Abstract
We investigated differences in brain activity in response to sleep-related pictures between chronic insomnia disorder (CID) patients and good sleepers (GS), and examined whether brain activity moderated the relationship between depressive symptoms and sleep disturbance in CID patients and GS. This study included 43 patients diagnosed with CID, based on the International Classification of Sleep Disorders-3, and 42 GS. The participants kept a sleep diary, underwent nocturnal polysomnography to measure sleep parameters, and completed self-report questionnaires to assess sleep and psychiatric symptoms. They underwent functional magnetic resonance imaging (fMRI) to examine differences in brain activity in response to sleep-related pictures compared to neutral pictures. A moderated moderation analysis was performed to investigate the moderating role of brain responses to sleep-related pictures in the association between depressive symptoms and sleep disturbance. Compared to GS, the brain responses to sleep-related stimuli were significantly lower in CID patients in the right lateral prefrontal cortex (LPFC) and dorsomedial prefrontal cortex (DMPFC). More severe depressive symptoms were significantly associated with longer sleep latency only when LPFC activity was low in CID patients, but not in GS. LPFC hypoactivity in response to sleep-related stimuli in CID patients could moderate the relationship between depression and sleep disturbance.
Similar content being viewed by others
Introduction
Insomnia is a common symptom and important risk factor for depression1. The relationship between insomnia and depression is bidirectional, and they have both been linked to structural and functional changes in the frontal cortex2,3. A large cohort study of 1,053 major depressive disorder (MDD) patients demonstrated that patients with more severe insomnia had smaller cortical surface areas in several frontal regions4. A recent neuroimaging review reported similar prefrontal abnormalities in MDD and insomnia5. However, structural and functional impairments in depressive disorder and insomnia disorder were inconsistent in two different neuroimaging meta-analyses6,7.
Espie et al.8 demonstrated that attentional bias toward external stimuli contributed to chronic insomnia through the attention-intention-effort model (AIE). Sleep-related attentional bias is defined as the propensity to selectively focus on sleep-related stimuli rather than neutral information. Selective attention to sleep-related stimuli inhibits sleep automaticity in individuals with hyperarousal insomnia, which leads to chronic insomnia due to sleep-related maladaptive behavior9. Hyperarousal, or overactivation of the arousal system, is a feature of both insomnia and depression10,11. Liu et al.12 reported an increase in the resting state amplitude of low-frequency fluctuation (ALFF) in the inferior frontal gyrus in relation to hyperarousal insomnia in MDD patients. Impairment of the salience network in depressive disorders has been described previously13,14. The amygdala, an important structure of the salience network, becomes hyper-responsive to negative stimuli in depressive patients, which leads to emotional dysregulation4,15. Therefore, it is necessary to explore the shared brain networks involved in the hyperarousal system and attentional bias, and their roles in the pathogenesis of insomnia and depression.
A few studies on sleep-related stimuli in insomnia patients have used functional magnetic resonance imaging (fMRI) to explore attentional bias. Moreover, the results have been inconsistent and none of these studies included depressive patients. Patients with psychophysiological insomnia exhibited greater activation of the precentral and prefrontal cortices in response to sleep-related pictures compared to good sleepers (GS) and the increased responses were reduced after cognitive-behavioral therapy16. This study supported a hyperarousal model of insomnia. Another study of 14 insomnia patients demonstrated decreased regional activity in the left middle temporal and left middle occipital gyri in response to sleep-related sounds following cognitive behavioral treatment for insomnia17. Spiegelhalder et al.18 suggested the use of sleep-related pictorial stimuli because the brain activity in insomnia patients, assessed using the emotional Stroop test, did not differ from that in controls. Brain imaging studies of patients with insomnia have examined dysfunction of the limbic system as well as the prefrontal region. Baglioni et al.19 demonstrated increased activity of the amygdala in response to insomnia-related images in insomnia patients. Huang et al.20 reported decreased functional connectivity between the amygdala and insula, striatum, and thalamus in insomnia patients in comparison to controls in the resting state. A resting state fMRI study of healthy adults with insomnia showed that sleep disturbance was associated with altered activity in the thalamus of the hyperarousal network21.
Previous fMRI studies using sleep-related stimuli had several limitations. None of the studies investigated the role of attentional bias, which is related to the hyperarousal system, in the relationship between depressive and insomnia symptoms. In addition, the number of patients was small and other sleep disorders, including obstructive sleep apnea and periodic limb movement disorder, were not excluded through polysomnography (PSG).
