Abstract
Schizophrenia patients (SZH) often show impaired cognition and reduced brain structural volumes; these deficits are also detectable in healthy relatives of SZH. However, there is considerable heterogeneity: a sizable percentage of SZH are relatively cognitively intact; clustering strategies have proved useful for categorising into cognitive subgroups. We used a clustering strategy to investigate relationships between subgroup assignment and brain volumes, in 102 SZH (N = 102) and 32 siblings of SZH (SZH-SIB), alongside 92 controls (CON) and 48 of their siblings. SZH had poorer performance in all cognitive domains, and smaller brain volumes within prefrontal and temporal regions compared to controls. We identified three distinct cognitive clusters (‘neuropsychologically normal’, ‘intermediate’, ‘cognitively impaired’) based on age- and gender-adjusted cognitive domain scores. The majority of SZH (60.8%) were assigned to the cognitively impaired cluster, while the majority of SZH-SIB (65.6%) were placed in the intermediate cluster. Greater right middle temporal volume distinguished the normal cluster from the more impaired clusters. Importantly, the observed brain volume differences between SZH and controls disappeared after adjustment for cluster assignment. This suggests an intimate link between cognitive performance levels and regional brain volume differences in SZH. This highlights the importance of accounting for heterogeneity in cognitive performance within SZH populations when attempting to characterise the brain structural abnormalities associated with the disease.
Similar content being viewed by others
Introduction
Positive and negative symptoms constitute the primary diagnostic criteria for schizophrenia, but cognitive deficits are also one of the hallmark features of SZH. These are present in 75–80% of patients, impacts on daily functioning1,2, are present at onset and remain relatively stable over the course of the illness3. Unfortunately, cognitive impairments are largely unresponsive to pharmacological therapy4. Cognitive impairment in SZH has been demonstrated across almost all cognitive domains, most notably executive function5, processing speed6, attention and vigilance7, and working memory8. Cognitive training has limited efficacy, although there is some evidence of benefit for executive function training, for example9. Since deficits in cognitive abilities in SZH are largely independent of clinical state and medication status, they have been proposed as a potential endophenotypic marker for SZH10. Further, they are familial in nature. Meta-analyses indicate impairments in unaffected first-degree relatives versus controls across a variety of cognitive tasks and particularly those tapping executive function11. This points to a genetic overlap between cognitive deficits and risk for SZH; polygenic schizophrenia risk scores have been associated with lower general cognitive ability10,12, supporting this.
Nevertheless, not all SZH show cognitive impairment. A cognitively relatively intact subgroup of SZH seems to exist with up to 25% of patients showing minimal evidence of impairment; also, there is significant heterogeneity in severity across those that are impaired13. Recently, studies have characterised this heterogeneity by implementing clustering strategies to identify subgroups of SZH patients based on cognitive function abilities13,14,15,16,17,18; this approach has also been utilised in first episode patients19,20. Cluster analysis allows the classification of individuals into subgroups based on their cognitive profiles, and such analyses have tended to identify 3 distinct subgroups: a relatively intact group, an intermediate group (a subgroup with the level of cognitive deficits sitting between normal performance and severe cognitive impairment), and a severely impaired group showing global deficits16,17. Although not all studies have identified a relatively intact cluster group21, a recent meta-analysis of relevant studies confirmed that a 3-cluster solution is the most supported outcome (17 of 22 studies reviewed)22. A very limited subset of such studies has included neuroimaging data alongside the cognitive assessments, to examine the relationships between group assignment and brain structure, and these have shown links to the underlying neurobiology. The intact subgroup has less pronounced cortical thinning23,24; conversely, clustering patients according to cortical thickness patterns differentiates those with greater impairment25. On brain volumes, Weinberg, et al.26 found widespread reductions (including significantly reduced total hippocampal, insula, superior temporal sulcus, and frontal) in gray matter volumes in the impaired cluster compared to controls. In contrast, patients in the relatively intact group differed from controls only on inferior parietal volume, whereas the intermediate group had reduced inferior parietal and insula volumes. Only one study has extended the clustering approach to include first-degree relatives15. Across their whole sample of SZH, relatives, and controls, a 3-factor solution was found. Cognitive performance in first-degree relatives, while impaired relative to controls, is not as impaired as that seen in SZH27; accordingly, more than half of the relatives were assigned to the ‘intermediate’ cluster. Between-cluster differences in brain volumes were identified in frontal, temporal, and limbic regions; amongst these, Ohi, et al.15 highlighted anterior cingulate cortex (ACC) volume as an important between-cluster factor since it was largely independent of diagnostic status. However, the Ohi, et al.15 study was limited by fairly small groups of relatives (N = 20) and controls (N = 25); also, by including any first-degree relative, the relatives were significantly older than the other groups as more than half were parents.
