Abstract
Impaired insight into illness is a common element of schizophrenia that contributes to treatment nonadherence and negative clinical outcomes. Previous studies suggest that impaired insight may arise from brain abnormalities. However, interpretations of these findings are limited due to small sample sizes and inclusion of patients with a narrow range of illness severity and insight deficits. In a large sample of patients with schizophrenia, the majority of which were designated as treatment-resistant, we investigated the associations between impaired insight and cortical thickness and subcortical volumes. A total of 94 adult participants with a schizophrenia spectrum disorder were included. Fifty-six patients (60%) had treatment-resistant schizophrenia. The core domains of insight were assessed with the VAGUS insight into psychosis scale. We obtained 3T MRI T1-weighted images, which were analysed using CIVET and MAGeT-Brain. Whole-brain vertex-wise analyses revealed impaired insight, as measured by VAGUS average scores, was related to cortical thinning in left frontotemporoparietal regions. The same analysis in treatment-resistant patients showed thinning in the same regions, even after controlling for age, sex, illness severity, and chlorpromazine antipsychotic dose equivalents. No association was found in non-treatment-resistant patients. Region-of-interest analyses revealed impaired general illness awareness was associated with cortical thinning in the left supramarginal gyrus when controlling for covariates. Reduced right and left thalamic volumes were associated with VAGUS symptom attribution and awareness of negative consequences subscale scores, respectively, but not after correction for multiple testing. Our results suggest impaired insight into illness is related to cortical thinning in left frontotemporoparietal regions in patients with schizophrenia, particularly those with treatment resistance where insight deficits may be more chronic.
Similar content being viewed by others
Introduction
Moderate-to-severe impairment of insight into illness commonly occurs in schizophrenia and contributes to treatment nonadherence and negative clinical outcomes1,2. Impaired insight is frequently associated with poor adherence to pharmacological treatment3,4,5,6, worse symptom severity7,8,9, and increased rates of relapse and hospitalisation10,11. Despite its clinical relevance, the pathophysiological mechanism(s) of impaired insight in patients with schizophrenia remain elusive.
Previous structural neuroimaging studies suggest that impaired insight may arise from underlying brain abnormalities. Impaired insight has been commonly associated with reduced brain volume in frontal regions12,13, but also temporal14, parietal, occipital15, and subcortical brain areas16. A recent coordinate-based meta-analysis similarly reported abnormalities in isolated brain-regions in relation to impaired insight, but with spatially diffuse global and frontal abnormalities17. However, the results of these earlier studies are largely inconsistent with some studies finding no structural correlates of impaired insight14,18,19.
Some explanations for the inconsistencies in the literature include small sample size, heterogeneity in patient samples, and inclusion of patients with a narrow range of illness severity and insight deficits. Studies have also used various measures of insight, with some using single- versus multi-item scales to assess only a single or multiple dimensions of insight into illness17,19, making it difficult to integrate and interpret these findings.
This study attempted to address some of the limitations of earlier studies. The aims were to assess the associations between impaired insight into illness and (i) cortical thickness, and (ii) regional subcortical volumes in patients with schizophrenia, the majority of which had treatment resistance. Treatment-resistant patients, as defined by partial or no response to first-line antipsychotic medications, i.e., typical or atypical non-clozapine antipsychotic drugs, are reported to have widespread reductions in cortical and subcortical volumes and cortical thickness20,21,22. We are not aware of any study that has explored the association between impaired insight and cortical thickness in this population. We employed the VAGUS scale to assess insight to illness, which is a brief, yet comprehensive measure that assesses the four core dimensions of insight, including general illness awareness, symptom attribution, awareness of the need for treatment, and awareness of the negative consequences of the disorder23.
We hypothesised cortical thinning in frontoparietal regions in relation to impaired insight with more pronounced thinning in these regions in patients with treatment-resistant schizophrenia. We also hypothesised a relationship between impaired insight and reduced subcortical volumes, as deficits in subcortical regions are widely reported in patients with schizophrenia19.