The present study aimed to investigate differences in brain activity in response to sleep-related pictures between patients with chronic insomnia disorder (CID) and GS, and to determine whether brain activity in specific regions moderated the relationship between depression and sleep disturbance. We tested the following two hypotheses. First, CID patients would show different activation in response to sleep-related pictures in insomnia-related regions compared to GS, especially in the frontal cortex and limbic structures. Second, we hypothesized that insomnia would be more severe in the presence of severe depressive symptoms and abnormal brain function.
Results
Demographic, clinical, and sleep variables
Table 1 summarizes the clinical and sleep variables of all participants. There were no significant differences in age (P = 0.067) or sex (P = 0.208) between CID patients and GS. However, CID patients had higher Pittsburgh Sleep Quality Index (PSQI), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), and Dysfunctional Beliefs and Attitudes about Sleep Scale-16 (DBAS-16) scores compared to GS (P < 0.05 for all). The range of BDI scores was 1–25 in the CID patients and 0–13 in GS. The median BDI value was 6 in both groups. The nocturnal PSG results showed that CID patients had a lower total sleep time (P = 0.014) and sleep efficiency (P = 0.007), and a longer wake time after sleep onset (WASO) (P = 0.011) compared to GS. The total sleep time and sleep efficiency, derived from the sleep diary, were significantly lower in CID patients than GS. The sleep latency was significantly higher (P < 0.001) and the WASO was longer in CID patients compared to GS, although these differences were not statistically significant.
Brain activation in response to sleep-related pictures
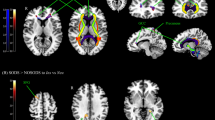
Brain activation in response to sleep-related pictures was significantly lower in CID patients than in GS in prefrontal regions (Table 2, Fig. 1) (false discovery rate [FDR] corrected P < 0.05), including the right lateral prefrontal cortex (LPFC), dorsomedial prefrontal cortex (DMPFC), and anterior cingulate cortex (ACC). There were no regions with significantly greater activation in CID patients compared to GS in response to sleep-related pictures. CID showed shorter sleep duration (i.e., total sleep time measured by PSG and sleep diary) than GS (see Table 1). Analyses of covariance were conducted to examine whether sleep duration (i.e., total sleep time) measured by PSG and sleep diary affected group differences in prefrontal response to sleep-related pictures. After controlling for sleep duration, most findings of the group differences remained significant (P < 0.01). However, the group difference in DMPFC response to sleep pictures was marginally significant (P = 0.07) after controlling for total sleep time measured by sleep diary.
Brain areas showing less activation in response to sleep-related pictures in the insomnia group. Brain regions that showed more activation in good sleepers than insomnia patients in response to sleep-related pictures. Higher levels of brain activation (red and yellow) were observed in the right inferior frontal (a) and superior medial frontal (b) gyri in the good sleepers (FDR correction; p < 0.05).
Moderation analysis
Parameter estimates were extracted from clusters (right LPFC, DMPFC, and ACC) that showed significant differences between groups.
Group (CID vs. GS) moderated the interaction between BDI score and right LPFC activity as predictors of sleep disturbance (Table 3). The moderating role of brain activity response to sleep-related pictures in the relationship between depression and sleep disturbance was significant in CID patients but not in GS. There was a significant, positive correlation between BDI and sleep latency determined from the sleep diary in CID patients with low-level right LPFC activity, but not in CID patients with high-level right LPFC activity (Fig. 2). No such significant correlations were found in GS. An analysis of DMPFC and ACC activity as moderators yielded no significant results.
Additional analyses
ROI-based analysis for group differences in the amygdala and anterior insula
The whole brain t test did not show any between-group differences in limbic structure activity, including the amygdala and anterior insula, in response to sleep-related pictures. Given that the amygdala and anterior insula are relatively small regions, FDR correction for multiple comparisons could hinder the identification of activity in these regions. Therefore, we performed a region of interest (ROI)-based analysis using a priori-defined regions of the amygdala and anterior insula. The ROIs were defined using automated anatomical labeling. Mean parameter estimates from each ROI were extracted and tested for between-group differences using a two-sample t test. The results showed no significant between-group differences in amygdala or anterior insula responses to sleep-related pictures vs. neutral pictures between CID patients and GS.
