Abstract
Growing evidence shows that insomnia is closely associated with schizophrenia (SCZ), but the neural mechanism under the association remains unclear. A direct comparison of the patterns of resting-state brain activities would help understand the above question. Using meta-analytic approach, 11 studies of insomnia vs. healthy controls (HC) and 39 studies of SCZ vs. HC were included to illuminate the common and distinct patterns between insomnia and SCZ. Results showed that SCZ and insomnia shared increased resting-state brain activities in frontolimbic structures including the right medial prefrontal gyrus (mPFC) and left parahippocampal gyrus. SCZ additionally revealed greater increased activities in subcortical areas including bilateral putamen, caudate and right insula and greater decreased activities in precentral gyrus and orbitofrontal gyrus. Our study reveals both shared and distinct activation patterns in SCZ and insomnia, which may provide novel insights for understanding the neural basis of the two disorders and enlighten the possibility of the development of treatment strategies for insomnia in SCZ in the future.
Similar content being viewed by others
Introduction
Schizophrenia(SCZ) is a chronic and severe mental disorder, manifested as positive symptoms such as hallucinations and delusions, negative symptoms like social withdrawal and apathy, and cognitive symptoms including deficits in working memory and executive function1. Although not included in the diagnostic criteria of schizophrenia, accumulating evidence has revealed that insomnia, one of the most prevalent health complaints worldwide2, is closely associated with schizophrenia3,4,5,6. Insomnia can be a predictive factor to estimate the probability that people at high risk develop into psychosis7. Moreover, insomnia plays an important role in exacerbating the core symptoms of schizophrenia, such as cognitive impairments8,9, auditory hallucinations and delusions10,11, social withdrawal and depression12. Clinical trials show an exciting prospect that treatment of insomnia may minimize the impact of psychotic symptoms and improve quality of life13,14. However, the neurobiological underpinnings for the pathogenesis of schizophrenia and insomnia are yet to be illuminated.
Several neurochemical studies5,15 have sought to elucidate the mechanism behind schizophrenia comorbid insomnia from different perspectives. For example, the hyperactivity of D2 receptors in the striatum16, reduction of GABA activity16, and lower levels of glutamate17 can disrupt circadian rhythm and increase wakefulness, ultimately leading to insomnia. Some researchers found that the deficits of sleep spindle activity, which is a representative thalamocortical oscillation, were observed in insomnia patients and also relate to the cognitive impairments in patients with schizophrenia18,19,20. However, it remains unclear whether insomnia and schizophrenia share similar deficits in neural networks.
Over the past decades, resting-state functional MRI (rs-fMRI), a neuroimaging technique that has provided a noninvasive and task-free method to explore intrinsic brain activity and functional connectivity in brain networks21, has been applied widely in both insomnia and schizophrenia to explore the neuropathological abnormalities behind22,23,24. The regional spontaneous brain activity can be commonly measured by three derived indices of rs-fMRI: the amplitude of low-frequency fluctuations (ALFF), fractional ALFF (fALFF), and regional homogeneity (ReHo) of the blood-oxygen-level-dependent (BOLD) signals25,26. ALFF and fALFF algorithms can measure the total power of the low-frequency BOLD signal, which is relevant to regional neural activity. Meanwhile, ReHo analysis reflects the synchrony of regional brain activity26. Although the principles and algorithms among these indices are different, all of them could reflect regional spontaneous brain activities in essence and their combination offers a more comprehensive assessment of brain dysfunction27,28. The decreased intrinsic brain activity measured by these indices are thought to indicate a functional deprivation or localized functional disruption by the disease, whereas increased brain activity may reflect disease-related excessive function state in the specific brain region29.
Previous studies of ALFF/fALFF or ReHo have shown similar functional abnormalities in insomnia and schizophrenia. For instance, studies of insomnia patients reported that ALFF and ReHo changes had been observed in brain areas associated with emotion, cognition, and sleeping-wake system, such as the prefrontal cortex30,31, occipital lobe30, limbic system32,33 and cerebellum30,31,33, which were also been consistently found abnormal in patients with schizophrenia34,35,36. However, no experimental studies have directly compared the patterns of brain activity change between insomnia and schizophrenia and it remains unclear whether there exists a shared neurobiological pattern between insomnia and schizophrenia. An alternative strategy is to calculate the overlapping and specific mechanisms by comparing single-disorder effects37,38, although the levels of comorbidity between schizophrenia and insomnia may confound the effects.
Therefore, we conducted a coordinate-based analysis of likelihood estimate (ALE) meta-analysis comparing rs-fMRI studies of insomnia patients with those of schizophrenia. Given the limited literature on directly comparing SCZ and insomnia, we included studies that separately compared each patient group (insomnia or SCZ) to healthy populations and combined the single group activations to explore the conjunction and contrast areas between the two diseases. This is intended to enable a better understanding of the common and distinct pathophysiology of insomnia and schizophrenia and ultimately enlighten the adaption and improvement of treatment for schizophrenia patients with insomnia.
Methods
Search strategy
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement39, we collected relevant studies by a search of the PubMed, EMBASE and Web of Science databases up to December 2021. Keywords for the search were as follows: (1) [(“rest-state” OR “resting state”) AND (“functional magnetic resonance” OR “fMRI” OR “functional MRI”) AND (“insomnia” OR “insomnia disorder”)]; (2) [(“schizophrenia” OR “schizoaffective disorder” OR “schizophrenic disorder”) AND (“amplitude of low-frequency fluctuation” OR “ALFF” OR “low-frequency fluctuation” OR “ReHo” OR “regional homogeneity”)]. We also checked the reference lists of the included studies and relevant review articles to identify potential studies that were not included in the systematic search. This meta-analysis is registered in the PROSPERO database (ID: CRD42021287152).