Methods
Study participants
In this cross-sectional study, inpatients or outpatients ≧18 years of age with a DSM-IV/Structured Clinical Interview (SCID-4)24 diagnosis of schizophrenia or schizoaffective disorder were recruited at the Centre for Addiction and Mental Health (CAMH), Toronto, Canada from 2014 to 2019. The data for this analysis was acquired from two studies: (1) a cross-sectional study investigating glutamatergic neurometabolite levels in treatment-resistant schizophrenia25, and (2) an intervention study that examined the effects of transcranial direct current stimulation on impaired insight in patients with schizophrenia (ClinicalTrials.gov Identifier: NCT02848885). The SCID was administered by two clinicians (YI and SN). Treatment resistance was defined as a suboptimal response to at least 2 previous non-clozapine antipsychotics, each attaining a chlorpromazine (CPZ) equivalent dose of 400 mg or more for 6 or more consecutive weeks based on the modified Treatment Response and Resistance in Psychosis Working Group Consensus criteria26,27,28. Both studies were approved by the CAMH Research Ethics Board. Capacity to consent to participate in the study was confirmed with the MacArthur Test of Competence29.
Exclusion criteria for both studies included: being unable to provide consent to participate in the research study; substance abuse or dependence within one month prior to entering the study (except caffeine and nicotine); a positive urine drug test for illicit drugs; any contraindication to magnetic resonance imaging (MRI); serious unstable medical illness or any concomitant major medical or neurological illness, including a history of seizures or a first degree relative with a history of a seizure disorder.
Study measures
Insight was measured using the VAGUS insight into psychosis scale (www.vagusonline.com), self-report version23. The VAGUS scale has four subscales that assess the core components of insight, including general illness awareness, accurate symptom attribution, awareness of the need for treatment, and awareness of negative consequences of the disorder. Each subscale score ranges from 0 to 10, with a higher score representing greater insight. An average score is derived from the subscale scores. The VAGUS self-report measure is stongly associated with the VAGUS clinician-rated version (r = 0.70 and p < 0.001)23. Illness severity was assessed using the Positive and Negative Syndrome Scale (PANSS). The PANSS total score was modified to exclude the PANSS G12 lack of judgement and insight item30. The WRAT-III reading subtest was used to measure premorbid IQ31. The R package, chlorpromazineR (version 0.1.2), was used to determine CPZ antipsychotic drug dose equivalents32. Self-reported smoking status was also collected.
Statistical analysis for demographic and clinical characteristics
SPSS Statistics version 21 (IBM Corporation) was used to carry out descriptive analyses for demographic and clinical data. Independent t-tests, χ2, and Fisher’s exact tests were employed to compare the demographic and clinical characteristics of treatment-resistant and non-treatment-resistant patients where appropriate. The significance level for these tests was set at p < 0.05.
Magnetic resonance imaging acquisition and processing
Three-dimensional IR-prepared T1-weighted MRI scans (BRAVO, TE = 3.00 ms, TR = 6.74 ms, TI = 650 ms, flip angle = 8°, FOV = 23 cm, 256 × 256 matrix, slice thickness = 0.9 mm) were obtained at CAMH using a 3T GE Discovery MR750 scanner (General Electric, Waukesha, WI).
T1-weighted images were preprocessed using the bpipe pipeline (http://github.com/CobraLab/minc-bpipe-library/), which consists of bias field correction33, neck cropping, and Brain Extraction based on nonlocal Segmentation Technique (BEaST) brain extraction34. All preprocessed images were visually inspected for quality control.
Cortical thickness analysis was performed using the CIVET processing pipeline (version 2.1.0; Montreal Neurological Institute). This procedure has been previously described in detail elsewhere35,36,37. Briefly, preprocessed T1-weighted images were linearly registered to the ICBM 152 average template using a nine-parameter transformation (i.e., 3 translations, rotations, and scales)38, and classified into grey matter (GM), white matter (WM) and cerebrospinal fluid39,40. The GM and WM surfaces were modelled onto the left and right hemispheres, each composed of 163,842 vertices41,42. Cortical thickness was determined by evaluating the distance between the WM and GM surfaces in the native space43,44. A 30 mm surfaced based diffusion kernel for vertex-wise analysis was used for smoothing and the results were nonlinearly registered to a surface template45. Regions of interest (ROI) were generated based on the Automated Anatomical Labelling (AAL) atlas46,47. ROI data was extracted from unsmoothed data. CIVET outputs were visually inspected for quality control.
Multiple Automatically Generated Templates (MAGeT-Brain) algorithm was used to carry out a fully-automated segmentation of subcortical volumes48,49. MAGeT-Brain is described in detail elsewhere19,50,51,52,53. The Colin-27 Subcortical Atlas54,55 was used in this analysis to obtain volumes of the striatum, thalamus, and globus pallidus. From the overall sample, scans from a subset of participants were selected as a template library via which the final segmentation was bootstrapped. In this analysis, 21 templates were used. The bootstrapping of the final segmentations through the template library resulted in the production of candidate labels for each subject. Candidate labels were then fused using a majority vote to complete the segmentation process. A version of the Automatic Normalization Tools (ANTS) registration technique, which is compatible with the minc toolkit (https://github.com/vfonov/mincANTS), was used for nonlinear registration56.