Moderation analysis using PSG data
Additional moderation analyses using PSG data were conducted to examine whether there were significant moderation effects of brain activity between BDI scores and sleep parameters collected from PSG in each CID patients and GS. The analyses did not find significant moderation effects.
Discussion
In the present study, brain activation in response to sleep-related pictures was significantly lower in prefrontal areas, including the right LPFC, DMPFC, and ACC, in CID patients than in GS. There was a positive association between depressive symptoms and sleep latency determined from the sleep diary only when right LPFC activity was low in CID patients. The association between depressive symptoms and sleep latency was not observed in CID patients with high LPFC activity or in GS regardless of brain activity. Right LPFC hypoactivity in response to sleep-related stimuli moderated the relationship between depressive symptoms and sleep disturbance in CID patients.
Previous studies demonstrated decreased prefrontal cortex (PFC) activity in insomnia patients during cognitive tasks. Insomnia patients showed hypoactivation of medial and inferior prefrontal cortical areas compared to controls during the performance of category and letter fluency tasks22. Drummond et al.23 reported lower right dorsolateral PFC activation in insomnia patients with increasing task difficulty. Prefrontal regions contribute to executive functions, including attention, problem solving, and planning24. Reduced PFC activation in insomnia patients has been associated with cognitive disinhibition, such as rumination and intrusive thoughts25. Our study used sleep-related pictures, which are thought to have relatively low cognitive demand, but CID patients still showed low PFC activation. The low PFC activation in response to sleep-related stimuli in CID patients represents abnormal brain function and can be considered a neurobiological marker for insomnia.
It is possible that lower PFC activation in CID patients may be related to sleep loss, which often reported in CID patients. Previous studies have shown that sleep deprivation itself (sleep loss) is associated with frontal hypoactivation during performing tasks. A meta-analysis of 11 sleep deprivation neuroimaging showed that the activity of the frontoparietal attention network including the prefrontal cortex and intraparietal sulcus significantly decreased during different attention tasks after sleep deprivation26. Sleep deprivation was also related to reduced activity of the frontoparietal network and the intrusion of the activity in the default mode network during working memory task27. Given that our CID patients showed short sleep duration, we conducted additional analyses to examine the effect of sleep loss in prefrontal differences between CID and GS. As we reported above, most findings remained significant or marginally significant after controlling for sleep loss.
Insomnia patients often complain of depressive symptoms, and insomnia and depression are known to have a bidirectional relationship1. These neurobiological markers in CID patients may moderate the relationship between depressive symptoms and insomnia. The results of moderated moderation analyses showed that low LPFC activity played a regulatory role in the association between depressive symptoms and sleep disturbance in CID patients in the present study. Our findings suggest that severe depressive symptoms and lower PFC activity are associated with longer subjective sleep latency. Previous studies have demonstrated that reduced cognitive function is characteristic of patients with depressive disorders, which is reflected in LPFC hypoactivation28. When depressive symptoms are severe, cognitive function declines and attentional bias may occur in relation to symptoms of insomnia. Our findings highlight the importance of LPFC function in the relationship between depressive symptoms and sleep disturbance in CID patients, suggesting that improvements in PFC function and the alleviation of depression may be important in the treatment of insomnia.
Neuroimaging studies have demonstrated PFC impairment in top-down modulation of emotional processing when sleep is disrupted29. When viewing negative emotional stimuli, a sleep deprivation group exhibited greater activation of the amygdala compared to the control group, while increased limbic activity was associated with decreased functional connectivity with the medial PFC30. Motomura et al.31 reported that sleep debt reduced functional connectivity between the amygdala and ventromedial PFC in an fMRI study using fearful face stimuli. Furthermore, Banks et al.32 reported that coupling of the medial PFC and amygdala moderated emotion regulation in reappraisal tasks. An analysis of appraisal processes using fMRI revealed that the right ventrolateral PFC was associated with emotion generation and regulation33. The right LPFC, which is a key node in the salience network, significantly influenced the insomnia symptoms associated with MDD12.