Study selection
A study was included in the meta-analysis if it satisfied the following criteria: (1) ALFF/fALFF or ReHo comparison of patients with schizophrenia/insomnia versus healthy controls(HC) was conducted; (2) whole‐brain coordinates in either Talairach or Montreal Neurological Institute (MNI) space can be acquired from the literature or Supplementary Materials; (3) The results were reported by using thresholds for significance that were corrected for multiple comparisons or uncorrected with spatial extent thresholds; (4) The study was written in English in the peer-reviewed journal.
The exclusion criteria were as follows: (1) case reports, conference abstracts, editorials, comments, or review studies without original data; (2) Studies using seed-voxel-based analysis or reporting only region-of-interest results; (3) The peak coordinates were not retrieved from the literature, even after contacting with the authors; (4) The number of each group in the study was <10; (5) studies of which data overlapped with those of other publications. In this case, we only included the study with the largest sample in our meta-analysis.
Data extraction
Two authors (ZG and YX) independently conducted the literature search, study selection, and data extraction. All information was double-checked. If a disagreement appeared, a third author mediated for the final decision. The demographic and clinical data, imaging parameters, statistical information, and complete stereotaxic peak coordinates were extracted from each included study. The coordinates reported in Talairach were converted into MNI space by the icbm2tal transform in the GingerALE40.
Notably, A study refers to an individual publication and an experiment refers to the single specific contrast producing some peak coordinates. To decrease within-group effects41, we merged the results of one study into a maximum of two experiments (increases and decreases in brain activity, i.e., patients > control and patients < control) in the meta-analysis.
Quality assessment
Each selected study was assessed for quality with a 12-point checklist which has been applied frequently in prior neuroimaging meta-analysis42,43. Two researchers (ZG and YX) independently evaluated the included studies from the following categories containing 12 items: participants (item 1–4), methods for image acquisition and analysis (item 5–10), and the results and conclusions (item 11,12). Each item received a score of 1, 0.5 or 0 based on the criteria that were fully, partially met, or not met, separately. More information of the checklist could be seen in Supplementary Materials.
Activation likelihood estimation (ALE) meta-analysis
The current coordinate-based meta-analysis was conducted by the GingerALE software version 3.0.2 (http://brainmap.org/ale/), which implements the latest ALE algorithm44. Specifically speaking, ALE is an approach of meta-analysis that collects activation foci reported in neuroimaging studies and creates three-dimensional Gaussian probability distributions for each focus to compute modeled activation (MA) maps. ALE images are then obtained by the union of MA maps. Finally, a convergence of foci is verified by testing against the null-hypothesis of random spatial association between experiments44,45.
All coordinates extracted from the included studies were integrated and separated into four groups based on the regional brain activity changes of patients compared with HC: SCZ > HC, SCZ < HC, insomnia > HC, and insomnia < HC. A two-step analysis plan was utilized. Firstly, we conducted first-level ALE analyses respectively for the four contrasts, with an initial uncorrected threshold of voxel-level p < 0.005 and a minimum cluster size of 20 mm3. Then, the ALE results of these first-level analyses were pooled into the second-level conjunction/subtraction analysis to identify the common and distinct brain activation between insomnia and schizophrenia. Two second-level models were built to examine our concern: one comparing increased activities (i.e., insomnia > HC and SCZ > HC) and the other comparing decreased activities (i.e., insomnia < HC and SCZ < HC). The second-level analysis involved quantitative conjunction analysis and conducted non-parametric permutation simulations (10,000 permutations) to draw statistical inferences of differences between insomnia and SCZ. Because of the explorative nature of this study, we utilized an uncorrected threshold of p < 0.05 with a minimum cluster volume of 20 mm3 to remove false small-size clusters as well as discovering more co-activated areas. To visualize the final results, the ALE cluster maps were exported into Mango (http://www.nitrc.org/projects/mango), utilizing an anatomical brain template (Colin27_T1_seg_ MNI.nii).
Subgroup analysis based on the phase of schizophrenia
The schizophrenia literature consists of both first-episode schizophrenia (FES) and long-term ill schizophrenia. Since growing evidence demonstrated that the patterns of structure and functional abnormalities may change during the course of schizophrenia46,47,48,49, whether illness course could exert an influence on the brain activation between insomnia and schizophrenia was worth investigating. To address this concern, we conducted an additional subgroup analysis of patients with first-episode and long-term ill schizophrenia. The classification of the phase of schizophrenia was based on criteria defined by the authors of the original study. Studies including both first-episode and long-term ill schizophrenia were excluded from the subgroup analysis.
Control analyses
To evaluate the robustness of our conjunction results and control the age-related effects, we conducted an additional control analysis. First, we excluded studies of children and adolescents and repeated conjunction and contrast analysis only for studies involving adults. Second, since the participants in insomnia studies were older than those in SCZ studies, we ran the same analysis but only included the schizophrenia literature solely to those studies involving participants whose ages are above 30 to narrow the age gap between participants of insomnia and SCZ.