Cortical thickness analyses
Vertex-wise analyses
Cortical thickness analyses were conducted using the RMINC package (https://github.com/mcvaneede/RMINC). First, whole-brain vertex-wise analyses were carried out using a general linear model for VAGUS average and subscale scores, controlling for age and sex. Maps of t-statistics at each vertex were projected onto an average brain template. Second, for any significant associations, we repeated the whole-brain vertex-wise analysis additionally controlling for illness severity and CPZ antipsychotic dose equivalents. Third, we performed separate analyses for treatment-resistant and non-treatment-resistant participants, including the same covariates. Total brain volume was not controlled for as cortical thickness and brain volume are poorly correlated57. Whole-brain analyses were corrected for multiple testing using a false discovery rate (FDR) < 0.1058. A lower threshold was used to reveal all affected brain regions.
Regions of interest analyses
ROI analyses were conducted using SPSS. The mean cortical thickness was generated for our ROIs based on the AAL atlas46,47. The supramarginal gyrus and the angular gyrus were selected as the a priori ROIs based on findings from previous neuroimaging studies that examined insight into illness in patients with schizophrenia59,60,61. Regression analyses were carried out to assess the relationship between VAGUS average and subscale scores with cortical thickness in the supramarginal and angular gyrus as separate dependent variables. The same covariates used in the vertex-wise analyses were included in the ROI analyses, i.e., age, sex, illness severity, and CPZ antipsychotic dose equivalents. The analyses were repeated for treatment-resistant and non-treatment-resistant patients. The threshold of significance level was established at p < 0.006 (0.05/8 (i.e., 2 separate regression models for each of the 4 ROIs)).
Subcortical volume analyses
Regression analyses were carried out using SPSS to assess the relationship between VAGUS average and subscale scores with left and right striatal, thalamic, and globus pallidus volumes62,63. Age, sex, illness severity, TBV, and CPZ antipsychotic dose equivalents were included as covariates. The analyses were repeated for treatment-resistant and non-treatment-resistant patients. The threshold of significance level was established at p < 0.004 (0.05/12 (i.e., 2 separate regression models for each of the 6 subcortical ROIs)).
Results
Demographic and clinical characteristics
A total of 94 participants were included. The demographic and clinical characteristics can be found in Table 1. Correlations between VAGUS scores and other demographic and clinical characteristics are shown in Supplemental Material 1. Supplemental Material 2 lists the antipsychotic medications used by participants.
Impaired insight and cortical thickness
Whole brain
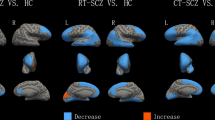
Impaired insight (i.e., lower VAGUS average scores) was associated with cortical thinning in the left middle and inferior frontal regions, temporal pole, and supramarginal gyrus, controlling for age and sex (Fig. 1A). No association was found when additionally controlling for illness severity and CPZ antipsychotic dose. No associations were found between VAGUS subscale scores and cortical thickness.
A Association between cortical thinning and impaired insight as measured by VAGUS average scores in patients with schizophrenia, controlling for age and sex (n = 94); B Association between cortical thinning and impaired insight as measured by VAGUS average scores in treatment-resistant patients with schizophrenia, controlling for age, sex, illness severity, and chlorpromazine dose equivalents (n = 56).
Subgroup analysis of only treatment-resistant patients showed cortical thinning in the same regions in relation to impaired insight (i.e., lower VAGUS average scores), controlling for age, sex, illness severity, and CPZ antipsychotic drug dose equivalents (Fig. 1B). No associations were found between VAGUS subscale scores and cortical thickness in treatment-resistant patients. There were no associations between VAGUS average and subscale scores and cortical thickness in non-treatment-resistant patients.
Regions of interest
There was an association between VAGUS average scores and cortical thickness in the left supramarginal gyrus, but not after correction for multiple testing (standardised β = 0.20, t = 2.06, p = 0.042). Impaired general illness awareness (i.e., lower VAGUS general illness awareness domain score) was associated with cortical thinning in the left supramarginal gyrus (standardised β = 0.34, t = 2.86, p = 0.005) (Table 2). There were no associations between VAGUS average or subscale scores with the right supramarginal gyrus, and the left and right angular gyrus.