The aim of this study was to provide further insights into cognitive subtypes within SZH and their first-degree relatives, and the relationship between subgroup assignment and brain volumes. The study expands upon and addresses shortcomings in the very limited pre-existing literature by utilising a better-matched and larger sample. For the relatives, only SZH-SIB were recruited, and a group of control siblings (CON-SIB) was also utilised to ensure ages were matched. We examined cognitive profiles of SZH and their siblings in comparison to age-matched control groups and then performed a cluster analysis on the cognitive parameters irrespective of the diagnostic group. Then, differences in brain volumes (within a set of predetermined brain regions implicated by previous studies28,29,30,31,32, incorporating frontal, temporal, insula, hippocampus, and 3rd ventricle) were assessed, comparing SZH to CON, and SZH-SIB with CON-SIB. We included the 3rd ventricle, rather than lateral ventricles, as it has been found that 3rd ventricle enlargement but not lateral ventricle enlargement corelates with cognitive deficits in SZH33. Then, cluster assignment was added as an additional covariate in these comparisons, to determine the extent to which between-group differences in brain volumes could be accounted for by cognitive cluster assignment.
Results
Differences in cognitive performance between the clusters
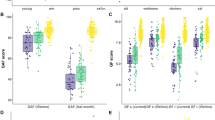
We adopted a k-means clustering approach, using the four cognitive domain measures (adjusted for age and gender) across all participants irrespective of diagnostic group. Three cognitive function clusters were identified (Clusters 1–3). As shown in Fig. 1, the neurocognitive profiles indicated a ‘neuropsychologically normal’ cluster (Cluster 3, N = 88), a cognitively impaired cluster (Cluster 1, N = 78), and an intermediate cluster (Cluster 2, N = 108). To test for gender distribution differences across clusters, we performed a Pearson Chi-Square test. Results revealed no statistically significant differences in distribution, clusters 1, 2 and 3 comprised 50, 48, and 51 male participants respectively. ANOVAs assessed differences in cognitive performance between the clusters. Results showed a main effect of cluster assignment on all cognitive domains (p < 0.001). Post hoc analyses revealed that those in Cluster 1 had poorer performance in all cognitive domains, compared to those in Cluster 2 (p < 0.001) and Cluster 3 (p < 0.001). Cluster 2 also had poorer performance in all domains compared to Cluster 3 (p < 0.001). In follow-up analyses, all results remained unchanged after additionally adjusting for education (ANCOVAs); see supplementary material for full statistics.
Groups (SZH, SZH-SIB, CON, or CON-SIB) were not evenly distributed between the three clusters (Fig. 1 and Table 1, χ2 = 109.19, p < 0.001). SZH were mainly distributed to Cluster 1 (60.8%), followed by Cluster 2 (30.4%), and Cluster 3 (8.8%). SZH-SIB were mainly distributed to Cluster 2 (65.6%), followed by Cluster 3 (21.9%), and Cluster 1 (12.5%). CON-SIB were mainly distributed to Cluster 2 (52.1%), followed by Cluster 3 (37.5%), and Cluster 1 (10.4%). CON were mainly distributed to Cluster 3 (58.7%), followed by Cluster 2 (33.7%), and Cluster 1 (7.6%).
No difference in age was observed between clusters, but the gender balance and years of education were significantly different (see Table 1). Considering just the SZH in each cluster, the mean age, gender ratio, age at onset, duration of illness, chlorpromazine equivalents of total antipsychotics (CPZ-eq), and SAPS/SANS scores in SZH did not differ by cluster (p > 0.05). Years of education in SZH differed significantly by cluster (p < 0.05). SZH in Cluster 1 had lower years of education (p < 0.05).