Hyperarousal is the main pathophysiology of insomnia, although no significant hyperactivation of key elements of the salience network, such as the amygdala and anterior insula, was found in our insomnia group. Additional ROI analysis of amygdala and anterior insula activity showed no significant differences between the CID patients and GS, which would have been because the degree of activation in these regions was very small in response to sleep-related pictures. In this study, activation in response to sleep-related pictures was significantly lower in CID patients than GS in the right LPFC, DMPFC, and ACC. In contrast, a previous fMRI study demonstrated higher activation of the prefrontal and precentral regions in response to sleep-related pictures in 14 psychophysiological insomnia patients compared to GS16. These discrepancies were probably due to differences in participant characteristics. Kim et al.16 reported no significant differences in BDI scores between insomnia and GS groups, whereas the difference in our study was significant. Insomnia patients with more severe depressive symptoms, demonstrated by higher BDI scores, were enrolled in this study. In addition, the study population was larger and we used a diagnosis of chronic insomnia based on the International Classification of Sleep Disorders-Third Edition (ICSD-3) rather than psychophysiological insomnia based on the ICSD-2.
The anxiety accompanying insomnia could be a considerable clinical factor related to rumination and worry34. We found a significant difference in BAI scores between CID patients and GS. However, moderation analysis of BAI scores as an independent variable showed that prefrontal brain activity did not significantly moderate the relationship between anxiety and sleep disturbance in either group. This result was not consistent with moderation analysis results using BDI scores. One possible reason is that depression may be more manifest than anxiety in insomnia patients. In support of this possibility, in several epidemiological studies, insomnia disorder was more strongly associated with depressive disorder than with anxiety disorder. For example, insomnia had stronger predictive power in relation to future depression (OR = 3.51) compared to future anxiety in a general population prospective study35. In addition, an epidemiological study of adolescents showed that prior insomnia was associated with the onset of depressive disorder, but no significant association was observed with the onset of anxiety disorder36.
Meanwhile, when further analysis was performed using PSG data, which is the objective sleep parameter, a significant moderation effect of brain activity between sleep disturbance and depression was not observed. It is because one-night polysomnography data may be insufficient to reflect usual sleep disturbance in insomnia patients37. Furthermore, these finding could be due to the first night effect of the participants characterized by decreased total sleep time and lower sleep efficiencies on the first night of PSG38.
The present study has several limitations. First, the severity of depressive symptoms was evaluated subjectively, and detailed evaluations of the duration, recurrence, and age at onset of depression were insufficient. A comprehensive mood evaluation should be performed in future studies. Second, although non-emotional sleep-related images were used, they could still cause distress to insomnia patients. Emotional and arousal levels should be controlled for more accurate assessment of between-group differences in future studies. Third, we excluded patients with MDD and negative results may be observed in further analyses performed in the limbic region. Our study primarily focused on the neural characteristics of attentional bias to sleep-related stimuli in CID patients. Including subjects with both CID and MDD may be difficult with regard to controlling for the effects of depressive symptoms on neural responses to sleep-related stimuli. Despite the relatively low range (mostly mild levels) of depressive symptoms in CID, we examined how the neural response to sleep-related stimuli moderated the relationship between depressive symptoms and sleep disturbance in patients with chronic insomnia. In addition, to overcome the validity issue that arises because the BDI score contains an insomnia item, we also analyzed the BDI score with exclusion of the 16th sub-item related to insomnia, and the results showed the same significant moderation effect.
This is the first fMRI study to explore the roles of possible mediating factors in the relationship between sleep disturbance and depressive symptoms. Our findings suggest that prefrontal hypoactivity in insomnia patients moderates the association between depressive symptoms and sleep disturbance in the presence of sleep-related stimuli. These results could improve our understanding of the shared neurobiological mechanisms underlying depression and insomnia.
Methods
Participants
Initially, 46 CID patients and 45 GS (27 women; mean age = 39.3 ± 11.9 years) were enrolled. The period of recruitment and data collection were from May 11, 2014, to July 14, 2020. Patients aged 18–65 years who met ICSD-3 criteria for CID were recruited from an outpatient clinic at the Department of Psychiatry and from the Center for Sleep and Chronobiology at Seoul National University Hospital39. Moreover, 23 GS were recruited from the advertisement. The exclusion criteria were as follows: history of serious neurological or medical illness; current neurological or medical illness; other major psychiatric disorders, including cognitive impairments and personality disorders, as defined by the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV); sleep disorders other than CID, such as obstructive sleep apnea (i.e., apnea–hypopnea index [AHI] ≥ 15) and periodic limb movement disorder (i.e., periodic limb movement index ≥ 15), based on ICSD-3 criteria; shiftwork; contraindications to MRI; and pregnancy. The study protocol was approved by the Ethics Committee of Seoul National University Hospital, in accordance with the Declaration of Helsinki. A complete description of the study protocol was provided to all participants and written informed consent was obtained.