Subgroup analysis of only treatment-resistant patients similarly found an association between lower VAGUS average scores and cortical thinning in the left supramarginal gyrus, but not after correction for multiple testing (standardised β = 0.20, t = 2.06, p = 0.021). There was no relationship between VAGUS subscale scores and cortical thinning in the left supramarginal gyrus in treatment-resistant patients. No associations were found between VAGUS average or subscale scores and the left supramarginal gyrus in non-treatment-resistant patients. In both groups, no relationships were found between VAGUS average or subscale scores and the right supramarginal gyrus, and the left and right angular gyrus.
Impaired insight and subcortical volume
There was an association between VAGUS symptom attribution subscale scores and right thalamic volume (t = –2.14, p = 0.035) and VAGUS awareness of negative consequences subscale scores and left thalamic volume (t = –2.11, p = 0.038), but not after correction for multiple testing. There were no associations between VAGUS average or subscale scores and the striatum, and globus pallidus, bilaterally. The results remained the same when analyzing treatment-resistant and non-treatment-resistant patients separately.
Discussion
This study examined the relationships between impaired insight and (i) cortical thickness, and (ii) subcortical volumes in patients with a schizophrenia spectrum disorder. We found cortical thinning in the left inferior and middle frontal regions, supramarginal gyrus, and the temporal pole in relation to impaired insight, particularly in treatment-resistant patients where insight deficits may be more chronic. Our results are consistent with prior studies that reported more pronounced cortical thinning and volume loss in treatment-resistant patients compared to non-treatment-resistant patients64,65. In treatment-resistant patients, the relationship remained significant even after controlling for illness severity and CPZ antipsychotic drug dose equivalents. The ROI analyses also identified an association between impaired general illness awareness (i.e., VAGUS general illness awareness domain) and cortical thinning in the left supramarginal gyrus. We found no associations between impaired insight and subcortical volumes.
A previous study by our group that examined the structural and functional correlates of insight found a direct link between cortical thinning and brain activity. In this study we found that higher brain activity in the left posterior parietal area during a functional MRI insight task in relation to insight impairment was associated with cortical thinning in the same region60. The results of other studies that have focused on examining the relationship between impaired insight and cortical thickness have been inconsistent14,17,18,19,66. The inconsistencies in the literature may be due to a number of factors, including limited sample size and different methods of measuring insight into illness14,17,18,19. To address some of these limitations, a study by Béland et al. included a large number of patients with schizophrenia (N = 110) to examine the association between clinical insight and cortical thickness. In their study, insight was determined by calculating two statistically derived factors from four different measures of insight. The two factors that emerged were: (i) awareness of illness and need for treatment, and (ii) awareness of symptoms and consequences of the disorder. The authors examined 8 brain regions previously implicated in impaired insight, including the inferior parietal area, but did not find any relationship between cortical thickness and the two insight factors19. There are several differences between Béland et al. and the present study that may explain the differences in the results. First, approximately half of the participants in the current study were on clozapine and considered to have treatment-resistant schizophrenia. Therefore, it is likely that our sample included more chronically ill patients with persistent insight deficits in comparison with the sample in the Béland et al. (2019) study. A separate investigation that included patients with first-episode psychosis, chronic, and treatment-resistant schizophrenia revealed extensive cortical thinning in brain regions with higher structural connectivity. The strength of structural connectivity between regions with cortical thinning increased with illness severity and was the highest in treatment-resistant schizophrenia67. Plausibly, cortical thinning in brain regions implicated in insight impairment is more apparent in treatment-resistant schizophrenia due to prolonged aberrant connectivity between these interconnected brain regions59,68. Second, we used a single measure of insight into psychosis rather than statistically derived factors. However, the VAGUS scale measures the same core domains of illness awareness, specifically general illness awareness, symptom attribution, awareness of the need for treatment, and awareness of negative consequences of the disorder23. Although there is evidence to suggest the neurobiological basis of insight is specific to insight types and its dimensions69, the relationship between insight impairment and cortical thinning in our study was mainly observed with VAGUS average scores. Only the VAGUS general illness awareness subscore was found to be associated with cortical thinnning in the left supramarginal gyrus in our ROI analyses.