Differences in cognitive performance between the diagnostic groups
ANOVAs assessed group differences in cognitive performance between CON, CON-SIB, SZH-SIB, and SZH; see Fig. 2. Results revealed significant main effects of the group for all four (age and gender-adjusted) cognitive domain measures (p < 0.001). Post hoc analyses revealed that SZH had poorer performance in all four cognitive domains, differences were significant between SZH compared to SZH-SIB in executive function, working memory, vocabulary (p < 0.05), and episodic memory (p < 0.001). Between SZH and CON, and between SZH and CON-SIB, significantly poorer performance was seen in SZH in all four domains (p < 0.001). (See supplementary material for full statistics).
Compared to CON, SZH-SIB had significantly poorer episodic memory (p = 0.001) and working memory performance (at trend level, p = 0.054). Although there were no statistically significant differences between SZH-SIB and CON-SIB, all the effect sizes were negative (episodic memory, Cohen’s d = −0.41, p = 0.41; executive function, d = −0.21, p = 0.88; working memory; d = −0.36, p = 0.54, vocabulary, d = −0.22, p = 0.77) pointing to lower scores in SZH-SIB compared to CON-SIB. There were no statistically significant cognitive performance differences between CON-SIB and CON in any domain (see Fig. 2).
None of these results were seen to change after re-running the ANCOVAs with education as an additional covariate.
Differences in ICV-Adjusted Volume between the diagnostic groups
Comparisons were conducted using ANCOVA with age and gender as covariates. Follow-up analyses then included cluster assignment as an additional covariate.
SZH vs CON
After FDR correction for multiple comparisons, smaller ICV-adjusted volumes were observed in SZH within bilateral DLPFC, right VLPFC, right superior temporal, bilateral inferior temporal, right middle temporal, and left insula compared to CON (p < 0.05). SZH had larger 3rd ventricle compared to CON (p < 0.05) (Table 2). SZH also had smaller left superior temporal volume (unadjusted P = 0.049), although this did not survive FDR correction. When cluster assignment was then added as an additional covariate, no significant differences were observed between SZH and CON in any ROIs (All FDR-corrected p values > 0.05) (Supplementary Table 1).
SZH-SIB vs. CON-SIB
There were no statistically significant differences between SZH-SIB and CON-SIB in any ROIs (All FDR corrected p values > 0.05) (Table 2). When cluster assignment was then added as an additional covariate, still no significant differences were observed between SZH-SIB and CON-SIB in any ROIs (All FDR corrected p values > 0.05) (Supplementary Table 1).
Differences in ICV-Adjusted Volume between the Clusters
Comparisons were conducted using ANCOVA with age and gender as covariates. Follow-up analyses then included diagnostic group as an additional covariate.
After FDR correction for multiple comparisons, larger ICV-adjusted volume was observed in Cluster 3 within right middle temporal compared to Cluster 1 and Cluster 2 (p < 0.05). Cluster 3 also had larger left middle temporal, bilateral inferior temporal and left insula compared to Cluster 1 and Cluster 2 although this did not survive FDR correction (All unadjusted p values < 0.05). Cluster 3 had smaller 3rd ventricle compared to Cluster 1 (unadjusted P = 0.013), but this did not survive FDR correction. Diagnosis (i.e., CON, CON-SIB, SZH-SIB, or SZH) was then added as an additional covariate. This abolished all between-cluster differences: even at an uncorrected threshold, no differences were observed between the clusters in any ROI (All uncorrected p values > 0.05) (Supplementary Table 2). As a follow-up analysis, we also examined brain volumetric differences between cluster groups for SZH only, using ANOVA. Results revealed that SZH within Cluster 1 (relatively intact group) have significantly larger right VLPFC volume compared to those within Cluster 2 and Cluster 3 (unadjusted p value = 0.041), however, this did not survive FDR correction. Differences in brain volumes between SZH in cluster 1 and cluster 2, assessed by ANCOVA, revealed no significant differences between clusters (all p values > 0.05) (Supplementary Table 4).