The participants were screened for psychiatric disorders by trained psychologists using the Structural and Clinical Interviews for DSM-IV axis I disorders40. Therefore, participants who met the diagnostic criteria for MDD through a clinical interview were excluded. Participants were instructed to stop taking any medications that could influence sleep or circadian rhythms, such as sedatives, hypnotics, and antidepressants. Individuals on these medications could participate in the study after a drug washout period of at least 5 days. Among the 46 CID patients, 3 were excluded due to periodic limb movement disorders on nocturnal PSG, while 3 of the 45 GS were excluded due to obstructive sleep apnea (n = 2) or periodic limb movement disorder (n = 1). Finally, 43 CID patients (33 women; mean age = 44.6 ± 13.9 years) and 42 GS (27 women; mean age = 39.3 ± 11.9 years) were included in the analysis. Two patients in the CID group and none in the GS group were taking zolpidem at the time of recruitment. Participants taking zolpidem discontinued before the study after consulting with a psychiatrist. None of the subjects were taking antidepressants, sedatives, or hypnotics.
Nocturnal polysomnography
At the Center for Sleep and Chronobiology of Seoul National University Hospital, overnight PSG (Profusion3; Compumedics, Charlotte, NC, USA) data, which consisted of EEG (with electrodes at F3, F4, C3, C4, O1, and O2, using A1 and A2 as reference sites), bilateral electrooculography (EOG), single-lead electrocardiography (ECG), submentalis and bilateral anterior tibialis electromyography (EMG), airflow measurements via nasal pressure transducers and oronasal thermal sensors, respiratory inductance plethysmography to monitor chest and abdominal movements, and finger pulse oximetry, were analyzed. PSG was scored by experienced technicians and physicians in accordance with the American Academy of Sleep Medicine (AASM) recommendations. The AHI, periodic limb movement index, time in bed, total sleep time, sleep latency, sleep efficiency, WASO, REM latency, and sleep percentage were recorded in each stage.
Clinical assessment
All participants completed self-report questionnaires, including the Epworth Sleepiness Scale (ESS), PSQI, BDI, BAI, and DBAS-16. The ESS, which is widely used to measure sleep propensity, was applied to assess daytime sleepiness41. The PSQI is a self-report questionnaire that measures overall quality of sleep42. The BDI and BAI were used to measure the severity of depressive symptoms and anxiety, respectively43,44. The DBAS-16 is a self-report questionnaire on sleep-disruptive cognition, such as faulty appraisals, unrealistic expectations, and perceptual bias regarding sleep45. All participants were instructed to keep a 1-week sleep diary, which included time in bed, total sleep time, sleep latency, sleep efficiency, and WASO. The average of the 1-week sleep diary written by the subject every day was used for the analysis.
fMRI experimental procedure
The fMRI experiment had a block design, and consisted of randomly intermixed sleep and neutral blocks. We selected 28 sleep-related and 28 neutral pictures from the Internet as stimuli. The pictures were presented to 25 insomnia patients for validation, and classified as “sleep-related” if they were identified as such by more than 80% of the patients. The insomnia patients who participated in the validation study, which was conducted separately, were not included in this study. Both the sleep-related and neutral pictures were designed to not trigger or depict any specific emotions. There were no emotional or facial expressions in the sleep-related and neutral pictures, and they were matched for size and brightness. This validation process was described previously by Kim et al.16 Each block began with fixation for 1 s followed by a 12-s picture presentation, 4-s dot sign, and 2-s sleep rating. In each block, four sleep-related or neutral pictures were presented for 3 s each. After the picture presentation phase (12 s in total; 4 pictures × 3 s), a dot was presented for 4 s followed by the rating scale for 2 s. Each block was followed by a crosshair, presented for 12–20 s, as a baseline. During presentation of the response cue, the participants were asked to judge whether or not the presented pictures were sleep-related. The participants were instructed to press the left button with their index finger for sleep-related pictures, and to press the right button with their middle finger if the pictures were not sleep-related.