Results
Study selection and characteristics
Figure 1 shows the flow diagram which depicts the process of reviewing and selecting included studies. Ultimately, we included 11 studies of insomnia and 39 studies of schizophrenia, respectively. The final datasets comprised 360 insomnia patients (mean age 43.1 years), 1945 SCZ patients (mean age 28.5 years), and 2216 HC (372 from insomnia vs. HC studies, mean age 41.6 years; and 1844 from SCZ vs. HC studies, mean age 29.1 years). The literature researching insomnia yield 60 foci of brain activation and the studies about SCZ provide 342 significant foci. For all included studies, 10 experiments reported HC > insomnia,10 for insomnia > HC, whereas 36 experiments reported HC > SCZ and 32 experiments for SCZ > HC. The detailed demographic, clinical and imaging characteristics of the included studies are summarized in Table 1.
The qualities of all included studies were considered to be similar and high. The mean quality scores of included studies for insomnia and SCZ were 11.8 (range 11.5–12) and 11.8 (range 11–12) respectively. The detailed quality scores of each included study are showed in Table S1.
Results of the main meta-analysis
Results from the first-level ALE analyses of insomnia and SCZ studies are presented in Supplementary Tables S2–S5. The conjunction and contrast analyses of insomnia and SCZ literature provide the clusters of overlapping increased and decreased brain activities, as well as the significant different brain activations between the two diseases. The second-level results are discussed below in detail.
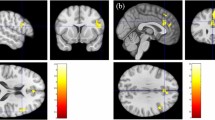
The conjunction analysis demonstrates the common region with increased activity in frontolimbic structures, including the right medial prefrontal gyrus (mPFC) and left parahippocampal gyrus (Fig. 2, Table 2A). We found no significant direct overlapping area in decreased activities between insomnia and SCZ likewise.
Subtractions of first-level analyses revealed distinct patterns of activation between insomnia and SCZ. Compared with those with SCZ, patients with insomnia exhibited greater increased activities in right posterior cingulate (Fig. 3, Table 2B) and greater decreased activities in right insula, frontal lobe, cerebellum and left cingulate gyrus (Fig. 4, Table 2D).
SCZ schizophrenia, L left, R right. Contrast analysis shows areas with increased activities which were significantly different between insomnia and SCZ patients. Clusters with greater increased activities in SCZ are shown in red, and clusters with greater increased activities in insomnia are shown in green. First-level analyses were formed using a threshold of p < 0.005, k = 20 mm3, and second analyses were conducted with a threshold of p < 0.05, k = 20 mm, 10,000 permutations.
SCZ schizophrenia, L left, R right. Contrast analysis shows areas with decreased activities which were significantly different between insomnia and SCZ patients. Clusters with greater decreased activities in SCZ are shown in red, and clusters with greater decreased activities in insomnia are shown in green. First-level analyses were formed using a threshold of p < 0.005, k = 20 mm3, and second analyses were conducted with a threshold of p < 0.05, k = 20 mm, 10,000 permutations.
In contrast, the analyses found greater increased activities in the subcortical area, including bilateral putamen and caudate body, right insula (Fig. 3, Table 2C), as well as greater decreased activities in the right precentral gyrus and left orbitofrontal gyrus (Fig. 4, Table 2E) in SCZ relative to insomnia.
Results of subgroup analysis
To estimate the effect of illness duration on the main results, we repeated the conjunction and contrast analysis for FES and long-term ill SCZ, respectively. In the FES subgroup, the distinct patterns of regional brain activities between insomnia and FES are similar to those in the main results. In the long-term ill SCZ subgroup, we found overlapping increased activities in medial prefrontal gyrus and parahippocampus remain significant, and patients with long-term ill SCZ demonstrate greater decreased activities in right paracentral lobule, precuneus and left occipital gyrus. The detailed results can be found in Table S6.
Control analysis
After excluding two adolescent schizophrenia studies, the main results with only adult participants remain largely unchanged. The detailed results were provided in Table S7. When the analyses were restricted to studies with participants with older age (15 experiments in HC > SCZ contrast; 15 experiments in SCZ > HC contrast), the common hyperactivation in parahippocampal gyrus remained significant, although the increased activity in mPFC were not replicated in the relatively low-power analysis. Moreover, the greater increased activities in subcortical areas in SCZ and decreased activities in precentral gyrus were also replicated in the subgroup analysis (Table S8).
Discussion
In the current study, we conducted a coordinate-based meta-analysis that investigates the common and distinct patterns of brain activity between insomnia and SCZ by comparing patients with healthy controls and contrasting the activation areas in insomnia and SCZ patients. The results displayed a convergence of increased activities within frontolimbic structures containing right mPFC and left parahippocampal gyrus. Moreover, the overlapping increased brain activities were accompanied by specific distinct increased activity within subcortical areas and decreased activativity within precentral gyrus and ventromedial prefrontal gyrus (VMPFC) in SCZ. These results support a hypothesis that explains the associations between SCZ and insomnia: the common abnormality in areas of attention, vigilance and emotion control and common impairments in areas of memory and cognition, but probably different mechanisms of sleep disturbance in SCZ compared with primary insomnia. Although the unknown comorbidity between the two diseases may limit our conclusion, our findings can offer novel insights into the complicated interplay between insomnia and schizophrenia to further explore the potential treatment target for schizophrenia patients with insomnia symptoms.