Discussion
The current study used a clustering strategy to characterise cognitive subtypes within SZH and their unaffected siblings, investigate brain volume differences between diagnostic groups, and the extent to which cluster assignment could account for these. In all cognitive domains, SZH had poorer performance compared to CON and CON-SIB, in line with evidence that cognitive impairment is a core feature of schizophrenia affecting almost all cognitive domains, including episodic memory34, working memory8, and executive function35. SZH were also impaired relative to SZH-SIB, but with smaller effect sizes. Although no significant differences were seen between SZH-SIB and CON-SIB, across the domains understudy, all effect sizes were in the direction of poorer cognitive performance in SZH-SIB. Likewise, Ohi, et al.15 only found performance differences between first-degree relatives and controls on the attention subscale of the BACS (symbol coding), but effect sizes across other domains were in the direction of poorer performance in relatives. However, other studies have identified more significant impairments on neurocognitive tests in unaffected first‑degree relatives of SZH36,37,38,39. This supports the notion that cognitive impairment is a potential endophenotype of schizophrenia, reflecting a pathophysiological basis of vulnerability to schizophrenia rather than an epiphenomenon of the disease process40,41. Here, although the pattern of effect sizes alluded to impairments, none of the statistical comparisons showed any significant cognitive performance differences between SZH-SIB and CON-SIB in this sample. This might be due to their relatively young age; Barch, et al.42 found cognitive differences in SZH-SIB compared to CON were only detectable in those aged over 21, but not in younger SZH-SIB. Thus, it might be that robust differences only emerge at a slightly later age point than that of our SZH-SIB sample. Barch, et al.42 also concluded that the inconsistency between studies might be due to sampling differences in the unaffected relatives of SZH. Rather than siblings, most previous studies used less strict recruitment criteria: many included any first-degree relative, some included offspring, who could well be impacted by pre-or peri-natal care issues41.
Across the entire sample, we identified three distinct cognitive clusters based on age- and gender-adjusted cognitive domain scores. These clearly showed a ‘neuropsychologically normal’ cluster, a ‘cognitively impaired cluster’, and an ‘intermediate cluster’. As expected, the majority of SZH were assigned to the cognitively impaired cluster while most CON were assigned to the neuropsychologically normal cluster. Less than 10% of SZH were in the neuropsychologically normal cluster. This accords with previous studies showing that around 10% of SZH tend to be classed as neuropsychologically normal based on a clustering analysis14,15,16, although a handful of studies have reported higher percentages: for example, Allen, et al.43 found that 20% of SZH were classed as neuropsychologically normal in their sample. On the other hand, most SZH-SIB were placed in the intermediate cluster, providing evidence of some cognitive impairment affecting SZH-SIB. This tallies with the results of Ohi, et al.15 who also found most relatives to be assigned to the intermediate cluster. The intermediate cluster (Cluster 2) performed worse than previously reported scores in healthy individuals, implying some impairment was present. For example, in WMS-III logical memory, Cluster 2 (mean raw scores: LNS: 9.23, LM-1: 9.90, LM-2: 9.94) performed significantly worse than a large control group (N = 330 mean age of 36, LNS: 12.72) from Cosgrove, et al.44 and performed worse even compared to a healthy older control group (N = 107, mean age = 71, LM-1: 10.77, LM-2: 11.52) from Foley, et al.45 reported average scores of 10.77 and 11.52 for the WMS-III logical memory (1 and 2) subtests in a sample of 107 older controls aged between 55 and 93 (mean age = 70.97). In contrast, our Cluster 2 performed well below these (mean raw scores: LNS: 9.23, LM-1: 9.90, LM-2: 9.94), despite the scores from Foley et al. (all p values < 0.05). Thus, Cluster 2 appears to be intermediately, albeit significantly, impaired.
As expected, significantly smaller brain volumes within many of the ROIs were evident in SZH, in bilateral DLPFC, right VLPFC, right middle and superior temporal, bilateral inferior temporal, and left insula, compared to CON. These results accord with the literature, showing widespread reduced gray matter volume across frontal and temporal brain regions in SZH46,47. In SZH, pronounced gray matter alterations are detectable at the first episode, and are progressive; this has been attributed to processes, including neuroinflammation48, and cumulative antipsychotic exposure49. We also observed larger third ventricles in SZH compared to CON. The ventricular expansion was one of the earliest macroscopic brain structural abnormalities identified in SZH, studies have consistently linked it to volume loss in local cortical/subcortical structures50,51.
