Abstract
Impaired cognition is associated with lower quality of life and poor outcomes in schizophrenia. Brain glutamate may contribute to both clinical outcomes and cognition, but these relationships are not well-understood. We studied a multicentre cohort of 85 participants with non-affective psychosis using proton magnetic resonance spectroscopy. Glutamate neurometabolites were measured in the anterior cingulate cortex (ACC). Cognition was assessed using the Brief Assessment for Cognition in Schizophrenia (BACS). Patients were categorised as antipsychotic responders or non-responders based on treatment history and current symptom severity. Inverted U-shaped associations between glutamate or Glx (glutamate + glutamine) with BACS subscale and total scores were examined with regression analyses. We then tested for an interaction effect of the antipsychotic response group on the relationship between glutamate and cognition. ACC glutamate and Glx had a positive linear association with verbal memory after adjusting for age, sex and chlorpromazine equivalent dose (glutamate, β = 3.73, 95% CI = 1.26–6.20, P = 0.004; Glx, β = 3.38, 95% CI = 0.84–5.91, P = 0.01). This association did not differ between good and poor antipsychotic response groups. ACC glutamate was also positively associated with total BACS score (β = 3.12, 95% CI = 0.01–6.23, P = 0.046), but this was not significant after controlling for antipsychotic dose. Lower glutamatergic metabolites in the ACC were associated with worse verbal memory, and this relationship was independent of antipsychotic response. Further research on relationships between glutamate and cognition in antipsychotic responsive and non-responsive illness could aid the stratification of patient groups for targeted treatment interventions.
Similar content being viewed by others
Introduction
Impaired cognition in psychotic disorders contributes to poor social and functional outcomes1,2,3. Cognitive deficits are observed in relatives of schizophrenia patients, clinical high-risk groups, and at the onset of psychosis4,5,6. Cognitive dysfunction is therefore an important target for research as it may precede potential confounds of prolonged antipsychotic treatment and illness chronicity. Antipsychotics primarily act as dopamine D2 receptor antagonists and have minimal impact on alleviating cognitive dysfunction7,8,9,10. This suggests that cognitive impairment in psychosis involves mechanisms other than dopamine. Investigating mechanisms of impaired cognitive function can advance our understanding of the aetiology of illness and the development of targeted treatments.
Converging lines of evidence implicate altered glutamatergic function in the aetiology of schizophrenia, particularly for negative and cognitive symptom domain11,12,13. A primary model of glutamate dysregulation centres on NMDA receptor hypofunction, which leads to excessive signalling of glutamatergic pyramidal neurons across the cortex and elevated glutamate release14. Administration of NMDA receptor antagonists increases cortical glutamate15,16,17, induces schizophrenia-like cognitive deficits in animal models and healthy human subjects18,19,20,21,22 and exacerbates cognitive impairment in schizophrenia23,24. Further, animal models provide some indication that cognitive impairments induced by NMDA antagonists may be partially reversed by moderate doses of glutamate modulating compounds25. The clinical efficacy of several compounds enhancing NMDA receptor signalling has been trialled, with meta-analysis demonstrating a small effect size (ES) for the reduction of PANSS-cognitive symptoms (ES = 0.28)26. One other meta-analysis found no evidence of improved cognitive function from antipsychotic treatment augmented with glutamatergic modulators27, which highlights the difficulties in translating findings from preclinical studies into effective glutamate drug therapies.
The relationship between glutamate and cognition in schizophrenia is not yet understood28,29. The anterior cingulate cortex (ACC) is involved in cognition30 and demonstrates abnormal activity during cognitive task performance in schizophrenia31. Six studies have examined the relationship between ACC glutamate neurometabolites and cognition in medicated patients32,33,34,35,36,37. One study reported a positive association between Glx and a composite measure of neurocognitive scores37. Three other studies assessed cognition across multiple domains using the Repeatable Battery for Neuropsychological Status (RBANS) and reported no association between glutamate or Glx (glutamate plus glutamine) concentrations and cognitive performance33,34,36. Another study found no evidence of an association between glutamate and performance on working memory and processing speed35. The sixth study found a positive association between Glx and cognitive flexibility, measured using the Wisconsin Card Sorting Task32. A positive association between dorsal ACC Glx and measures of working memory and attention was also reported in a large unmedicated patient sample38. Within the medial prefrontal cortex more broadly, no association between Glx and working memory was found in a sample of medicated and unmedicated patients39, whilst another study reported a negative association between the ratio of glutamine to glutamate and measures of cognitive flexibility, verbal working memory and attention40. Notably, the majority of these investigations included relatively small sample sizes and did not account for potential confounding effects of age, sex and antipsychotic dose on brain glutamate concentrations41,42.