Analogous to anosognosia, a neurological phenomenon characterised by deficits in illness awareness resulting from parietal and/or frontal lesions, impaired insight in patients with schizophrenia has been proposed to arise from deficits in these same brain regions12,13,70,71,72,73,74. Studies in patients with first-episode psychosis showed reduced right dorsolateral prefrontal cortex volume in patients with impaired insight71,73. Similarly, a longitudinal study found that volume deficits in frontal and parietal regions in patients with first-episode psychosis predicted insight impairments two years after the onset of the first-episode75. Consistent with these results, our study found cortical deficits in frontal and parietal regions, specifically the left inferior and middle frontal gyrus and the supramarginal gyrus, in patients with impaired insight. Cortical thinning was also observed in the left temporal pole. The left inferior frontal region is frequently implicated in self-referential processing where several functional neuroimaging studies in healthy controls and patients with schizophrenia have shown increased activity in this region during self-reflection tasks76,77. Moreover, a previous functional neuroimaging study by our group in patients with schizophrenia found an association between greater self-certainty and reduced resting-state functional connectivity with the left inferior frontal cortex in the dorsal attention network, suggesting a deficit in this region may reflect a lack of mental flexibility in patients with impaired insight59. The temporal pole, a component of the paralimbic circuit along with the orbitofrontal cortex and the insular cortex, is engaged in various cognitive functions including language, multisensory integration, and social and emotional behaviours, all of which have been found to be impaired in patients with schizophrenia78,79. Its direct link with impaired insight requires further exploration.
A number of investigations suggest that impaired insight in patients with schizophrenia likely arises from disrupted brain networks rather than solely from deficits in specific brain regions18,68,80,81. Resting-state functional connectivity studies have shown aberrant connections within the default mode network and the self-reflection network in patients with impaired insight59,82. A diffusion tensor imaging study by our group also showed reduced posterior corpus callosal tract integrity in patients with impaired insight compared to those with intact insight and healthy controls, possibly reflecting disrupted communication between key brain regions associated with impaired insight68.
Although our study did not find a statistically significant association between impaired insight and thalamic volume, we suspect our study may have been underpowered to detect this relationship. We theorise that cortical abnormalities in frontoparietal regions in patients with impaired insight may reflect deficits in the indirect frontoparietal pathway that connects the frontal and posterior parietal regions via the basal ganglia and the thalamus83,84,85. Further studies are needed to examine the links among cortical and subcortical structural and functional deficits in patients with impaired insight in schizophrenia.
The present study has several limitations. First, this study was conducted retrospectively. As a result, measures that would have helped further validate our results, such as using a clinician-rated measure of insight, were not collected. The lack of a clinician-rated measure is somewhat mitigated by the strong correlation between the VAGUS-SR and the clinician-rated version and other established clinician-rated measures of insight into illness in schizophrenia23. Arguably, self-report measures obviate clinician factors, such as bias, that may negatively influence the accurate assessment of insight into illness in schizophrenia86,87. Second, we did not compare treatment-resistant and non-treatment resistant participants as our variable of interest was insight into illness and to do so adequately would require a sophisticated matching analysis based on degree of insight and other relevant variables. Third, due to the cross-sectional design of the study, we were unable to account for the effects of prior antipsychotic drug use (e.g., specific drug and duration of use) on cortical thickness and subcortical volumes. As all of the treatment-resistant patients were on clozapine, it is possible that clozapine contributed to cortical thinning in these patients88,89. That being said, there is more evidence to support the theory that illness chronicity or resistance contributes to cortical thinning in schizophrenia20,21,22,23. Last, the sample size in the non-treatment-resistant group was relatively small. Therefore, it is possible that we did not have enough statistical power to detect significant associations in this group. Relatedly, there were likely patients in the non-treatment-resistant group who are non-responsive to their current treatment but did not meet our criteria for treatment resistance.
Conclusions
Overall, our findings suggest that cortical thinning in the left frontal, temporal pole, and posterior parietal regions is associated with insight impairment in patients with a schizophrenia spectrum disorder, and more so in patients with treatment resistance. Future longitudinal studies with well-characterised samples representative of the full spectrum of insight and illness severity may be needed to better understand the neuroanatomical correlates of insight into illness in schizophrenia.
References
Amador, X. F. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch. Gen. Psychiatry 51, 826–836 (1994).
Buckley, P. F. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs 21, 129–141 (2007).
Czobor, P. et al. Treatment adherence in schizophrenia: a patient-level meta-analysis of combined CATIE and EUFEST studies. Eur. Neuropsychopharmacol. 25, 1158–1166 (2015).
Kim, J. et al. Insight and medication adherence in schizophrenia: An analysis of the CATIE data. Neuropharmacology https://doi.org/10.1016/j.neuropharm.2019.05.011 (2019).