Then, we added cognitive cluster as an additional covariate to these analyses. This was seen to abolish all observed volumetric differences between SZH and CON. This highlights the importance of patients’ cognitive status in relation to the brain volume differences associated with schizophrenia: it seems that once cognitive status is accounted for, these differences are significantly diminished. Thus, brain volume reductions in SZH seem to be intimately tied to individual differences in cognitive performance levels, and therefore studies that aim to better characterise brain volume differences in SZH would be advised to consider cognitive performance in addition to the more typical measures of positive and negative symptom severity. Likewise, Ohi, et al.15 also found that adding cognitive cluster as a covariate significantly diminished the brain volume differences between their SZH, first-degree relative, and control samples, although insula and frontal differences remained significant. However it should be noted that Ohi, et al.15 used volume-based morphometry rather than the Freesurfer-based ROI approach used here, and their sample characteristics differed in that they included any first degree relatives (rather than just siblings); this group was significantly older than both their SZH and CON groups, and their CON group was only 25 in number. Nevertheless, both the current study and that of Ohi, et al.15 observed that adding diagnostic group as an additional covariate completely abolished the effects of cognitive cluster on regional volumes. This important finding underlines differences in cognitive profiles as a potent explanatory factor for the abnormalities in brain anatomy seen in schizophrenia. There is considerable heterogeneity in the literature around these abnormalities; the contribution of cognitive performance differences is underappreciated and often unaccounted for in these studies: the current finding supports a recommendation that comprehensive cognitive testing be included in all structural investigations going forward, to allow these effects to be accounted for and add clarity to the field.
No volumetric differences were seen between SZH-SIB and CON-SIB. This is in contrast with previous studies reporting frontotemporal gray matter reductions in unaffected relatives of SZH versus controls52,53, including in inferior and medial frontal cortex54,55,56, and in inferior and superior temporal gyrus57, suggesting a genetic contribution. We did not detect differences, and this could be attributed to sampling differences. In our study, we included only unaffected siblings of SZH as relatives. By contrast, some of the aforementioned studies also recruited offspring of SZH54,57 and those with a history of Axis 1 diagnoses (i.e. mood disorders)53; some studies have also contrasted with a control group at low genetic risk for schizophrenia52. However, familial and genetic bases of structural alterations should be interpreted with caution. Even though some studies have reported frontotemporal volume reduction in relatives, there are also some longitudinal studies showing these reductions are most evident in early adolescence and become less marked thereafter58,59.
The strengths of the current study include better size samples compared to similar previous work, and the inclusion of siblings only, in the unaffected relatives group. Previous studies have often included any first-degree relatives and thus the current sample is more homogenous; the inclusion of age- and gender-matched control siblings as a comparison group helps strengthen confidence in the inferences. However, the current study considered only a limited set of cognitive domains: other cognitive domains that are known to be impaired in SZH such as motor speed, verbal fluency, and social cognition should be investigated in future studies, to determine whether the current findings are generalisable to these. Another limitation is that the SZH sample predominantly consisted of males which is a common issue amongst SZH study samples60,61. Moreover, SZH had lower level of education compared to CON. Although we adjusted all analyses for education, this might have affected results. However, unmatched education level is also a common issue in the SZH literature62,63.
In conclusion, this study provides further insight into the cognitive heterogeneity of SZH, an age- and gender-matched control group, and their siblings. Unlike most previous investigations, groups were well-matched on demographic variables, only siblings were included as relatives, and brain volume measures were considered. Importantly, the observed brain volume differences between SZH and controls disappeared after adjustment for cluster assignment. This shows how closely linked cognitive performance levels are to regional brain volume differences in SZH, and points to the importance of accounting for heterogeneity in cognitive performance within SZH populations by incorporating a comprehensive battery of cognitive measures alongside brain imaging. Previous work has often neglected to do this, and this could contribute to discrepancies and inconsistencies in the literature: such an approach would allow better characterisation of the brain structural abnormalities associated with the disease.
