUINA ratio in schizophrenia11. Increases in KYN levels and TDO2 expession were found in the prefrontal cortex and anterior cingulate cortex of individuals affected by schizoprenia44,45. More recently, Kindler et al. 202046 found that KYNA levels, the KYN:TRY ratio, and the transcripts of TDO and KATI/II were increased in the prefrontal cortex of a “high cytokine schizophrenia subgroup” identified by measurements of proinflammatory cytokine mRNA, and there was a positive correlation between KAT and glial fibrillary acidic protein mRNA. This suggests that central neuroinflammation causes the activation of the kynurenine pathway in schizophrenia, and that the increase in KYNA levels is secondary to reactive gliosis. The conclusion of a systematic review on peripheral and central kynurenine metabolites in psychiatric disorders was that brain/CSF KYNA levels are increased, whereas metabolites of the KMO-driven branch of the kynurenine pathway (e.g., 3-HK, XA, and QUINA) are unchanged in schizophrenia47.
Our results diverge from this conclusion. We found that levels of all kynurenine metabolites, with the exception of XA, were increased in the white matter, and levels of KYN, 3-HK, ANA, and 3-HANA were increased in the gray matter of the DLPFC of individuals affected by schizophenia. The homogenous activation of the kynurenine pathway in the white matter is in line with a large body of evidence suggesting that white matter abnomalities are associated with schizophrenia48,49,50,51,52,53,54. Fractional anisotropy MI analysis exploring age-related white matter trajectories in a large cohort of patients affected by schizophrenia and healthy controls demonstrates that white matter is affected in all stages of schizophrenia in a tract-specific manner since the early phases of myelin maturation50. It is believed that inflammation contributes to the pathophysiology of white matter damage in schizophrenia55,56,57. If so, the white matter might represent a preferential site of activation of the kynurenine pathway, explaining our data. Activation of the kynurenine pathway in the white matter of schizophrenic patients might represent a compensatory mechanism aimed at restraining immune activation and neuroinflammation58,59,60,61,62. The increase in the KYNA:QUINA ratio found in the white matter of individuals affected by schizophenia might limit the endogenous activation of NMDA receptors expressed by oligodendrocytes, thus limiting the excitotoxic component of myelin damage63,64,65,66. It is important to note that the between-subject differences in the abundance of glia cells, particularly astrocytes and microglia, could potentially contribute to the measured variations in kynurenine metabolite concentrations. Future research is needed to explore both the abundance of glia cells and markers of their activation state to potentially unravel the intricate interplay between kynurenine metabolites and glia cells, and delineate their potential role as biomarkers, if found at circulant levels in the periphery.
Data obtained in the DLPFC gray matter of individuals affected by schizophrenia were partially unexpected. There was a trend to an increase KYNA levels and KYNA to QUINA ratio, which was consistent with previous findings and with the hypoglutamatergic hypothesis of schizophrenia (see above). However, we were surprised to find a significant incease in KYN, 3-HK, 3-HANA, and ANA levels, considering that 3-HK is formed by KMO, which is known to be defective in schizophrenia11,34,67. One possible explanation is that these metabolites accumulate because of a defective activity of 3-HAO, which converts 3-HANA into QUINA11. ANA is formed by KYN through a reaction catalyzed by kynureninase, and then hydroxylated into 3-HANA20. Interestingly, a 2-fold increase in ANA levels has been reported in the serum of patients affected by schizophrenia, and has been considered as a potential biomarker and treatment marker for schizophrenia68.