Our meta-analysis demonstrated a convergent increased activation pattern within right mPFC. mPFC is a crucial region for integrating input information and delivering integrated information to output structures50. It has been proved to play an important role in emotion control, attention and vigilance51,52,53, in which deficits have been shown in SCZ patients31,54,55. Interestingly, mPFC is also found to generate non-rapid eye movement (NREM) slow-wave oscillations and as a result, to participate in mediating the induction and maintenance of different sleep stage56,57. In addition, abnormal increased brain activities in the mPFC have been reported in both SCZ54,58,59,60,61 and insomnia patients31,55,62previously. Since mPFC is a key node for default network (DMN), it is likely that the dysregulation of DMN plays a pivotal role in the common pathophysiology of insomnia and SCZ. The increased activities of mPFC may disrupt the decoupling of DMN during sleep63 and lead to aberrant thalamocortical sleep-wakefulness rhythms64,65. Similarly, the failure of deactivation in DMN could be observed in SCZ patients66 and correlated to dysfunctional connectivity to thalamus67. This can enlighten future studies on exploring the main circuit responsible for insomnia symptoms in SCZ.
Besides neuroimaging studies, genetic studies have indicated mPFC as a potential key region in the pathophysiological mechanisms behind insomnia problems in SCZ. Shen et al. found that deleting FXR1 gene, of which the variants are linked to shorter sleep duration68, from parvalbumin interneurons of mPFC leads to impaired mPFC gamma oscillation and SCZ-like behaviors69. The large GWAS study in 1,331,010 individuals also showed insomnia-related gene set enrichments in frontal cortex, which have correlations with psychiatric traits70. Considering together with those findings, our results consolidate the hypothesis that mPFC is a key neuroanatomic structure that is responsible for insomnia symptom in SCZ patients.
In addition to the hyperactivation in mPFC, our study also revealed the convergent increased activity in the parahippocampal gyrus. Increased activity in the parahippocampal region has been consistently reported by previous meta-analyses on functional activation patterns in both SCZ and insomnia71,72. As an important component of the limbic system, hippo/parahippocampal gyrus has been demonstrated to be associated with lots of cognitive processes, including semantic processing, visuospatial navigation, working memory and episodic memory73,74,75,76. Our results are in agreement with the theory that the aberrant activations of parahippocampal gyrus are correlated with impaired cognition and memory, which has been found in both SCZ35,77,78 and insomnia patients79. Moreover, the medial prefrontal cortex and parahippocampal gyrus are interconnected by cingulum bundle structurally80 and belong to DMN functionally. Our results revealed that the abnormal activation of DMN was presumed to be the common target for the neurobiological mechanisms of SCZ and insomnia, which was found to be altered in the two diseases separately81,82.
Apart from the functional convergent pattern between SCZ and insomnia patients, our study also demonstrated the main greater increased activities in the subcortical regions in SCZ compared to insomnia. Increased activities in subcortical regions including insula, striatum, and caudate body have been extensively reported by resting-state fMRI-based meta-analyses in SCZ49,83. Broad evidence from fMRI studies has revealed that the subcortical areas containing insula extending into the striatal regions are implicated in autonomic, affective, cognitive and behavioral processing84,85,86,87, deficits of which are the core manifestations of SCZ. Previous studies have also unraveled the association between activation of subcortical regions and impairment of cognitive-emotional control in SCZ88. Another possibility is that antipsychotic medication could also increase activities in basal ganglia region89,90. The increased activities of subcortical regions may cause high emotional vulnerability84 and other psychiatric symptoms91, as well as dysregulation of autonomic control, which will regulate cardiovascular and neural activities during sleep and waking92. These abnormalities constitute the “sleep stressor” and lead the brain into a hyper-arousal state, preventing the SCZ patients from sound sleep. This hypothesis could be supported by the findings that sedating atypical antipsychotics like olanzapine and paliperidone may ameliorate sleep parameters in SCZ patients93,94. Thus, more studies targeting the neurobiological function of subcortical regions in SCZ comorbid insomnia are warranted to be conducted.
As for decreased brain activities, our results showed the different patterns between SCZ and insomnia patients. In SCZ patients, precentral gyrus and orbitofrontal gyrus were found to be deactivated to a higher degree. Previous fMRI studies have found decreased ReHo/ALFF values in the OFC and precentral gyrus in either first-episode or long-term ill schizophrenia35,95,96,97. OFC and precentral gyrus are both participated in sensorimotor integration and their hypoactivations are correlated with negative symptoms including motor retardation and blunted affection in SCZ98,99, which are commonly not appeared in insomnia. Conversely, the decreased activation regions in insomnia patients are distributed more broadly. These regions including cerebellum, insula, cingulate gyrus and frontal gyrus were found to play a role in sleep disorders. Their morphological and functional changes indicated abnormal neural activity and may be correlated with disturbance of neural electrical activities in REM and NREM sleep57,100,101,102. More studies combined with EEG and neuroimaging techniques are required to investigate how these regions regulate the sleep-wake circle and cause sleep disturbance in the future.
Furthermore, our subgroup analysis found the effect of illness duration in SCZ on our results. Most results in the main analysis were still robust even in consideration of illness duration. The distinct patterns of brain activation in FES and long-term illness SCZ are also consistent with those reported in the previous meta-analyses49,103. The activation difference between different phases of SCZ may reflect the progressive impairment and change of brain function along the illness course. However, the effects of medication and lifestyle are still difficult to control and the clinical sleep-disturbance patterns in FES and long-term ill SCZ remains unclear, so more clinical and neuroimaging studies should be conducted to unravel the problem.