Methods
Subjects
The present study includes data for 102 SZH (32 females, aged between 17 and 61, mean age 33.6), age- and gender-matched 92 CON (41 females, aged between 14 and 66, mean age 30.7), 48 CON-SIB (34 females, aged between 15 and 28, mean age 20.3), and 32 SZH-SIB (18 females, aged between 14 and 28, mean age 21.8, see Table 3). The data for subjects were obtained from the publicly available Northwestern University Schizophrenia Data Sharing for SchizConnect (NUSDAST) database64 and downloaded from http://schizconnect.org website. The Scale for the Assessment of Positive Symptoms (SAPS)65 and Scale for the Assessment of Negative Symptoms (SANS)66 were used to assess symptom severity. All patients were stabilized on antipsychotics for at least two weeks prior to the study67 and doses of medications were converted to chlorpromazine equivalents (see Table 3). The research centre defined exclusion criteria as having an intellectual disability based on DSM-IV, having a severe medical disorder or head injury, and having met the criteria for substance use based on DSM- IV, and written informed consent was obtained from all participants before participation67. More information on data sampling and recruitment has been described elsewhere64,68.
MRI Acquisition
NUSDAST collected MRI scans with a Siemens 1.5 T Vision Scanner. The details of the acquisition process are described elsewhere64,68. Following parameters were defined by the research centre to collect high-resolution T1-weighted structural images using an MPRAGE sequence: TR = 9.7 ms, TE = 4 ms, flip = 10°, ACQ = 1, 256 × 256 matrix, 1 × 1 mm in-plane resolution, 128 slices, slice thickness 1.25 mm, 5:36 min scan time each68. All images were processed via FreeSurfer 3.0.469 and the cortical parcellations were derived based on the Destrieux atlas70. Regional gray matter volumes were derived from the Destrieux parcellation. We calculated intra-cranial (ICV)-adjusted volumes by dividing the volume in each ROI by the total ICV and multiplying by 100 ((volume in ROI/ICV)*100). We then defined the frontal ROI volumes as follows: DLPFC (sum of superior frontal, and rostral and caudal middle frontal), VLPFC (sum of pars opercularis, pars orbitalis, and pars triangularis), OFC (sum of lateral and medial orbital frontal). The temporal ROIs were superior temporal, middle temporal, and inferior temporal. We also included insula volume and hippocampus volume. All the ROIs were assessed separately as left and right-sided.
Cognitive Measures
All participants completed a neuropsychological battery testing executive function, episodic memory, working memory, and vocabulary. Tasks were as follows.
From the Wechsler Memory Scale—Third Edition (WMS-III). Digit Span (total forward and backwards): participants recite back a sequence of numbers in the same order, and in reverse order71. Spatial Span (total forward and backwards): participants are asked to repeat a spatial sequence demonstrated by examiner in the same order (forward) and reverse order (backward)72. Letter Number Sequencing: participants are asked to repeat a mixed list of letters and numbers in alphabetic and ascending orders72. Logical Memory subtest: participants are asked to verbally recall a given story immediately (LM I, immediate version) and after a delay interval (LM II, delayed version)72, and Family Pictures subtest: subjects are shown a series of pictures of scenarios which participants are required to recall72.
Wisconsin Card Sorting Test (WCST)73: subjects sort 64 cards based on the colour, shape, and numbers, perseverative errors occur when a participant persists in using wrong rule despite negative feedback74.
Wechsler Adult Intelligence Scale75. Matrix Reasoning subtest: participants select images for pattern completion76. Vocabulary subtest: subjects define presented words77.
To create the following cognitive domains, we summed z-scores as follows (as recommended by NUSDAST researchers78):
-
Working Memory: Sum of z-scores from Wechsler Memory Scale—Third Edition (WMS-III); including Digit Span (total forward and backwards), Spatial Span (total forward and backwards), and Letter-Number Sequencing
-
Episodic Memory: WMS-III Logical Memory and Family Pictures subtests
-
Executive Function: perseverative errors on the WCST and WAIS-III Matrix Reasoning subtest
-
Vocabulary: scores from the Wechsler Adult Intelligence Scale Vocabulary subtest
Each domain score was converted to standardized z-scores using the mean and SDs of the whole sample and adjusted for age and gender by firstly fitting linear regression models to these (with age and gender as predictors), and then using the residuals from these linear regression models as the basis for subsequent analyses.