We have already reported that cinnabarinic acid is detectable at very low levels in the human DLPFC, is reduced in patients affected by schizophrenia, and this reduction did not correlate with age, sex, duration of the disease, and duration and type of antipsychotic medications31. Here we quantitated kynurenine metabolites in the gray and white matter of males and females and we showed that KYN, 3-HK, ANA, and 3-HANA are significantly increased both in the gray and white matter of the DLPFC of patients affected by schizophrenia. We run a thorough analysis correlating age and sex between controls and subjects affected by schizophrenia. The analysis revealed no significant differences in these demographic variables between the two cohorts, confirming the validity of our control group. The analysis run on male gray matter confirmed the increase of KYN, 3-HK, 3-HANA, and QUINA, whereas white matter showed an increase of all metabolites, but not XA. These significant increases were not found in female samples, suggesting that there could be a gender difference, although we have to note that the number of female samples are 4 in control subjects and 7 in the group of patients affected by schizophrenia. Moreover, we correlated kynurenine metabolite levels with age, duration of treatments (typical or atypical antipsychotic drugs) and neuropathology in patients affected by schizophrenia. We did not observe any correlation with these parameters, but a significant negative correlation between age and TRY and XA levels in the gray matter of DLPFC patients affected by schizophrenia, but not in control subjects. This is peripherally paralleled with the reported reduction of XA levels in the serum of patients affected by schizophrenia and their first-degree relatives30. It has been reported that activity of 3-hydroxyanthranilic acid dioxygenase (3-HAO), the enzyme that synthetizes QUINA, is significantly reduced in the DLPFC of schizophrenic patients. In line with this observation, we now report that 3-HANA levels are increased by about 3-fold in the gray matter, and about 4-fold in the white matter in the DLPFC of schizophrenic patients. This increase could be due to the accumulation of 3-HANA in response to the reduced activity of 3-HAO in patients affected by schizophrenia11.
In conclusion, this is the first report on measurements of all the metabolites of the kynurenine pathway in the DLPFC of control subjects and patients affected by schizophrenia. The results show increased levels of almost all kynurenine pathway metabolites suggesting a hyperactive pathway that could play a relevant role in the pathophysiology of schizophrenia. The parallelism between peripheral, as reported in the literature, and central levels of some metabolites could suggest their use as valuable predictive and prognostic biomarker candidates for schizophrenia31. In our experience, cinnabarinic acid is the only metabolite of all kynurenine metabolites to be reduced in the prefrontal cortex of individuals affected by schizophrenia31.
Our study has a number of limitations, including the presence of amyloid, tau or vascular patology in some samples of the CTRL and SCZ group, which might have caused local inflammation, thereby activating the kynurenine pathway. In addition, all samples from the SCZ groups were obtained from patients with a history of antipsychotic medication, which might have affected the activation of the kynurenine pathway. Finally, although there was no correlation between any of the kynurenine metabolite and the PMI, we cannot exclude that ante-mortem variables, such as the extent of the agonic state, duration of hypoxia, and the presence of fever might have influenced our findings.
Materials and methods
Human brain samples
Samples of the frontal lobe dorsolateral prefrontal cortex (DLPFC, Brodmann area 46, 9) from individuals affected by schizophrenia and non-schizophrenic controls were kindly provided by the Harvard Brain Tissue Resource Center, funded through NIH-NeuroBiobank HHSN-271-2013-00030C.
UPLC/MS-MS analysis of kynurenine pathway metabolites
Measurements of kynurenine pathway metabolite levels were carried out in samples of DLFC of individuals affected by schizophrenia and non-schizophrenic controls. Detection and quantification of kynurenine pathway metabolites in tissue extracts were performed by ultra-performance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS). Tissue extracts were prepared from about 40–50 mg of brain tissues, either white and gray matter, of patients affected by schizophrenia and healthy controls. White and gray matter tissues were sonicated in 0.1 N perchloric acid (weight/volume), homogenates were centrifuged at maximal speed in a microfuge for 30 min and supernatants were placed into vials for automatic injection into the UPLC system. The analysis was performed by the 1260 Infinity II Agilent Liquid Chromatography System separating molecules on a reversed-phase column (Poroshell 120, EC-C18, 1.9 µm, 2.1 × 50 mm - Agilent, Santa Clara, CA). Five μl were injected and the separation was obtained by a gradient using the eluent A (0.1% aqueous formic acid) and the eluent B (100% methanol) at a flow of 200 μl/min, using 10% solvent A for the first min and 100% solvent B for the following 3 min.