Lincoln, T. M. et al. The impact of negative treatment experiences on persistent refusal of antipsychotics. Compr. Psychiatry 70, 165–173 (2016).
Misdrahi, D. et al. Determination of adherence profiles in schizophrenia using self-reported adherence: results from the FACE-SZ dataset. J. Clin. Psychiatry 77, e1130–e1136 (2016).
Lincoln, T. M., Lullmann, E. & Rief, W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr. Bull. 33, 1324–1342 (2007).
Lysaker, P. H., Pattison, M. L., Leonhardt, B. L., Phelps, S. & Vohs, J. L. Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry 17, 12–23 (2018).
Mintz, A. R., Dobson, K. S. & Romney, D. M. Insight in schizophrenia: a meta-analysis. Schizophr. Res. 61, 75–88 (2003).
Novick, D. et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 176, 109–113 (2010).
Svarstad, B. L. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 52, 805–811 (2001).
Sapara, A. et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr. Res. 89, 22–34 (2007).
Shad, M. U. Neurobiological underpinnings of insight deficits in schizophrenia. Int. Rev. Psychiatry (Abingdon, England) 19, 437–446 (2007).
Emami, S., Guimond, S., Mallar Chakravarty, M. & Lepage, M. Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophr. Res. 170, 66–72 (2016).
Sapara, A., Ffytche, D. H., Cooke, M. A., Williams, S. C. & Kumari, V. Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J. Psychiatry 6, 311–321 (2016).
McFarland, J. et al. Association of grey matter volume deviation with insight impairment in first-episode affective and non-affective psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 263, 133–141 (2013).
Pijnenborg, G. H. M. et al. Brain areas associated with clinical and cognitive insight in psychotic disorders: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 116, 301–336 (2020).
Buchy, L., Makowski, C., Malla, A., Joober, R. & Lepage, M. Longitudinal trajectory of clinical insight and covariation with cortical thickness in first-episode psychosis. J. Psychiatr. Res. 86, 46–54 (2017).
Béland, S. et al. Clarifying associations between cortical thickness, subcortical structures, and a comprehensive assessment of clinical insight in enduring schizophrenia. Schizophr. Res. 204, 245–252 (2019).
Anderson, V. M., Goldstein, M. E., Kydd, R. R. & Russell, B. R. Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int. J. Neuropsychopharmacol. 18, pyv016 (2015).
Kim, J. et al. Neuroanatomical profiles of treatment-resistance in patients with schizophrenia spectrum disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 99, 109839 (2020).
Shah, P. et al. Glutamatergic neurometabolites and cortical thickness in treatment-resistant schizophrenia: Implications for glutamate-mediated excitotoxicity. J. Psychiatr. Res. 124, 151–158 (2020).
Gerretsen, P. et al. The VAGUS insight into psychosis scale – Self-report and clinician-rated versions. Psychiatry Res. 220, 1084–1089 (2014).
First, M., Spitzer, R., Gibbon, M. & Williams, J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P). (Biometrics Research Dept, New Yoork State Psychiatric Institute, 2001).
Iwata, Y. et al. Glutamatergic neurometabolite levels in patients with ultra-treatment-resistant schizophrenia: a cross-sectional 3T proton magnetic resonance spectroscopy study. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2018.09.009 (2018).
Howes, O. D. et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 174, 216–229 (2017).
Demjaha, A., Murray, R. M., McGuire, P. K., Kapur, S. & Howes, O. D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 169, 1203–1210 (2012).
Demjaha, A. et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol. Psychiatry. 75, e11–e13 (2014).
Appelbaum, P. S. & Grisso, T. The MacArthur Treatment Competence Study. I: mental illness and competence to consent to treatment. Law Hum. Behav. 19, 105–126 (1995).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Wilkinson, G. S. WRAT-3: Wide Range Achievement Test Administration Manual (Wide Range, Inc., 1993).
Brown E., Shah P., & Kim J. ChlorpromazineR: Convert Antipsychotic Doses to Chlorpromazine Equivalents. https://docs.ropensci.org/chlorpromazineR/, https://github.com/ropensci/chlorpromazineR (2019).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Eskildsen, S. F. et al. BEaST: brain extraction based on nonlocal segmentation technique. Neuroimage 59, 2362–2373 (2012).
Chakravarty, M. M. et al. DISC1 and striatal volume: a potential risk phenotype for mental illness. Front. Psychiatry 3, 57 (2012).