Brain glutamate concentrations may vary across patients and there is some evidence that elevations in ACC glutamate neurometabolites are associated with a higher illness severity, worse clinical course, poorer functioning and treatment resistance36,42,43,44,45,46,47,48,49. Neuroimaging studies provide some evidence that when cortical glutamatergic metabolite concentrations are higher within schizophrenia groups compared to healthy controls the direction of associations between glutamate and cognition are negative29,50. Conversely, in studies where cortical glutamatergic metabolite concentrations are comparable or lower in schizophrenia groups than in healthy controls, associations between glutamate and cognition are positive32,37,51,52,53,54. One explanation for these different findings may be that deficient or excess cortical glutamate beyond some optimal range leads to cognitive impairments in schizophrenia. Taken together, these findings could potentially suggest an inverted ‘U’ shaped relationship between glutamate and cognition in schizophrenia.
One study has examined the relationship between glutamate and cognition in treatment-resistant and treatment-responsive illness, which found no relationship between dorsal ACC glutamate and cognition across the whole sample or within the patient subgroups36. However, the study used linear correlational analysis, which meant potential non-linear relationships between glutamate and cognition were not assessed and possible confounds were not controlled for. Further, although ACC Glx was higher in the treatment-resistant group compared to healthy controls, the difference in Glx between treatment and treatment-responsive groups did not differ significantly, which may explain why the relationships between Glx and cognition also did not differ between groups36.
This study investigated the relationship between ACC glutamatergic metabolites and cognitive data in the STRATA-1 patient cohort, who were recruited according to their response to antipsychotic treatment. Within this cohort we previously reported higher glutamate levels in the ACC of antipsychotic non-responders compared to responders when controlling for age and sex45, but no group differences in cognition55. Based on the evidence above, our primary hypothesis was that there would be an inverted U-shaped association between ACC glutamate and cognition across the whole sample. Second, we hypothesised an interaction effect of antipsychotic response group on the relationship between glutamate and cognition, such that the association would be positive in antipsychotic responders and negative in antipsychotic non-responders.
Results
Demographic and clinical characteristics of the cohort are reported in Table 1. In total, 85 participants had both 1H-MRS and cognitive data. One participant did not complete the task assessing motor processing speed (Token Motor Task). All other participants completed each task. The mean number of days between the collection of both measures was 4 (SD = 8.00, range = 0–40 days). An example MR spectrum is presented in Supplementary Fig. 1.
The relationship between cognition and clinical and demographic variables
Supplementary Table 1 reports the relationship between cognition and clinical and demographic characteristics of the sample. Sex, age of onset, CPZE dose, current cannabis use, current smoking, current benzodiazepine and current SSRI use were not associated with BACS composite t and z or subdomain scores. Age was negatively correlated with motor speed and attention and information processing speed. BACS composite t and z-scores, verbal memory, verbal fluency, working memory and attention and information processing speed were negatively correlated with PANSS negative subscale scores (Supplementary Table 2). As reported in our recent publication in the same cohort55, there was no difference in cognitive performance between groups prescribed antipsychotics with no or low versus high anticholinergic effects (see ref. 55 and Supplementary Materials).
Cognition and glutamatergic metabolites
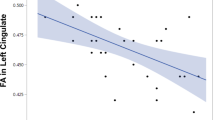
Tables 2 and 3 display results from all multivariable regression models. The direction of relationship between ACC Glucorr and Glxcorr with cognition was positive across all cognitive domains (Supplementary Figs. 2 and 3). ACC Glucorr and Glxcorr were positively associated with verbal memory after adjusting for age and sex. Both associations remained significant after adjusting for CPZE (Glucorr, β = 3.73, 95% CI = 1.26–6.20, P = 0.004; Glxcorr, β = 3.38, 95% CI = 0.84–5.91, P = 0.01). Visual inspection of the data indicated a linear relationship between glutamate neurometabolites and verbal memory (Fig. 1A, C); non-linear associations for both Glucorr and Glxcorr with verbal memory were not significant (Glucorr2: β = −0.87, 95% CI = −2.42, 0.68, P = 0.27; Glxcorr2: β = −0.65, 95% CI = −1.91, 0.61, P = 0.31) and the LR test suggested a linear model was a better fit to data (Glucorr: LR X2 (1) = 1.33, P = 0.25; Glxcorr: LR X2 (1) = 1.12, 0.29). There was no evidence of an inverted U-shaped relationship between glutamate (t = 0.10, P = 0.46) or Glx (t = 0.07, P = 0.47) and verbal memory. Subsequent analyses including an antipsychotic response group × metabolite interaction term found no significant effects, such that the relationship between verbal memory and Glucorr or Glxcorr did not differ as a function of antipsychotic response group (Glucorr × group: β = 3.43, 95% CI = −1.51, 8.36, P = 0.17; Glxcorr × group: β = 1.12, 95% CI = −3.92, 6.16, P = 0.66).