MRI data acquisition
A 3-Tesla whole-body Siemens scanner (TrioTim Syngo; Siemens, Erlangen, Germany) with a 12-channel birdcage head coil was used for functional image acquisition with an interleaved T2-weighted echo-planar imaging gradient echo sequence (repetition time = 3,000 ms, echo time = 30 ms, flip angle = 90°; slice thickness = 3.0 mm; in-plane resolution = 3.4 × 3.4 mm; field of view = 220 mm; matrix size = 64 × 64). The experiment was performed in an fMRI room on an 8-inch screen, and the stimuli were presented using DMDX software. For each participant, 174 functional volumes were acquired. Anatomical images were also acquired using a T1-weighted 3D gradient echo pulse sequence with magnetization-prepared rapid gradient echo (repetition time = 1,670 ms, echo time = 1.89 ms, flip angle = 9°, slice thickness = 1.0 mm, in-plane resolution = 1 × 1 mm, field of view = 250 mm, matrix size = 256 × 256). The method of MRI data acquisition process was cited from Kim et al. study16.
fMRI preprocessing and analysis
The fMRI data were analyzed using SPM12 (Wellcome Department of Cognitive Neurology, London, UK). The first six volumes from all runs were discarded to prevent the non-equilibrium effect. The fMRI volumes were slice-time corrected. To correct for inter-scan rigid body motion, the volumes were realigned to the first image in the time series. After realignment, the volumes images were co-registered to the T1-weighted anatomical images and normalized to Montreal Neurological Institute space using a transformation matrix derived from T1 anatomical image segmentation. After normalization, the volumes were spatially smoothed using a 6-mm full width at half maximum isotropic Gaussian kernel, to compensate for residual inter-participant variability and increase statistical sensitivity. The resulting fMRI time series was high-pass filtered using a cut-off time of 128 s to remove low-frequency drifts. An artifact detection tool (http://www.nitrc.org/projects/artifact_detect/) was used to identify outlier volumes. Volumes with significant head motion (> 2 mm composite motion or large global mean intensity (i.e., a large difference in global mean intensities across functional volumes > 3 SD) were identified for each participant. The outlier volumes did not exceed 20% of the total volume in any participant, so no volumes were excluded from the final analysis.
A voxel-based general linear model was used to estimate parameters, including six motion parameters. Outliers were treated as covariates of no interest. Two regressors were used to model the sleep-related and neutral stimuli. The rating and fixation were also modeled to reduce the residuals. The design matrix was temporally convolved using a canonical hemodynamic response function to improve fit. Finally, contrast images associated with the conditions of interest [sleep-related or neutral stimuli] were created. The above method description of fMRI data preprocessing was cited from Kim et al. study16.
To evaluate differences in brain responses to sleep-related pictures between CID patients and GS, the contrast images [sleep-related pictures vs. neutral pictures] were analyzed using a whole-brain two-sample t-test. The significance level was set at p < 0.05, and was corrected for multiple comparisons using the false discovery rate (FDR). We extracted parameter estimates from brain regions showing differences in responses to sleep-related pictures (vs. neutral pictures) between the two groups.
Statistical analysis
Continuous and categorical variables were analyzed using the t test and chi-square test, respectively, performed with SPSS Statistics software (version 24.0; IBM Corp., Armonk, NY, USA).
A moderated moderation analysis was performed to assess the moderating role of brain responses to sleep-related pictures in the association between depressive symptoms and sleep disturbance in CID patients and GS, using the PROCESS macro (v3.0) for SPSS46. The responses to sleep-related pictures and groups (CID vs. GS) were used as continuous and categorical moderators, respectively (Fig. 3). Depressive symptoms (BDI score) and sleep disturbance (sleep latency from the sleep diary) were included as independent and dependent variables, respectively. In between-group comparisons, behavioral and neuroimaging data were analyzed by adjusting for age, which could affect sleep disturbance. Conditional process analysis was performed using bootstrapping resampling (10,000 samples) to estimate 95% confidence intervals. Significant interactions were further tested using the Johnson–Neyman procedure implemented in PROCESS.
Moderated moderation model. A moderated moderation analysis was performed using the PROCESS macro (v3.0) for SPSS. In the model, group was entered as a moderator (Z) of the moderating effect of brain activity in response to sleep-related stimuli (W) on the association between sleep disturbances (dependent variable, Y) and depression (independent variable, X).