The mass spectrometry analysis was carried out on the 6470 LC/TQ Agilent triple quadrupole system equipped with a turbo ion spray source. The detector was set in the positive ion mode and the ion spray voltage was set at 5000 V (with a source temperature of 300 °C). Nitrogen was used as collision gas and the collision activation dissociation gas was set at medium value. A dynamic multiple reaction monitoring (dMRM) method was applied to detect and quantify kynurenine metabolites. The instrument was set in the dMRM mode, checking the transition m/z (in parenthesis collision energy) for TRY 204.7 → 172.7 (4); 204.7 → 76.9 (72); KYN 209 → 145.8 (20); 209 → 64.9 (56); ANA 138.2 → 119.9 (8); 138.2 → 64.9 (36); KYNA 190 → 143.9 (20); 190 → 88.8 (48); 3-HK 225.1 → 208 (8); 225.1 → 110 (16), XA 206.2 → 159.8 (20); 206.2 → 131.8 (36), 3-HANA 154.1 → 135.8 (12); 154.1 → 79.9 (32); QUINA 168.03 → 150 (8); 168.03 → 78 (24). Each analyte was monitored with a dwell time of 100 ms in the transitions from the precursor ion into the product ion and the mass spectrometer was tuned to obtain the best sensitivity for all transitions. The Mass Hunter software (Agilent, Santa Clara, CA) was used to analyze data. The calibration curve was tuned by dissolving different amounts of all kynurenine metabolites in acetonitrile and processing them in the identical way of tissue samples.
Statistical analysis
Data were analyzed by Student’s t test. The Shapiro-Wilk normality test was run to met the criteria for normal distribution and the following Grubbs’s test was used to identify outliers. Correlation between kynurenine metabolite levels and different variables was carried out by linear regression analysis. Analyses were carried out by GraphPad software v. 8. The ANCOVA Analysis was carried out by IBM SPSS Statistics software v. 26.
Data availability
The datasets generated and analysed during the current study are available in the repository NEUROMED.
References
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19, 37–38 (2014).
Cerveri, G., Gesi, C. & Mencacci, C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr. Dis. Treat. 15, 1525–1535 (2019).
Yohn, S. E., Weiden, P. J., Felder, C. C. & Stahl, S. M. Muscarinic acetylcholine receptors for psychotic disorders: bench-side to clinic. Trends Pharmacol. Sci. 43, 1098–1112 (2022).
Halff, E. F., Rutigliano, G., Garcia-Hidalgo, A. & Howes, O. D. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 46, 60–74 (2023).
Barichello, T., Simoes, L. R., Quevedo, J. & Zhang, X. Y. Microglial activation and psychotic disorders: evidence from pre-clinical and clinical studies. Curr. Top. Behav. Neurosci. 44, 161–205 (2020).
Perkins, M. N. & Stone, T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 247, 184–187 (1982).
Perkins, M. N. & Stone, T. W. Actions of kynurenic acid and quinolinic acid in the rat hippocampus in vivo. Exp. Neurol. 88, 570–579 (1985).
Stone, T. W. & Connick, J. H. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience 15, 597–617 (1985).
Aoyama, N. et al. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes Brain Behav. 5, 364–368 (2006).
Erhardt, S., Schwieler, L., Imbeault, S. & Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 112, 297–306 (2017).
Sathyasaikumar, K. V. et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 37, 1147–1156 (2011).
Wonodi, I. et al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry 68, 665–674 (2011).
Byrne, G. I., Lehmann, L. K. & Landry, G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53, 347–351 (1986).
Pfefferkorn, E. R., Rebhun, S. & Eckel, M. Characterization of an indoleamine-2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J. Interferon Res. 6, 267–279 (1986).
Hissong, B. D., Byrne, G. I., Padilla, M. L. & Carlin, J. M. Upregulation of interferon-induced indoleamine-2,3-dioxygenase in human macrophage cultures by lipopolysaccharide, muramyl tripeptide, and interleukin-1. Cell. Immunol. 160, 264–269 (1995).