There was a significant association between ACC Glucorr and BACS-t scores when controlling for age and sex (β = 3.12, 95% CI = 0.01, 6.23, P = 0.049). Visual inspection of the data indicated a linear relationship between glutamate neurometabolites and BACS-t (Fig. 1). Consistent with visual inspection, the non-linear association between Glucorr and BACS-t was not significant (Glucorr2: β = −2.25, 95% CI = −5.07, 0.57, P = 0.17), the LR test suggested a linear model was a better fit to the data (LR X2 (1) = 4.77, P = 0.09) and the utest confirmed no evidence of an inverted U-shaped relationship between Glucorr and BACS-t (t = 1.31, P = 0.10). When the linear model was further adjusted for CPZE, the association between BACS-t and Glucorr became non-significant. There was no significant relationship between BACS-t and ACC Glxcorr when controlling for age, sex and CPZE. No significant antipsychotic response group × metabolite interaction effects were found for BACS-t scores and performance on all other cognitive domains (Supplementary Table 3 and Supplementary Figs. 4 and 5).
One participant had low values on both BACS-t and ACC Glucorr (Fig. 1B). Both raw data entry and the BACS-t composite score calculation were checked for accuracy. We did not exclude this participant from analyses because, relative to the overall STRATA-1 cognition cohort, the BACS-t score fell within the range of the sample55. 1H-MRS spectra were visually inspected, and the scan passed all our standard quality control procedures45.
Discussion
This study investigated the relationship between glutamatergic metabolites and cognition in a sample of medicated participants with non-affective psychosis. We also explored whether there was a group difference in the relationship between glutamatergic metabolites and cognition between antipsychotic responders and non-responders. Our main finding was a positive linear association between verbal memory performance and both ACC Glucorr and Glxcorr after adjusting for age, sex and CPZE dose. Interaction analyses found no effect of antipsychotic response on the relationship between Glucorr or Glxcorr and verbal memory. ACC Glucorr was also associated with BACS-t after controlling for age and sex, but further adjustments for CPZE dose rendered this association non-significant. We did not find evidence to support the hypothesised inverted U-shaped relationship between glutamate and cognition. Overall, our results indicate that higher levels of glutamate may be associated with better verbal memory performance in schizophrenia and that this relationship does not differ according to the degree of antipsychotic response.
The finding of a positive association between ACC glutamatergic metabolites and verbal memory performance is broadly consistent with some previous studies on unmedicated first episode psychosis and chronically medicated schizophrenia cohorts, which have reported positive associations between ACC Glx and measures of working memory, attention and executive function32,37,38. Conversely, four other studies on medicated cohorts have reported no association between ACC glutamatergic metabolites and performance on tasks measuring broad neurocognitive status, working memory and information processing speed33,34,35,36. Most of these investigations included relatively small samples and did not control for possible effects of age, sex and antipsychotic dose were not controlled for. Results from the largest medicated cohort published to date (n = 104 schizophrenia patients, 97 healthy controls)56 also found no significant association between performance across the MATRICS57 cognitive battery and Glx concentrations in supraventricular white matter regions, which included a portion of the ACC but was not limited to this brain region56. Overall, between-study differences in the cognitive tasks employed, method of adjusting glutamatergic metabolites (scaling to creatine or correcting for voxel tissue content), neural region of interest (including voxel positioning in the ACC) and the clinical characteristics of the patient samples make direct comparisons difficult.