Several limitations should be considered when interpreting the findings in our meta-analysis. First, the proportion of schizophrenia patients with comorbid insomnia in the included studies is unavailable, which limited the explanation of the shared neural activity pattern of the two diseases. Further investigations directly focused on the comorbidity of SCZ and insomnia are required to confirm our findings. Second, for the explorative nature of this study, we adopted a relatively loose statistical threshold, which might increase false positive probability of the results. Third, our meta-analysis is based on the peak coordinates from the selected literature rather than on raw data, which includes nonsignificant results, which may limit the accuracy of our results. Fourth, on account of the limitation in the amount of literature, the studies on insomnia included in this meta-analysis are relatively less compared with the fMRI studies of SCZ. The difference in datasets of the two diseases may cause concerns of differential statistical power in the first-level analysis. Lastly, some factors including sex, age, and severity of the disease can impact brain activation. Considering our intention to identify the common and distinct pattern of brain activation between SCZ and insomnia by separately comparing the patients with healthy controls who matched the factors mentioned before, the effect of those factors between the SCZ studies and insomnia studies is limited.
Conclusion
Despite those limitations, our current coordinate-based meta-analysis, drawing upon a large number of studies from both SCZ and insomnia literature, offers some novel findings on how the two diseases converge and differ from each other in the brain activation area. We identify the convergent increased brain activity pattern including mPFC and parahippocampal gyrus, providing a possible common pathway of SCZ with comorbid insomnia. Furthermore, we demonstrate the distinct activation patterns between the two diseases, most prominently including the increased activities of subcortical regions in SCZ, indicating the specific mechanisms of sleep disturbance in SCZ. In the future, more experiments are required to verify our conclusions and explore the potential value of these neurobiological patterns in the treatment of sleep problems in SCZ.
References
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97 (2016).
Ohayon, M. M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev. 6, 97–111 (2002).
Benson, K. L. Sleep in schizophrenia: impairments, correlates, and treatment. Psychiatr. Clin. North Am. 29, 1033–1045, abstract ix-x (2006).
Meyer, N. et al. Sleep and circadian rhythm disturbance in remitted schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr. Bull. 46, 1126–1143 (2020).
Robertson, I., Cheung, A. & Fan, X. Insomnia in patients with schizophrenia: current understanding and treatment options. Prog Neuropsychopharmacol. Biol. Psychiatry 92, 235–242 (2019).
Pocivavsek, A. & Rowland, L. M. Basic neuroscience illuminates causal relationship between sleep and memory: translating to schizophrenia. Schizophr. Bull. 44, 7–14 (2018).
Ruhrmann, S. et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch. Gen. Psychiatry 67, 241–251 (2010).
Bromundt, V. et al. Sleep-wake cycles and cognitive functioning in schizophrenia. Br. J. Psychiatry 198, 269–276 (2011).
Laskemoen, J. F. et al. Do sleep disturbances contribute to cognitive impairments in schizophrenia spectrum and bipolar disorders? Eur. Arch. Psychiatry Clin. Neurosci. 270, 749–759 (2020).
Mulligan, L. D., Haddock, G., Emsley, R., Neil, S. T. & Kyle, S. D. High resolution examination of the role of sleep disturbance in predicting functioning and psychotic symptoms in schizophrenia: a novel experience sampling study. J. Abnorm. Psychol. 125, 788–797 (2016).
Blanchard, J. J., Andrea, A., Orth, R. D., Savage, C. & Bennett, M. E. Sleep disturbance and sleep-related impairment in psychotic disorders are related to both positive and negative symptoms. Psychiatry Res. 286, 112857 (2020).
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26, R432–R443 (2016).
Freeman, D. et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry 2, 975–983 (2015).
Chiu, V. W. et al. Sleep profiles and CBT-I response in schizophrenia and related psychoses. Psychiatry Res. 268, 279–287 (2018).
Kaskie, R. E., Graziano, B. & Ferrarelli, F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat. Sci. Sleep 9, 227–239 (2017).
Monti, J. M. et al. Sleep and circadian rhythm dysregulation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 43, 209–216 (2013).
Korenic, S. A. et al. Sleep quality is related to brain glutamate and symptom severity in schizophrenia. J. Psychiatr. Res. 120, 14–20 (2020).
Cosgrave, J., Wulff, K. & Gehrman, P. Sleep, circadian rhythms, and schizophrenia: where we are and where we need to go. Curr. Opin. Psychiatry 31, 176–182 (2018).
Ferrarelli, F. Sleep abnormalities in schizophrenia: state of the art and next steps. Am. J. Psychiatry 178, 903–913 (2021).
Ferrarelli, F. & Tononi, G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr. Res. 180, 36–43 (2017).
Barkhof, F., Haller, S. & Rombouts, S. A. Resting-state functional MR imaging: a new window to the brain. Radiology 272, 29–49 (2014).
Fasiello, E. et al. Functional connectivity changes in insomnia disorder: a systematic review. Sleep Med. Rev. 61, 101569 (2021).
Mwansisya, T. E. et al. Task and resting-state fMRI studies in first-episode schizophrenia: a systematic review. Schizophr. Res. 189, 9–18 (2017).
Chen, L., Xia, C. & Sun, H. Recent advances of deep learning in psychiatric disorders. Precis. Clin. Med. 3, 202–213 (2020).
Margulies, D. S. et al. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. Magma 23, 289–307 (2010).
Lv, H. et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. Am J Neuroradiol. 39, 1390–1399 (2018).
Zheng, J. X. et al. Disrupted spontaneous neural activity related to cognitive impairment in postpartum women. Front. Psychol. 9, 624 (2018).