ROI Approach
The ROIs focused on fronto-temporal regions since metanalyses have identified consistent volumetric reductions in these in SZH vs. CON. Frontal ROIs were dorsolateral prefrontal and ventrolateral prefrontal regions, the temporal ROIs were superior temporal, middle temporal, and inferior temporal29,31,32. We also included insula volume30 and hippocampus volume28 as these have also been shown to be reduced in SZH compared to CON. Finally, we included 3rd ventricle volume which is consistently shown to be increased in SZH29.
Statistical Analysis
Data were analysed using IBM SPSS Statistics 21.0. P values < 0.05 (two-tailed) were considered statistically significant. On demographic and age- and gender-corrected cognitive domains, groups (within diagnostic groups and clusters separately) were compared using ANOVAs (for age, years of schooling, and cognitive domains), and a χ2 test (for gender) and ANOVAs followed by Tukey’s post hoc tests to assess significant differences among the diagnostic groups and cluster groups. Cohen’s d was used to evaluate standardized effect sizes (https://lbecker.uccs.edu/). The possible effect of the diagnostic group on ICV-adjusted volumes was tested using ANCOVAs in which age and gender were included as covariates. SZH vs CON and SZH-SIB vs CON-SIB comparisons were conducted separately, to ensure results were not confounded by differences in age. This was important given strong ageing effects on brain volumes both in controls79 and schizophrenia80. To assess the extent to which between-groups differences could be accounted for by cognitive cluster assignment, a follow-up analysis included cluster assignment as an additional covariate. To assess differences in SZH brain volumes according to cluster assignment, we compared brain volumes of SZH in cluster 1 with SZH in cluster 2 using ANCOVA, with age and gender as covariates. Because of the small number of cluster 3 (N = 9) in SZH we did not include cluster 3 in this analysis as it is insufficient for ANCOVA.
Multiple comparisons correction was conducted using false discovery rate (FDR) with the Benjamini-Hochberg method81, and p < 0.05 was considered statistically significant. A k-means clustering approach was performed to identify distinct clusters in cognitive performance across all participants, based on all four of the cognitive domain Z-scores (adjusted for age and gender), and independent of diagnostic status. The k-means clustering partitioned the sample into clusters with each participant assigned to the cluster with the nearest mean; a k-means approach was selected so as to be parsimonious with most previous similar investigations, also it is more suitable for continuous variables than hierarchical clustering, which is better suited to categorical variables82. A 3-cluster solution was found to be optimal. A range of cluster solutions (2–5) was tested, and a 3-cluster solution resulted in the largest Gap statistic, optimality was also indicated based on the Elbow Curve. A 3-cluster solution accords with that employed by Ohi, et al.15, and the overwhelming majority of previous studies have also identified a 3-cluster solution22.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Holshausen, K., Bowie, C. R., Mausbach, B. T., Patterson, T. L. & Harvey, P. D. Neurocognition, functional capacity, and functional outcomes: the cost of inexperience. Schizophr. Res. 152, 430–434 (2014).
Keefe, R. S. E., Poe, M., Walker, T. M. & Harvey, P. D. The Relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to Functional Capacity and Real-world Functional Outcome. J. Clin. Exper. Neuropsychol. 28, 260–269 (2006).
Bowie, C. R. & Harvey, P. D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat 2, 531–536 (2006).
Tripathi, A., Kar, S. K. & Shukla, R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 16, 7–17 (2018).
Zhang, B. et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci. Rep. 7, 11821 (2017).
Knowles, E. E., David, A. S. & Reichenberg, A. Processing speed deficits in schizophrenia: reexamining the evidence. Am. J. Psychiatry 167, 828–835 (2010).
Nuechterlein, K. H. et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr. Res. 163, 38–46 (2015).