We found that higher glutamate was associated with better verbal memory across the whole cohort. This finding appears to be at odds with data from animal and human studies which suggest that compounds that increase cortical glutamate levels disrupt cognitive function19,21,22,23,24,25. Further, this finding also conflicts with observations that schizophrenia groups displaying the highest levels of illness severity, including cognitive dysfunction, may also have higher brain glutamate than less symptomatic patients and healthy controls18,36,46,47. However, neuroimaging studies collectively suggest that the relationship between glutamate and cognition may vary depending on whether glutamate in schizophrenia is elevated or comparable to healthy controls32,38,40,51,52,53,54,56,58. Preclinical models and proof-of-concept clinical studies also give some indication that glutamate modulating compounds improve cognition at moderate doses, whereas low and high doses may have suboptimal or negative effects, respectively, on cognition25,59,60,61. These observations raise the possibility that insufficient or excessive glutamate outside of an optimal range may be associated with worse cognitive functioning. Within our sample the positive relationship between cognition and glutamate appeared linear, which would be consistent with findings in patient cohorts with glutamate levels comparable or lower to those in healthy controls32,38,51,52,53,54. Future research could specifically examine whether the relationship between glutamate and cognition differs in patients with glutamate levels above or below healthy control values.
The largest effect size was found for the association between glutamate and verbal memory. Impairments within verbal cognitive domains are robust across the illness course of schizophrenia62,63. Impaired verbal memory function is also evident in the clinical high-risk phase of psychosis and is more severe in high-risk subjects who later progress to psychosis64,65. A recent meta-analysis found that verbal memory impairments are more apparent in treatment-resistant compared to treatment-responsive schizophrenia66, and prospective investigation suggests this group difference is detectable from the first episode of psychosis67. However, cognitive performance did not differ between the good and poor antipsychotic response groups in our cohort55. We found no evidence that the relationship between glutamate and verbal memory differed between good and poor antipsychotic responders. Whilst both worse verbal cognition and higher ACC glutamate may be associated with treatment resistance66,67,68, the lack of interaction suggests that there is no qualitative difference in the relationship between glutamate and cognition between antipsychotic response groups, at least within the ranges of antipsychotic response, glutamate, and cognitive function measured in the current study. Alternatively, it is possible that the lack of group difference in the relationship between glutamate and cognition may be due to the relative similarity in cognitive function between good and poor antipsychotic responders in our sample55. One other study found no association between ACC Glx and cognition within treatment-resistant and -responsive groups despite the treatment-resistant sample having worse cognitive function36. However, patient groups did not differ in Glx concentrations and the interaction between group and glutamatergic metabolite concentrations on cognition was not directly investigated. Whether the marked cognitive impairments observed in treatment-resistant schizophrenia66 represent a distinct or exaggerated pathophysiological mechanism compared to those displaying good treatment response warrants further investigation.
Strengths and limitations
We present data from a multicentre investigation, which provides a representative sample of medicated patients across the UK and a larger sample size than most previous studies. We used the BACS to assess cognitive function within several domains directly relevant to schizophrenia69,70, which is quicker to administer than other broad neuropsychological batteries (~30 min versus a couple of hours) whilst remaining as sensitive in detecting cognitive impairments69. 1H-MRS acquisition sequences were harmonised across research sites, and we were able to control for site effects present in MRS data by standardising metabolite values. Further, in vitro phantom data and a healthy control pilot scan confirmed good data quality across research sites (see ref. 45 and Supplementary Discussion).
While several statistical tests were run, we did not correct for multiple comparisons given preclinical and human imaging evidence for associations between glutamate and task performance measured across several independent cognitive domains18,19,20,21,22,23,24,29. In terms of study limitations, not all brain scans and cognitive assessments were performed on the same day, which may have affected the relationship we observed between glutamate and cognition. Further, our study did not include the measurement of glutamatergic metabolites in other brain regions relevant for cognition, such as the dorsolateral prefrontal cortex and the hippocampus29. Another caveat is that CPZE dose does not account for variations in medication adherence, although we only recruited participants demonstrating at least a moderate level of adherence by applying the CRS scale. Future research would benefit from the collection of antipsychotic plasma levels to confirm treatment adherence and exclude cases of pseudo-treatment resistance. It is also important to note that our patient sample had an average illness duration of 5 years and therefore we cannot make inferences on the relationship between glutamate and cognition in initial stages of illness or the first episode of psychosis. This is important given evidence that the relationship between glutamate and cognition may change after antipsychotic treatment71, although how this may also relate to antipsychotic response is still unstudied.
Conclusion
This study found a positive association between glutamate and cognition in schizophrenia and this relationship did not differ between good and poor antipsychotic responders. Our findings support a role of ACC glutamate for cognition in schizophrenia.