Yao, L. et al. A multimodal meta-analysis of regional structural and functional brain alterations in type 2 diabetes. Front. Neuroendocrinol. 62, 100915 (2021).
Iwabuchi, S. J. et al. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci. Biobehav. Rev. 51, 77–86 (2015).
Li, C. et al. Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 12, 1371–1378 (2016).
Zhou, F., Huang, S., Zhuang, Y., Gao, L. & Gong, H. Frequency-dependent changes in local intrinsic oscillations in chronic primary insomnia: a study of the amplitude of low-frequency fluctuations in the resting state. NeuroImage Clin. 15, 458–465 (2017).
Wang, T. et al. Regional homogeneity changes in patients with primary insomnia. Eur. Radiol. 26, 1292–1300 (2016).
Dai, X. J. et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 10, 2163–2175 (2014).
Kühn, S. & Gallinat, J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 39, 358–365 (2013).
Lui, S. et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol. Med. 45, 97–108 (2015).
Ren, W. et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am. J. Psychiatry 170, 1308–1316 (2013).
Saletin, J. M., Jackvony, S., Rodriguez, K. A. & Dickstein, D. P. A coordinate-based meta-analysis comparing brain activation between attention deficit hyperactivity disorder and total sleep deprivation. Sleep 42 (2019).
McGrath, L. M. & Stoodley, C. J. Are there shared neural correlates between dyslexia and ADHD? A meta-analysis of voxel-based morphometry studies. J. Neurodev. Disord. 11, 31 (2019).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Lancaster, J. L. et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205 (2007).
Turkeltaub, P. E. et al. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 33, 1–13 (2012).
Gao, X. et al. Association between structural and functional brain alterations in drug-free patients with schizophrenia: a multimodal meta-analysis. J. Psychiatry Neurosci. 43, 131–142 (2018).
Zhang, W. et al. Brain structural correlates of familial risk for mental illness: a meta-analysis of voxel-based morphometry studies in relatives of patients with psychotic or mood disorders. Neuropsychopharmacology 45, 1369–1379 (2020).
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F. & Fox, P. T. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361 (2012).
Eickhoff, S. B. et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85 (2016).
Ellison-Wright, I., Glahn, D. C., Laird, A. R., Thelen, S. M. & Bullmore, E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am. J. Psychiatry 165, 1015–1023 (2008).
Chan, R. C., Di, X., McAlonan, G. M. & Gong, Q. Y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull. 37, 177–188 (2011).
Li, T. et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr. Bull. 43, 436–448 (2017).
Gong, J. et al. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: a meta-analysis of resting-state functional MRI. J. Psychiatry Neurosci. 45, 55–68 (2020).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Kim, H., Ährlund-Richter, S., Wang, X., Deisseroth, K. & Carlén, M. Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218 (2016).
Duverne, S. & Koechlin, E. Rewards and cognitive control in the human prefrontal cortex. Cereb. Cortex 27, 5024–5039 (2017).
Burk, J. A., Blumenthal, S. A. & Maness, E. B. Neuropharmacology of attention. Eur. J. Pharmacol. 835, 162–168 (2018).
Yan, W. et al. Relationships between abnormal neural activities and cognitive impairments in patients with drug-naive first-episode schizophrenia. BMC Psychiatry 20, 283 (2020).
Shao, Z. et al. Dysfunction of the NAc-mPFC circuit in insomnia disorder. Neuroimage Clin. 28, 102474 (2020).
Muzur, A., Pace-Schott, E. F. & Hobson, J. A. The prefrontal cortex in sleep. Trends Cogn. Sci. 6, 475–481 (2002).
Mander, B. A. et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 16, 357–364 (2013).
Hoptman, M. J. et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 117, 13–20 (2010).
Yu, R. et al. Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS ONE 8, e57516 (2013).
Yu, R. et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum. Brain Mapp. 35, 627–637 (2014).
Turner, J. A. et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front. Neurosci. 7, 137 (2013).
Zhang, S. et al. Effects of transcutaneous auricular vagus nerve stimulation on brain functional connectivity of medial prefrontal cortex in patients with primary insomnia. Anat. Rec. 304, 2426–2435 (2021).
Horovitz, S. G. et al. Decoupling of the brain’s default mode network during deep sleep. Proc. Natl Acad. Sci. USA 106, 11376–11381 (2009).
Tagliazucchi, E. & Laufs, H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 82, 695–708 (2014).
Khazaie, H. et al. Functional reorganization in obstructive sleep apnoea and insomnia: a systematic review of the resting-state fMRI. Neurosci. Biobehav. Rev. 77, 219–231 (2017).
Hu, M. L. et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 33, 73–84 (2017).
Harrison, B. J. et al. Dynamic subcortical modulators of human default mode network function. Cereb. Cortex bhab487 (2021).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10, 1100 (2019).
Shen, M. et al. FXR1 regulation of parvalbumin interneurons in the prefrontal cortex is critical for schizophrenia-like behaviors. Mol. Psychiatry 26, 6845–6867 (2021).
Jansen, P. R. et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 51, 394–403 (2019).
Jiang, B., He, D., Guo, Z. & Gao, Z. Effect-size seed-based d mapping of resting-state fMRI for persistent insomnia disorder. Sleep Breath 24, 653–659 (2020).
McTeague, L. M. et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 177, 411–421 (2020).
Crespo-Facorro, B., Barbadillo, L., Pelayo-Terán, J. M. & Rodríguez-Sánchez, J. M. Neuropsychological functioning and brain structure in schizophrenia. Int. Rev. Psychiatry 19, 325–336 (2007).