Plitman, E. et al. Glutamatergic metabolites, volume and cortical thickness in antipsychotic-naive patients with first-episode psychosis: implications for excitotoxicity. Neuropsychopharmacology 41, 2606–2613 (2016).
Talpalaru, A., Bhagwat, N., Devenyi, G. A., Lepage, M. & Chakravarty, M. M. Identifying schizophrenia subgroups using clustering and supervised learning. Schizophrenia Research https://doi.org/10.1016/j.schres.2019.05.044 (2019).
Collins, D. L., Neelin, P., Peters, T. M. & Evans, A. C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist Tomogr. 18, 192–205 (1994).
Tohka, J., Zijdenbos, A. & Evans, A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 23, 84–97 (2004).
Zijdenbos, A., Forghani, R. & Evans, A. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT. in Medical Image Computing and Computer-Assisted Intervention— MICCAI’98 (eds. Wells, W. M., Colchester, A. & Delp, S.) 439–448 (Springer Berlin Heidelberg, 1998).
Kim, J. S. et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27, 210–221 (2005).
MacDonald, D., Kabani, N., Avis, D. & Evans, A. C. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12, 340–356 (2000).
Ad-Dab’bagh Y. et al. In: Zilles K (ed) Native space cortical thickness measurement and the absence of correlation to cerebral volume, 11th Annual Meeting of the Organization for Human Brain Mapping, Toronto (2005).
Lerch, J. P. & Evans, A. C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24, 163–173 (2005).
Boucher, M., Whitesides, S. & Evans, A. Depth potential function for folding pattern representation, registration and analysis. Med. Image Anal. 13, 203–214 (2009).
Lyttelton, O., Boucher, M., Robbins, S. & Evans, A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage 34, 1535–1544 (2007).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Chakravarty, M. M. et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum. Brain Mapp. 34, 2635–2654 (2013).
Pipitone, J. et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 101, 494–512 (2014).
Caravaggio, F. et al. Amotivation is associated with smaller ventral striatum volumes in elderly patients with schizophrenia. Int. J. Geriatr. Psychiatry 33, 523–530 (2018).
Chung, J. K. et al. Depressive symptoms and small hippocampal volume accelerate the progression to dementia from mild cognitive impairment. J. Alzheimer’s Dis. 49, 743–754 (2016).
Plitman, E. et al. Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr. Bull. 42, 415–424 (2016).
Tullo, S. et al. MR-based age-related effects on the striatum, globus pallidus, and thalamus in healthy individuals across the adult lifespan. Hum. Brain Mapp. 0, 5269–5288 (2019).
Chakravarty, M. M., Bertrand, G., Hodge, C. P., Sadikot, A. F. & Collins, D. L. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30, 359–376 (2006).
Tullo, S. et al. Warping an atlas derived from serial histology to 5 high-resolution MRIs. Sci. Data 5, 180107 (2018).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Sowell, E. R. et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex 17, 1550–1560 (2007).
Genovese, C. R., Lazar, N. A. & Nichols, T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. NeuroImage 15, 870–878 (2002).
Gerretsen, P. et al. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: Resting state functional connectivity. Schizophr. Res. 160, 43–50 (2014).
Gerretsen, P. et al. Illness denial in schizophrenia spectrum disorders: a function of left hemisphere dominance. Hum. Brain Mapp. 36, 213–225 (2015).
Kim, J. et al. Modulation of brain activity with transcranial direct current stimulation: Targeting regions implicated in impaired illness awareness in schizophrenia. Eur. Psychiatry 61, 63–71 (2019).
Chakravarty, M. M., Bertrand, G., Hodge, C. P., Sadikot, A. F. & Collins, D. L. The creation of a brain atlas for image guided neurosurgery using serial histological data. NeuroImage 30, 359–376 (2006).
Tullo, S., Devenyi, G. A., Patel, R., Park, M. T. M., Collins, D. L. & Chakravarty, M. M. Warping an atlas derived from serial histology to 5 high-resolution. MRIs Abstract Scientific Data 5, https://doi.org/10.1038/sdata.2018.107 (2018).
Okada, N. et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 21, 1460–1466 (2016).
van Erp, T. G. M. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 21, 547–553 (2016).
Buchy, L. et al. Cognitive insight is associated with cortical thickness in first-episode psychosis. Schizophr. Res. 172, 16–22 (2016).
Wannan, C. M. J. et al. Evidence for network-based cortical thickness reductions in schizophrenia. Am. J. Psychiatry 176, 552–563 (2019).