Methods
Ethics
The study had NHS Research Ethics Committee approvals (15/LO/0038). All participants provided written informed consent.
Participants
Participants were recruited across four sites: Cardiff University (CU), University of Edinburgh (UoE), University of Manchester (UoM) and King’s College London (KCL). Inclusion criteria were being aged between 18 and 65, meeting Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for schizophrenia or schizophreniform disorder and the ability to understand and consent to study procedures, including a sufficient level of English. Exclusion criteria were poor medication adherence (defined as a score of <3 on the Compliance Rating Scale (CRS))72, current pregnancy, previous severe head injury involving loss of consciousness for >5 min, currently meeting the international Classification of Diseases (ICD) criteria for harmful substance misuse or psychotic disorder secondary to substance misuse and any Magnetic Resonance imaging (MRI) contraindications, such as implanted electronic devices or metallic objects. Participants were also excluded if they had received treatment with clozapine in the last 3 months prior to study screening because clozapine may affect brain glutamate concentrations73.
This patient group is a subsample of the STRATA-1 imaging cohort presented in a previous publication45. As well as a brain MRI scan with proton magnetic resonance spectroscopy (1H-MRS), participants included in the current study completed a battery of cognitive assessments55. Scans and cognitive assessments were performed on the same day where possible. For each antipsychotic dose, chlorpromazine equivalent (CPZE) doses were calculated according to methods outlined by Davis and Chen74, except for amisulpride which used defined daily dose (https://www.whocc.no/atc_ddd_index/).
Defining good and poor antipsychotic response
Participants displaying good and poor antipsychotic responses were recruited based on a priori criteria for antipsychotic treatment response. Recruitment aimed for a 1:1 ratio of antipsychotic responders and non-responders. Treatment history and current symptom severity were assessed through structured interviews and review of medical records. The antipsychotic response was defined as (1) having treatment with only one antipsychotic since illness onset, or treatment changes that were due to adverse effects rather than non-response; (2) clinical Global Impression-Schizophrenia Scale (CGI-SCH) severity score <4; (3) positive and Negative Syndrome Scale (PANSS) total score <60. Antipsychotic non-response was defined as (1) documented treatment with at least two antipsychotics for >4 weeks each, at doses above the minimum therapeutic doses as defined by the British National Formulary; (2) a CGI-SCH score >3; (3) PANSS total score of at least 70.
1H-MRS and quality control procedures
Metabolite concentrations were measured using 1H-MRS at 3 Tesla according to the protocol described by Egerton and colleagues45. Spectra were analysed in LCModel version 6.3.1 L using a standard LCModel basis set. Extracted metabolite estimates were water-referenced and corrected for voxel tissue content (denoted by Glucorr and Glxcorr)45. We chose not to scale metabolite values to creatine given evidence that these metabolite values may vary in schizophrenia cohorts42. The spectral linewidths and signal-to-noise ratio were reviewed as part of quality control procedures, and spectra were excluded if the linewidth was 2 standard deviations above or the signal-to-noise ratio was 2 standard deviations below the overall mean for the voxel across all participants at all sites. Individual metabolite concentrations were excluded if their Cramér–Rao lower bounds (CRLB) value was 20% or higher. CRLBs for glutamate and Glx did not differ between good and poor antipsychotic response groups45, confirming that CRLB filtering did not result in different levels of exclusion of metabolite estimates between the groups75. All metabolite concentrations were converted to z-scores to account for site effects in metabolite concentration estimates, which were present due to differences in the MRI scanners used across sites (see ref. 45 and Supplementary Discussion for a detailed discussion of scanner and site effects).
Cognition
Cognition was assessed using the Brief Assessment for Cognition in Schizophrenia69 (BACS). Performance was evaluated across six cognitive domains: executive function, working memory, motor processing speed, verbal memory, verbal fluency and attention and information process speed. Overall cognitive function was assessed using composite BACS-t and z-scores, which were standardised against normative data and calculated according to equations provided by Keefe and colleagues55,70. A z-score of 0 represents average performance with reference to the healthy control population of the same age range and sex, while each point represents 1 standard deviation. Higher scores reflect better cognitive performance on each domain and for the composite measures.