Data availability
All data generated or analysed during this study are included in this published article.
References
Baglioni, C. et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 135, 10–19. https://doi.org/10.1016/j.jad.2011.01.011 (2011).
Altena, E., Vrenken, H., Van Der Werf, Y. D., van den Heuvel, O. A. & Van Someren, E. J. Reduced orbitofrontal and parietal gray matter in chronic insomnia: A voxel-based morphometric study. Biol. Psychiat. 67, 182–185. https://doi.org/10.1016/j.biopsych.2009.08.003 (2010).
Yu, S. et al. The orbitofrontal cortex gray matter is associated with the interaction between insomnia and depression. Front. Psychiat. 9, 651. https://doi.org/10.3389/fpsyt.2018.00651 (2018).
Leerssen, J. et al. Brain structural correlates of insomnia severity in 1053 individuals with major depressive disorder: Results from the ENIGMA MDD Working Group. Transl. Psychiat. 10, 425. https://doi.org/10.1038/s41398-020-01109-5 (2020).
Bagherzadeh-Azbari, S. et al. Neuroimaging insights into the link between depression and Insomnia: A systematic review. J. Affect. Disord. 258, 133–143. https://doi.org/10.1016/j.jad.2019.07.089 (2019).
Muller, V. I. et al. Altered brain activity in unipolar depression revisited: Meta-analyses of neuroimaging studies. JAMA Psychiat. 74, 47–55. https://doi.org/10.1001/jamapsychiatry.2016.2783 (2017).
Tahmasian, M. et al. A lack of consistent brain alterations in insomnia disorder: An activation likelihood estimation meta-analysis. Sleep Med. Rev. 42, 111–118. https://doi.org/10.1016/j.smrv.2018.07.004 (2018).
Espie, C. A., Broomfield, N. M., MacMahon, K. M., Macphee, L. M. & Taylor, L. M. The attention-intention-effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Med. Rev. 10, 215–245. https://doi.org/10.1016/j.smrv.2006.03.002 (2006).
Riemann, D. et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 14, 19–31. https://doi.org/10.1016/j.smrv.2009.04.002 (2010).
Riemann, D., Krone, L. B., Wulff, K. & Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 45, 74–89. https://doi.org/10.1038/s41386-019-0411-y (2020).
Nofzinger, E. A. et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry 161, 2126–2128. https://doi.org/10.1176/appi.ajp.161.11.2126 (2004).
Liu, C. H. et al. Increased salience network activity in patients with insomnia complaints in major depressive disorder. Front. Psychiatry 9, 93. https://doi.org/10.3389/fpsyt.2018.00093 (2018).
Bressler, S. L. & Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 14, 277–290. https://doi.org/10.1016/j.tics.2010.04.004 (2010).
Uddin, L. Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. https://doi.org/10.1038/nrn3857 (2015).
Hamilton, J. P. et al. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry 169, 693–703. https://doi.org/10.1176/appi.ajp.2012.11071105 (2012).
Kim, S. J. et al. Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci. Rep. 7, 12528. https://doi.org/10.1038/s41598-017-13065-0 (2017).
Kim, N. et al. Decreased regional brain activity in response to sleep-related sounds after cognitive behavioral therapy for psychophysiological insomnia. Psychiatry Clin. Neurosci. 73, 254–261. https://doi.org/10.1111/pcn.12822 (2019).
Spiegelhalder, K. et al. Brain reactivity and selective attention to sleep-related words in patients with chronic insomnia. Behav. Sleep Med. 16, 587–600. https://doi.org/10.1080/15402002.2016.1253014 (2018).
Baglioni, C. et al. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 37, 1907–1917. https://doi.org/10.5665/sleep.4240 (2014).
Huang, Z. et al. Abnormal amygdala connectivity in patients with primary insomnia: Evidence from resting state fmri. Eur. J. Radiol. 81, 1288–1295 (2012).
Liu, C. et al. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 1648, 317–324 (2016).
Altena, E. et al. Prefrontal hypoactivation and recovery in insomnia. Sleep 31, 1271–1276 (2008).
Drummond, S. P. et al. Neural correlates of working memory performance in primary insomnia. Sleep 36, 1307–1316. https://doi.org/10.5665/sleep.2952 (2013).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167 (2001).