Hissong, B. D. & Carlin, J. M. Potentiation of interferon-induced indoleamine-2,3-dioxygenase mRNA in human mononuclear phagocytes by lipopolysaccharide and interleukin-1. J. Interf. Cytokine Res. 17, 387–393 (1997).
Babcock, T. A. & Carlin, J. M. Transcriptional activation of indoleamine dioxygenase by interleukin1 and tumor necrosis factor in interferon-treated epithelial cells. Cytokine 12, 588–594 (2000).
Campbell, B. M., Charych, E., Lee, A. W. & Möller, T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 8, 12 (2014).
Amori, L., Guidetti, P., Pellicciari, R., Kajii, Y. & Schwarcz, R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 109, 316–325 (2009).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H.-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Parsons, C. G. et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 283, 1264–1275 (1997).
Elmslie, K. S. & Yoshikami, D. Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res. 330, 265–272 (1985).
Hilmas, C. et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 21, 7463–7473 (2001).
Huang, X. et al. Modeling subtype-selective agonists binding with alpha4beta2 and alpha7 nicotinic acetylcholine receptors: effects of local binding and long-range electrostatic interactions. J. Med. Chem. 28, 7661–7674 (2006).
Kapolka, N. J. & Isom, D. G. HCAR3: an underexplored metabolite sensor. Nat. Rev. Drug Discov. 19, 745 (2020).
Stone, T. W. & Perkins, M. N. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 72, 411–412 (1981).
de Carvalho, L. P., Bochet, P. & Rossier, J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 28, 445–452 (1996).
Fazio, F. et al. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol. Pharmacol. 81, 643–656 (2012).
Fazio, F. et al. Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice. Neuropharmacology 81, 237–243 (2014).
Fazio, F. et al. 2015. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci. Rep. 5, 17799 (2015).
Ulivieri, M. et al. The trace kynurenine, cinnabarinic acid, displays potent antipsychotic-like activity in mice and its levels are reduced in the prefrontal cortex of individuals affected by schizophrenia. Schizophr. Bull. 46, 1471–1481 (2020).
Plitman, E. et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 43, 764–777 (2017).
Wang, A. K. & Miller, B. J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 44, 75–83 (2018).
Wonodi, I. et al. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr. Res. 160, 80–87 (2014).
Belforte, J. E. et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 13, 76–83 (2010).
Homayoun, H. & Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 27, 11496–11500 (2007).
Jackson, M. E., Homayoun, H. & Moghaddam, B. 2004. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 101, 8467–8472 (2004).
Moghaddam, B. & Javitt, D. 2012. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15 (2012).
Savitz, J. The kynurenine pathway: a finger in every pie. Mol. Psychiatry 25, 131–147 (2020).
Nilsson, L. K., Linderholm, K. R. & Erhardt, S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J. Neural. Transm. 113, 557–571 (2006).
Schwarcz, R. et al. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 50, 521–530 (2001).
Erhardt, S. et al. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 313, 96–98 (2001).
Linderholm, K. R. et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 38, 426–432 (2012).
Miller, C. L. et al. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 15, 618–629 (2004).
Miller, C. L., Llenos, I. C., Dulay, J. R. & Weis, S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 16, 1073–1074 (2006).
Kindler, J. et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 25, 2860–2872 (2020).
Skorobogatov, K. et al. Brain versus blood: a systematic review on the concordance between peripheral and central kynurenine pathway measures in psychiatric disorders. Front. Immunol. 12, 716980 (2021).
Davis, K. L. et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry 60, 443–456 (2003).
Kubicki, M., McCarley, R. W. & Shenton, M. E. Evidence for white matter abnormalities in schizophrenia. Curr. Opin. Psychiatry. 18, 121–134 (2005).
Cetin-Karayumak, S. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol. Psychiatry 25, 3208–3219 (2020).
Erkol, C. et al. White matter measures and cognition in schizophrenia. Front. Psychiatry 11, 603 (2020).