Statistical analysis
All analyses were performed using STATAv.1576. Multivariable linear regression models were used to examine the effect of Glucorr and Glxcorr on BACS composite and subdomain scores. Both composite and subdomain scores were considered as primary outcomes of interest given evidence of potential relationships between glutamate and cognition on several cognitive tasks measuring distinct processes29. Models were adjusted for age and sex based on their relationship with brain glutamate concentration41,45,77, To explore any potential effects of antipsychotic medication on glutamate and cognitive function42,78,79, CPZE dose was also entered as a covariate. We then tested whether a linear or non-linear model provided a better fit to our data by expanding models to include a quadratic term (metabolite2). We used the likelihood-ratio (LR) test to compare the fit of linear and non-linear models as well as the STATA command utest80,81 to examine whether non-linear relationships between metabolites and cognition were inverted U-shape. To test whether the relationship between glutamatergic metabolites and cognition differed between responder and non-responder groups, we subsequently included the interaction term antipsychotic response group × metabolite to fully adjusted linear models. All tests for statistical significance were two-sided with an alpha of 0.05.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. At the time of submission, the data governance frameworks are being put in place to make a fully anonymized version of the data available to the wider research community via TranSMART data-sharing platform: https://transmartfoundation.org/, which will be hosted at King’s College London. To apply for access to the data, please contact the chief investigator J.H.M. at james.maccabe@kcl.ac.uk.
References
Heinrichs, R. W. & Zakzanis, K. K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 12, 426–445 (1998).
Nielsen, R. E. Cognition in schizophrenia—a systematic review. Drug Discovery Today: Therapeutic Strategies. 8, 43–48 (2011).
Ventura, J., Hellemann, G. S., Thames, A. D., Koellner, V. & Nuechterlein, K. H. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr. Res. 113, 189–199 (2009).
Bora, E. et al. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr. Scand. 130, 1–15 (2014).
Fatouros-Bergman, H., Cervenka, S., Flyckt, L., Edman, G. & Farde, L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr. Res. 158, 156–162 (2014).
Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V. & Seidman, L. J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 23, 315–336 (2009).
Goldberg, T. E. et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect. Arch. Gen. Psychiatry. 64, 1115–1122 (2007).
Kaar, S. J., Natesan, S., McCutcheon, R. & Howes, O. D. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 172, 107704 (2020).
Mailman, R. B. & Murthy, V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity. Curr Pharm Des. 16, 488–501 (2010).
Woodward, N. D., Purdon, S. E., Meltzer, H. Y. & Zald, D. H. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 8, 457–472 (2005).
Javitt, D. C. Glutamatergic theories of schizophrenia. Isr. J. Psychiatry Relat. Sci. 47, 4–16 (2010).
McCutcheon, R. A., Krystal, J. H. & Howes, O. D. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 19, 15–33 (2020).
Moghaddam, B. & Krystal, J. H. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 38, 942–949 (2012).
Moghaddam, B. & Javitt, D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 37, 4–15 (2012).
Bojesen, K. B. et al. Glutamate levels and resting cerebral blood flow in anterior cingulate cortex are associated at rest and immediately following infusion of S-ketamine in healthy volunteers. Front. Psychiatry. 9, 22 (2018).
Liu, J. & Moghaddam, B. Regulation of glutamate efflux by excitatory amino acid receptors: evidence for tonic inhibitory and phasic excitatory regulation. J. Pharmacol. Exp. Ther. 274, 1209–1215 (1995). https://www.ncbi.nlm.nih.gov/pubmed/7562490.
Javitt, D. C. et al. Utility of imaging-based biomarkers for glutamate-targeted drug development in psychotic disorders: a randomized clinical trial. JAMA Psychiatry 75, 11–19 (2018).
Amitai, N., Semenova, S. & Markou, A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology 193, 521–537 (2007).
Beck, K. et al. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-analysis. JAMA Network Open 3, e204693–e204693 (2020).
Goff, D. C. & Coyle, J. T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry. 158, 1367–1377 (2001).
Krystal, J. H. et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214 (1994).
Jentsch, J. D., Tran, A., Le, D., Youngren, K. D. & Roth, R. H. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17, 92–99 (1997).
Lahti, A. C., Koffel, B., LaPorte, D. & Tamminga, C. A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13, 9–19 (1995).
Malhotra, A. K. et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17, 141–150 (1997).
Castner, S. A. et al. Relationship between glycine transporter 1 inhibition as measured with positron emission tomography and changes in cognitive performances in nonhuman primates. Neuropsychopharmacology 39, 2742–2749 (2014).
Tsai, G. E. & Lin, P. Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 16, 522–537 (2010).