Tekin, S. & Cummings, J. L. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J. Psychosom. Res. 53, 647–654. https://doi.org/10.1016/s0022-3999(02)00428-2 (2002).
Ma, N., Dinges, D. F., Basner, M. & Rao, H. How acute total sleep loss affects the attending brain: A meta-analysis of neuroimaging studies. Sleep 38, 233–240 (2015).
Krause, A. J. et al. The sleep-deprived human brain. Nat. Rev. Neurosci. 18, 404–418 (2017).
George, M. S., Ketter, T. A. & Post, R. M. Prefrontal cortex dysfunction in clinical depression. Science 2, 59–72. https://doi.org/10.1002/depr.3050020202 (1994).
Krause, A. J. et al. The sleep-deprived human brain. Nat. Rev. Neurosci. 18, 404–418. https://doi.org/10.1038/nrn.2017.55 (2017).
Yoo, S. S., Gujar, N., Hu, P., Jolesz, F. A. & Walker, M. P. The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr. Biol. 17, R877-878. https://doi.org/10.1016/j.cub.2007.08.007 (2007).
Motomura, Y. et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS ONE 8, e56578. https://doi.org/10.1371/journal.pone.0056578 (2013).
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J. & Phan, K. L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2, 303–312. https://doi.org/10.1093/scan/nsm029 (2007).
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A. & Ochsner, K. N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. https://doi.org/10.1016/j.neuron.2008.09.006 (2008).
Neckelmann, D., Mykletun, A. & Dahl, A. A. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 30, 873–880 (2007).
Jansson-Fröjmark, M. & Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 64, 443–449 (2008).
Johnson, E. O., Roth, T. & Breslau, N. The association of insomnia with anxiety disorders and depression: Exploration of the direction of risk. J. Psychiatr. Res. 40, 700–708 (2006).
Coleman, R. M. et al. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA 247, 997–1003 (1982).
Le Bon, O. et al. The first-night effect may last more than one night. J. Psychiatr. Res. 35, 165–172 (2001).
American Academy of Sleep Medicine. International Classification of Sleep Disorders-Third Edition (ICSD-3) https://learn.aasm.org/Public/Catalog/Details.aspx?id=%2FgqQVDMQIT%2FEDy86PWgqgQ%3D%3D (2014).
First MB. User's Guide for the Structured Clinical Interview for DSM-IV axis I disorders: SCID-I. (Biometrics Research Dept., New York State Psychiatric Institute, 2002).
Cho, Y. W. et al. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath 15, 377–384. https://doi.org/10.1007/s11325-010-0343-6 (2011).
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Hahn, H., Yum, T. & Shin, Y. Standardization study of Beck Depression Inventory in Korea. J. Korean Neuropsychiatr. Asso. 25, 487–502 (1982).
Yook, S. & Kim, Z. A clinical study on the Korean version of Beck Anxiety Inventory: Comparative study of patient and non-patient. Korean J. Clin. Psychol. 16, 185–197 (1997).
Morin, C. M., Vallieres, A. & Ivers, H. Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS-16). Sleep 30, 1547–1554. https://doi.org/10.1093/sleep/30.11.1547 (2007).
Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach 335–7 (The Guilford Press, 2014).
Acknowledgements
This study was supported by the National Research Foundation of Korea Technology (NRF) grant funded by the Korea government Technology (MSIT) (NRF-2022R1A2C1008209) and the Technology Innovation Infrastructure Program through the Korea Institute for Advancement of Technology (The Ministry of Trade, Industry and Energy, Study No.: P0014279).
Author information
Authors and Affiliations
Contributions
K.H.L. and Y.J.L. conceived the study. M.H.L., K.H.L., S.M.O., H.Y.L., M.C.S, and J.E.J. contributed the data acquisitions. M.H.L., K.H.L., H.Y.L., J.E.J., and Y.J.L. contributed the analyses. M.H.L. and Y.J.L. drafted the manuscript and K.H.L., S.M.O., H.Y.L. and J.E.J. revised it critically for intellectual contents. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, M.H., Lee, K.H., Oh, S.M. et al. The moderating effect of prefrontal response to sleep-related stimuli on the association between depression and sleep disturbance in insomnia disorder. Sci Rep 12, 17739 (2022). https://doi.org/10.1038/s41598-022-22652-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22652-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.