Gerretsen, P. et al. Impaired illness awareness in schizophrenia and posterior corpus callosal white matter tract integrity. NPJ Schizophr. 5, 8 (2019).
Xavier, R. M. & Vorderstrasse, A. Neurobiological basis of insight in schizophrenia: a systematic review. Nurs. Res. 65, 224–237 (2016).
Gerretsen, P. et al. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum. Brain Mapp. 34, 1035–1043 (2013).
Shad, M. U., Muddasani, S., Prasad, K., Sweeney, J. A. & Keshavan, M. S. Insight and prefrontal cortex in first-episode Schizophrenia. NeuroImage 22, 1315–1320 (2004).
Flashman, L. A. et al. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J. Neuropsychiatry Clin. Neurosci. 13, 255–257 (2001).
Shad, M. U., Muddasani, S. & Keshavan, M. S. Prefrontal subregions and dimensions of insight in first-episode schizophrenia—a pilot study. Psychiatry Res Neuroimaging 146, 35–42 (2006).
Gerretsen, P., Plitman, E., Rajji, T. K. & Graff-Guerrero, A. The effects of aging on insight into illness in schizophrenia: a review. Int. J. Geriatr. Psychiatry 29, 1145–1161 (2014).
Parellada, M. et al. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr. Bull. 37, 38–51 (2011).
van der Meer, L. et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 39, 1288–1295 (2013).
Shad, M. U. & Keshavan, M. S. Neurobiology of insight deficits in schizophrenia: An fMRI study. Schizophr. Res. 165, 220–226 (2015).
Crespo-Facorro, B. et al. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. Neuroimaging 132, 107–115 (2004).
Xu, Y. et al. Selective functional disconnection of the orbitofrontal subregions in schizophrenia. Psychol Med. 47, 1637–1646 (2017).
Antonius, D. et al. White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr. Res. 128, 76–82 (2011).
Peters, B. D., Blaas, J. & de Haan, L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J. Psychiatric Res. 44, 993–1004 (2010).
Ćurčić-Blake, B., van der Meer, L., Pijnenborg, G. H. M., David, A. S. & Aleman, A. Insight and psychosis: functional and anatomical brain connectivity and self-reflection in schizophrenia. Hum. Brain Mapp. 36, 4859–4868 (2015).
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann. Rev. Neurosci. 9, 357–381 (1986).
Clower, D. M., Dum, R. P. & Strick, P. L. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb. Cortex 15, 913–920 (2005).
Ekman, M., Fiebach, C. J., Melzer, C., Tittgemeyer, M. & Derrfuss, J. Different roles of direct and indirect frontoparietal pathways for individual working memory capacity. J. Neurosci. 36, 2894–2903 (2016).
Möller, H. J. Rating depressed patients: observer- vs self-assessment. Eur. Psychiatry 15, 160–172 (2000).
Zimmerman, M., Walsh, E., Friedman, M., Boerescu, D. A. & Attiullah, N. Are self-report scales as effective as clinician rating scales in measuring treatment response in routine clinical practice? J. Affect. Disord. 225, 449–452 (2018).
Ahmed, M. et al. Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacol 40, 2409–2417 (2015).
Mattai, A. et al. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr. Res. 116, 44–48 (2010).
Acknowledgements
The authors would like to thank Wanna Mar, research coordinator. This work was supported by the Centre for Addiction and Mental Health (CAMH) Discovery Fund (J.K.), the Ontario Mental Health Foundation (OMHF) operating grant, and the Canadian Institute of Health Research (CIHR) (MOP-142493 and 14196 to A.G.-G.).
Author information
Authors and Affiliations
Contributions
J.K. and J.S. formulated the research aims, assisted with designing the research methodology, conducted statistical analyses, and wrote the first draft of manuscript. Y.K. conducted statistical analyses and assisted with writing the first draft of the manuscript. E.P. and P.S. formulated the research aims, assisted with research methodology, and data interpretation. Y.I., F.C., and E.B. assisted with designing the research methodology, data interpretation, and edited the manuscript. S.N. and M.C. assisted with designing the research methodology and data interpretation. V.D., G.R., and A.G. assisted with formulating the research aims, interpreting the data, and edited the manuscript. P.G. formulated the research aims, designed the research methodology, assisted with data interpretation, provided oversight, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J., Song, J., Kambari, Y. et al. Cortical thinning in relation to impaired insight into illness in patients with treatment resistant schizophrenia. Schizophr 9, 27 (2023). https://doi.org/10.1038/s41537-023-00347-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00347-y