Iwata, Y. et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol. Psychiatry 20, 1151–1160 (2015).
Merritt, K., McGuire, P. & Egerton, A. Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Front. Psychiatry. 4, 151 (2013).
Reddy-Thootkur, M., Kraguljac, N. V. & Lahti, A. C. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders—a systematic review of magnetic resonance spectroscopy studies. Schizophr. Res. https://doi.org/10.1016/j.schres.2020.02.001 (2020).
Bush, G., Luu, P. & Posner, M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000).
Adams, R. & David, A. S. Patterns of anterior cingulate activation in schizophrenia: a selective review. Neuropsychiatr Dis. Treat. 3, 87–101 (2007).
Ohrmann, P. et al. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr. Res. 106, 156–163 (2008).
Reid, M. A. et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol. Psychiatry 68, 625–633 (2010).
Rowland, L. M. et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr. Bull. 39, 1096–1104 (2013).
Rowland, L. M. et al. Frontal glutamate and γ-aminobutyric a cid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. JAMA Psychiatry 73, 166–174 (2016).
Tarumi, R. Y. et al. Levels of glutamatergic neurometabolites in patients with severe treatment-resistant schizophrenia: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology 45, 632–640 (2020).
Coughlin, J. M. et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol. Psychiatry 26, 3502–11 (2021).
Bojesen, K. B. et al. Associations between cognitive function and levels of glutamatergic metabolites and gamma-aminobutyric acid in antipsychotic-naïve patients with schizophrenia or psychosis. Biol. Psychiatry 89, 278–287 (2021).
Kegeles, L. S. et al. Elevated prefrontal cortex γ-aminob utyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 69, 449–459 (2012).
Shirayama, Y. et al. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage 49, 2783–2790 (2010).
Marsman, A. et al. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr. Bull. 39, 120–129 (2013).
Merritt, K. et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatry 78, 667–681 (2021).
Egerton, A. et al. Anterior cingulate gluta mate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology 37, 2515–2521 (2012).
Egerton, A. et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1H-MRS study (OPTiMiSE). Mol. Psychiatry 23, 2145–2155 (2018).
Egerton, A. et al. Dopamine and glutamate in antipsychotic-responsive compared with antipsychotic-nonresponsive psychosis: a multicenter positron emission tomography and magnetic resonance spectroscopy study (STRATA). Schizophr. Bull. 47, 505–516 (2021).
Goldstein, E., Anderson, V. M., Pillai, A., Kydd, R. R. & Russell, B. R. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int. J. Neuropsychopharmacol. 18, https://doi.org/10.1093/ijnp/pyu117 (2015).
Iwata, Y. et al. Glutamatergic neurometabolite levels in patients with ul tra-treatment-resistant schizophrenia: a cross-sectional 3t proton magnetic resonance spectroscopy study. Biol. Psychiatry 85, 596–605 (2019).
Merritt, K. et al. Remission from antipsychotic treatment in first episode psychosis related to longitudinal changes in brain glutamate. npj Schizophr. 5, 12 (2019).
Mouchlianitis, E. et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr. Bull. 42, 744–752 (2016).
Rüsch, N. et al. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr. Res. 99, 155–163 (2008).
Bustillo, J. R. et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol. Psychiatry 69, 19–27 (2011).
Falkenberg, L. E. et al. Impact of glutamate levels on neuronal response and cognitive abilities in schizophrenia. Neuroimage Clin. 4, 576–584 (2014).
Ohrmann, P. et al. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J. Psychiatr. Res. 41, 625–634 (2007).
Wang, A. M. et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 76, 314–323 (2019).
Millgate, E. et al. Cross-sectional study comparing cognitive function in treatment responsive versus treatment non-responsive schizophrenia: evidence from the STRATA study. BMJ Open. 11, e054160 (2021).
Bustillo, J. R. et al. Glutamatergic and neuronal dysfunction in gray and white matter: a spectroscopic imaging study in a large schizophrenia sample. Schizophr. Bull. 43, 611–619 (2017).
Nuechterlein, K. H. et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry 165, 203–213 (2008).
Rowland, L. M. et al. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 6, e967 (2016).
Eddins, D. et al. The relationship between glycine transporter 1 occupancy and the effects of the glycine transporter 1 inhibitor RG1678 or ORG25935 on object retrieval performance in scopolamine impaired rhesus monkey. Psychopharmacology 231, 511–519 (2014).