Lee, D. K. et al. Common gray and white matter abnormalities in schizophrenia and bipolar disorder. Plos One 15, e0232826 (2020).
Joo, S. W. et al. White matter impairments in patients with schizophrenia: a multisite diffusion MRI study. Prog. Neuropsychopharmacol Biol. Psychiatry. 111, 110381 (2021).
Smigielski, L. et al. White matter microstructure and the clinical risk for psychosis: a diffusion tensor imaging study of individuals with basic symptoms and at ultra-high risk. Neuroimage Clin 35, 103067 (2022).
Najjar, S. & Pearlman, D. M. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr. Res. 161, 102–112 (2015).
Di Biase, M. A. et al. Large-scale evidence for an association between peripheral inflammation and white matter free water in schizophrenia and healthy individuals. Schizophr. Bull. 47, 542–551 (2021).
Li, H. et al. The relationship between TLR4/NF-κB/IL-1β signaling, cognitive impairment, and white-matter integrity in patients with stable chronic schizophrenia. Front. Psychiatry 13, 966657 (2022).
Grohmann, U., Fallarino, F. & Puccetti, P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 24, 242–248 (2003).
Gargaro et al. Indoleamine 2,3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication. Immunity 55, 1032–1050.e14 (2022).
Pallotta, M. T. et al. Indoleamine 2,3-dioxygenase 1 (IDO1): an up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 289, 6099–6118 (2022).
Stone, T. W. et al. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 16, 1002004 (2024).
Stone, T. W. & Williams, R. O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 44, 442–456 (2023).
Bakiri, Y., Hamilton, N. B., Káradóttir, R. & Attwell, D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia 56, 233–240 (2008).
Burzomato, V., Frugier, G., Pérez-Otaño, I., Kittler, J. T. & Attwell, D. The receptor subunits generating NMDA receptor mediated currents in oligodendrocytes. J. Physiol. 588, 3403–3414 (2010).
Zhang, J., Liu, J., Fox, H. S. & Xiong, H. N-methyl-D-aspartate receptor-mediated axonal injury in adult rat corpus callosum. J. Neurosci. Res. 91, 240–248 (2013).
Doyle, S. et al. Vesicular glutamate release from central axons contributes to myelin damage. Nat. Commun. 9, 1032 (2018).
Holtze, M. et al. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 37, 53–57 (2012).
Oxenkrug, G., van der Hart, M., Roeser, J. & Summergrad, P. Anthranilic acid: a potential biomarker and treatment target for schizophrenia. Ann. Psychiatry Ment. Health 4, 1059 (2016).
Acknowledgements
The authors thank the Harvard Brain Tissue Resource Center, funded through NIH-NeuroBiobank HHSN-271-2013-00030C, the National Institute of Mental Health (NIMH), National Institute of Neurological Diseases and Stroke (NINDS), National Institute on Aging (NIA), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The authors are also grateful to the brain donors and their families for the tissue samples used in the studies and to Prof. Sabina Berretta (Harvard Medical School; Director of the Translational Neuroscience Laboratory, McLean Hospital; Scientific Director of the Harvard Brain Tissue Resource Center, McLean Hospital) for her valuable help. This work was supported by the Italian Ministry of Health (Project code: RF-2019-12368646, 2019).
Author information
Authors and Affiliations
Contributions
N.A., G.D., F.F.: kynurenine metabolite measurements and data analysis; F.N., V.B., G.B.: design and coordination of the study, writing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Autoptic human brain samples were obtained from the NIH-NeuroBiobank HHSN-271-2013-00030C after signing a material transfer agreement (MTA) according to the Uniform Biological Materials Transfer Agreement (UBMTA).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antenucci, N., D’Errico, G., Fazio, F. et al. Changes in kynurenine metabolites in the gray and white matter of the dorsolateral prefrontal cortex of individuals affected by schizophrenia. Schizophr 10, 27 (2024). https://doi.org/10.1038/s41537-024-00447-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-024-00447-3