Aminoff, E. M., Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17, 379–390 (2013).
Epstein, R. A., Patai, E. Z., Julian, J. B. & Spiers, H. J. The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci. 20, 1504–1513 (2017).
Hasselmo, M. E. & Stern, C. E. Mechanisms underlying working memory for novel information. Trends Cogn. Sci. 10, 487–493 (2006).
Ragland, J. D. et al. Prefrontal activation deficits during episodic memory in schizophrenia. Am. J. Psychiatry 166, 863–874 (2009).
Thermenos, H. W. et al. Hyperactivity of caudate, parahippocampal, and prefrontal regions during working memory in never-medicated persons at clinical high-risk for psychosis. Schizophr. Res. 173, 1–12 (2016).
Li, Y. et al. Abnormal neural network of primary insomnia: evidence from spatial working memory task fMRI. Eur. Neurol. 75, 48–57 (2016).
Bubb, E. J., Metzler-Baddeley, C. & Aggleton, J. P. The cingulum bundle: anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 92, 104–127 (2018).
Marques, D. R., Gomes, A. A., Caetano, G. & Castelo-Branco, M. Insomnia disorder and brain’s default-mode network. Curr. Neurol. Neurosci. Rep. 18, 45 (2018).
Whitfield-Gabrieli, S. & Ford, J. M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8, 49–76 (2012).
Xu, Y., Zhuo, C., Qin, W., Zhu, J. & Yu, C. Altered spontaneous brain activity in schizophrenia: a meta-analysis and a large-sample study. Biomed. Res. Int. 2015, 204628 (2015).
Pessoa, L. A network model of the emotional brain. Trends Cogn. Sci. 21, 357–371 (2017).
Jung, W. H. et al. Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state FMRI. PLoS ONE 9, e106768 (2014).
Brooks, D. J. et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET. Brain 113, 1539–1552 (1990).
Looi, J. C. & Walterfang, M. Striatal morphology as a biomarker in neurodegenerative disease. Mol Psychiatry 18, 417–424 (2013).
Larabi, D. I., van der Meer, L., Pijnenborg, G. H. M., určić-Blake, B. & Aleman, A. Insight and emotion regulation in schizophrenia: a brain activation and functional connectivity study. Neuroimage Clin. 20, 762–771 (2018).
Lui, S. et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry 67, 783–792 (2010).
Hu, M. L. et al. Short-term Effects of Risperidone Monotherapy on Spontaneous Brain Activity in First-episode Treatment-naïve Schizophrenia Patients: A Longitudinal fMRI Study. Sci. Rep. 6, 34287 (2016).
Kesby, J. P., Eyles, D. W., McGrath, J. J. & Scott, J. G. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl. Psychiatry 8, 30 (2018).
Chouchou, F. et al. How the insula speaks to the heart: cardiac responses to insular stimulation in humans. Hum. Brain Mapp. 40, 2611–2622 (2019).
Monti, J. M., Torterolo, P., Pandi & Perumal, S. R. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med. Rev. 33, 51–57 (2017).
Atkin, T., Comai, S. & Gobbi, G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol. Rev. 70, 197–245 (2018).
He, Z. et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol. Med. 43, 769–780 (2013).
Huang, X. Q. et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. NeuroImage. 49, 2901–2906 (2010).
Liu, H. et al. Cigarette smoking and schizophrenia independently and reversibly altered intrinsic brain activity. Brain Imaging Behav. 12, 1457–1465 (2018).
Kanahara, N. et al. Orbitofrontal cortex abnormality and deficit schizophrenia. Schizophr. Res. 143, 246–252 (2013).
Vanes, L. D. et al. Neural correlates of positive and negative symptoms through the illness course: an fMRI study in early psychosis and chronic schizophrenia. Sci. Rep. 9, 14444 (2019).
DelRosso, L. M. & Hoque, R. The cerebellum and sleep. Neurol. Clin. 32, 893–900 (2014).
Dai, X. J. et al. Plasticity and susceptibility of brain morphometry alterations to insufficient sleep. Front. Psychiatry. 9, 266 (2018).
Shao, Y. et al. Spindle-related brain activation in patients with insomnia disorder: an EEG-fMRI study. Brain Imaging Behav. 16, 659–670 (2022).
Qiu, X. et al. Regional homogeneity brain alterations in schizophrenia: an activation likelihood estimation meta-analysis. Psychiatry Investig 18, 709–717 (2021).
Dai, X. J. et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J. Clin. Sleep Med. 12, 363–374 (2016).
Liu, C. H. et al. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 1648, 317–324 (2016).
Ran, Q. et al. Abnormal amplitude of low-frequency fluctuations associated with rapid-eye movement in chronic primary insomnia patients. Oncotarget 8, 84877–84888 (2017).
Wang, Y. K. et al. Evaluation of the age-related and gender-related differences in patients with primary insomnia by fractional amplitude of low-frequency fluctuation: a resting-state functional magnetic resonance imaging study. Medicine 99, e18786 (2020).
Zhao, B. et al. The instant spontaneous neuronal activity modulation of transcutaneous auricular vagus nerve stimulation on patients with primary insomnia. Front. Neurosci. 14, 205 (2020).
Pang, R. et al. Altered regional homogeneity in chronic insomnia disorder with or without cognitive impairment. Am. J. Neuroradiol. 39, 742–747 (2018).