Leung, S., Croft, R. J., O’Neill, B. V. & Nathan, P. J. Acute high-dose glycine attenuates mismatch negativity (MMN) in healthy human controls. Psychopharmacology 196, 451–460 (2008).
Umbricht, D. et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry 71, 637–646 (2014).
Aas, M. et al. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front. Psychiatry 4, 182 (2014).
Aleman, A., Hijman, R., de Haan, E. H. & Kahn, R. S. Memory impairment in schizophrenia: a meta-analysis. Am. J. Psychiatry 156, 1358–1366 (1999).
Catalan, A. et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry 78, 859–867 (2021).
Fusar-Poli, P. et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry 69, 562–571 (2012).
Millgate, E. et al. Neuropsychological differences between treatment-resistant and treatment-responsive schizophrenia: a meta-analysis. Psychological Med. 52, 1–13, https://doi.org/10.1017/S0033291721004128 (2022).
Kravariti, E. et al. Neuropsychological function at first episode in treatment-resistant psychosis: findings from the ÆSOP-10 study. Psychol. Med. 49, 2100–2110 (2019).
Gillespie, A. L., Samanaite, R., Mill, J., Egerton, A. & MacCabe, J. H. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry 17, 12 (2017).
Keefe, R. S. et al. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 68, 283–297 (2004).
Keefe, R. S. et al. Norms and standardization of the brief assessment of cognition in schizophrenia (BACS). Schizophr. Res. 102, 108–115 (2008).
Dempster, K. et al. Glutamatergic metabolite correlations with neuropsychological tests in first episode schizophrenia. Psychiatry Res. 233, 180–185 (2015).
Kemp, R., Hayward, P., Applewhaite, G., Everitt, B. & David, A. Compliance therapy in psychotic patients: randomised controlled trial. BMJ 312, 345–349 (1996).
McQueen, G. et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophr. Bull. 47, 662–671 (2021).
Davis, J. M. & Chen, N. Dose response and dose equivalence of antipsychotics. J. Clin. Psychopharmacol. 24, 192–208 (2004).
Kreis, R. The trouble with quality filtering based on relative Cramér‐Rao lower bounds. Magn. Resonance Med. 75, 15–18 (2016).
Stata Statistical Software: Release 15 (StataCorp LLC, 2015).
Sailasuta, N., Ernst, T. & Chang, L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn. Reson. Imaging 26, 667–675 (2008).
Désaméricq, G. et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur. J. Clin. Pharmacol. 70, 127–134 (2014).
Mishara, A. L. & Goldberg, T. E. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol. Psychiatry 55, 1013–1022 (2004).
Lind, J. T., Mehlum, H. UTEST: Stata module to test for a U-shaped relationship. Statistical Software Components S456874, Boston College Department of Economics, (2007).
Lind, J. T. & Mehlum, H. With or without U? The appropriate test for a U-shaped relationship. Oxford Bull. Econ. Stat. 72, 109–118 (2010).
Acknowledgements
This research was supported by the Medical Research Council (MRC) UK, Stratified Medicines Initiative, reference MR/L011794/1 ‘STRATA’. Research at the London site was supported by the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry, Psychology and Neuroscience at King’s College London. K.G. is in receipt of a PhD studentship funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
K.G., E.M., A.E. and J.H.M. conceptualised the manuscript. K.G. was responsible for the statistical analysis and write-up of the manuscript, including the first draft plus editing and formatting in preparation for publication. A.E., J.H.M. and E.M. contributed to the statistical analysis, interpretation of the data, and assisted in the preparation of the manuscript. A.E., J.H.M., O.D.H., J.T.W., S.L., S.M.L., D.L., G.J.B. and P.M. contributed to the conceptualisation and design of the STRATA study. All other authors have contributed to data collection, interpretation of results and critically reviewed the manuscript. All authors revised and agreed upon the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.J.B. receives honoraria for teaching from GE Healthcare. In the last 3 years, S.M.L. has received personal payment from Sunovion for chairing an educational meeting. The remaining authors declare no financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41537_2022_265_MOESM1_ESM.docx
Supplementary Materials. Impaired verbal memory function is related to anterior cingulate glutamate levels in schizophrenia: findings from the STRATA study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Griffiths, K., Egerton, A., Millgate, E. et al. Impaired verbal memory function is related to anterior cingulate glutamate levels in schizophrenia: findings from the STRATA study. Schizophr 8, 60 (2022). https://doi.org/10.1038/s41537-022-00265-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00265-5