Zhang, Y. et al. Dysfunctional beliefs and attitudes about sleep are associated with regional homogeneity of left inferior occidental gyrus in primary insomnia patients: a preliminary resting state functional magnetic resonance imaging study. Sleep Med. 81, 188–193 (2021).
Alonso-Solis, A. et al. Altered amplitude of low frequency fluctuations in schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 189, 97–103 (2017).
Bai, Y. et al. Altered resting-state regional homogeneity after 13 weeks of paliperidone injection treatment in schizophrenia patients. Psychiatry Res. Neuroimaging 258, 37–43 (2016).
Cui, L. B. et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr. Res. 173, 13–22 (2016).
Fryer, S. L. et al. Reduced amplitude of low-frequency brain oscillations in the psychosis risk syndrome and early illness schizophrenia. Neuropsychopharmacology 41, 2388–2398 (2016).
Gao, B. et al. Spontaneous activity associated with delusions of schizophrenia in the left medial superior frontal gyrus: a resting-state fMRI study. PLoS ONE 10, e0133766 (2015).
Gao, S. et al. Distinguishing between treatment-resistant and non-treatment-resistant schizophrenia using regional homogeneity. Front. Psychiatry 9, 282 (2018).
Gao, S. et al. Enhanced prefrontal regional homogeneity and its correlations with cognitive dysfunction/psychopathology in patients with first-diagnosed and drug-naive schizophrenia. Front. Psychiatry. 11, 580570 (2020).
Gou, N. et al. Effects of DISC1 polymorphisms on resting-state spontaneous neuronal activity in the early-stage of schizophrenia. Front. Psychiatry 9, 137 (2018).
Jin, K. et al. Distinguishing hypochondriasis and schizophrenia using regional homogeneity: a resting-state fMRI study and support vector machine analysis. Acta Neuropsychiatrica 33, 182–190 (2021).
Lei, W. et al. Sex-specific patterns of aberrant brain function in first-episode treatment-naive patients with schizophrenia. Int. J. Mol. Sci. 16, 16125–16143 (2015).
Li, F. et al. Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology 279, 867–875 (2016).
Li, Z. et al. Aberrant spontaneous neural activity and correlation with evoked-brain potentials in first-episode, treatment-naïve patients with deficit and non-deficit schizophrenia. Psychiatry Res. Neuroimaging 261, 9–19 (2017).
Lian, N. et al. A comparative study of magnetic resonance imaging on the gray matter and resting-state function in prodromal and first-episode schizophrenia. Am. J. Med. Genet. Part B, Neuropsychiatric Genet. 177, 537–545 (2018).
Liu, C. et al. Abnormally increased and incoherent resting-state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophr. Res. 171, 158–165 (2016).
Liu, H. et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport 17, 19–22 (2006).
Liu, Y. et al. Abnormal neural activity as a potential biomarker for drug-naive first-episode adolescent-onset schizophrenia with coherence regional homogeneity and support vector machine analyses. Schizophr. Res. 192, 408–415 (2018).
Salvador, R. et al. Non redundant functional brain connectivity in schizophrenia. Brain Imaging Behav. 11, 552–564 (2017).
Shan, X. et al. Increased regional homogeneity modulated by metacognitive training predicts therapeutic efficacy in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 271, 783–798 (2021).
Tang, Y. et al. Altered functional connectivity and low-frequency signal fluctuations in early psychosis and genetic high risk. Schizophr. Res. 210, 172–179 (2019).
Wang, P. et al. Amplitude of low-frequency fluctuation (ALFF) may be associated with cognitive impairment in schizophrenia: a correlation study. BMC Psychiatry 19, 30 (2019).
Wu, R. et al. Reduced brain activity in the right Putamen as an early predictor for treatment response in drug-naive, first-episode schizophrenia. Front. Psychiatry 10, 741 (2019).
Xie, Y. et al. rTMS induces brain functional and structural alternations in schizophrenia patient with auditory verbal hallucination. Front. Neurosci. 15, 722894 (2021).
Yang, F. et al. Correlation of abnormalities in resting state fMRI with executive functioning in chronic schizophrenia. Psychiatry Res. 299, 113862 (2021).
Yang, Y. et al. Common and specific functional activity features in schizophrenia, major depressive disorder, and bipolar disorder. Front. Psychiatry 10, 52 (2019).
Zhao, X. et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging Behav. 13, 503–513 (2019).
Zheng, J. et al. Disrupted amplitude of low-frequency fluctuations in antipsychotic-naïve adolescents with early-onset schizophrenia. Psychiatry Res. Neuroimaging 249, 20–26 (2016).
Zhou, C. et al. Altered patterns of the fractional amplitude of low-frequency fluctuation and functional connectivity between deficit and non-deficit schizophrenia. Front. Psychiatry. 10, 680 (2019).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project Nos. 82120108014, 82071908, 81901705, 81621003, and 81761128023), Chinese Academy of Medical Sciences (Project No. 2021-I2M-C&T-A-022), Ministry of Science and Technology of the People’ s Republic of China (2021ZD0201900), Sichuan Science and Technology Program (Project No. 2021JDTD0002 and 2020YFS0116), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 and ZYJC18020). Dr. Su Lui acknowledges the support from Humboldt Foundation Friedrich Wilhelm Bessel Research Award and Chang Jiang Scholars (Program No. T2019069).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Z., Xiao, Y., Zhang, Y. et al. Comparisons of resting-state brain activity between insomnia and schizophrenia: a coordinate-based meta-analysis. Schizophr 8, 80 (2022). https://doi.org/10.1038/s41537-022-00291-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00291-3