Forbes, N. F., Carrick, L. A., McIntosh, A. M. & Lawrie, S. M. Working memory in schizophrenia: a meta-analysis. Psychol. Med. 39, 889–905 (2009).
Best, M. W., Milanovic, M., Iftene, F. & Bowie, C. R. A Randomized Controlled Trial of Executive Functioning Training Compared With Perceptual Training for Schizophrenia Spectrum Disorders: Effects on Neurophysiology, Neurocognition, and Functioning. Am. J. Psychiatry 176, 297–306 (2019).
Lencz, T. et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol. Psychiatry 19, 168–174 (2014).
Snitz, B. E., Macdonald, A. W. 3rd & Carter, C. S. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr. Bull. 32, 179–194 (2006).
Habtewold, T. D. et al. Association of schizophrenia polygenic risk score with data-driven cognitive subtypes: A six-year longitudinal study in patients, siblings and controls. Schizophr. Res. 223, 135–147 (2020).
Carruthers, S. P., Van Rheenen, T. E., Gurvich, C., Sumner, P. J. & Rossell, S. L. Characterising the structure of cognitive heterogeneity in schizophrenia spectrum disorders. A systematic review and narrative synthesis. Neurosci. Biobehav. Rev. 107, 252–278 (2019).
Lewandowski, K. E., Sperry, S. H., Cohen, B. M. & Ongür, D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol. Med. 44, 3239–3248 (2014).
Ohi, K. et al. Cognitive clustering in schizophrenia patients, their first-degree relatives and healthy subjects is associated with anterior cingulate cortex volume. NeuroImage. Clin. 16, 248–256 (2017).
Van Rheenen, T. E. et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol. Med. 47, 1848–1864 (2017).
Vaskinn, A. et al. Cognitive Heterogeneity across Schizophrenia and Bipolar Disorder: A Cluster Analysis of Intellectual Trajectories. J. Int. Neuropsychol. Soc. 26, 860–872 (2020).
Wexler, B. E. et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am. J. Psychiatry. 166, 189–195 (2009).
Reser, M. P., Allott, K. A., Killackey, E., Farhall, J. & Cotton, S. M. Exploring cognitive heterogeneity in first-episode psychosis: What cluster analysis can reveal. Psychiatry Res. 229, 819–827 (2015).
Uren, J., Cotton, S. M., Killackey, E., Saling, M. M. & Allott, K. Cognitive clusters in first-episode psychosis: Overlap with healthy controls and relationship to concurrent and prospective symptoms and functioning. Neuropsychology 31, 787–797 (2017).
Fernández-Linsenbarth, I. et al. Neurobiological underpinnings of cognitive subtypes in psychoses: A cross-diagnostic cluster analysis. Schizophr. Res. 229, 102–111 (2021).
Habtewold, T. D. et al. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl. Psychiatry 10, 244 (2020).
Yasuda, Y. et al. Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry Clin. Neurosci.74, 191–203 (2020).
Cobia, D. J., Csernansky, J. G. & Wang, L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr. Res. 133, 68–76 (2011).
Pan, Y. et al. Morphological Profiling of Schizophrenia: Cluster Analysis of MRI-Based Cortical Thickness Data. Schizophr. Bull. 46, 623–632 (2020).
Weinberg, D. et al. Cognitive Subtypes of Schizophrenia Characterized by Differential Brain Volumetric Reductions and Cognitive Decline. JAMA Psychiatry 73, 1251–1259 (2016).
Hill, S. K. et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry 170, 1275–1284 (2013).
Adriano, F., Caltagirone, C. & Spalletta, G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist 18, 180–200 (2012).
Haijma, S. V. et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 39, 1129–1138 (2013).
Saze, T. et al. Insular volume reduction in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 257, 473–479 (2007).
van Erp, T. G. M. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 21, 547–553 (2016).
Wright, I. C. et al. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry 157, 16–25 (2000).
Bornstein, R. A., Schwarzkopf, S. B., Olson, S. C. & Nasrallah, H. A. Third-ventricle enlargement and neuropsychological deficit in schizophrenia. Biol. Psychiatry 31, 954–961 (1992).
Danion, J.-M., Huron, C., Vidailhet, P. & Berna, F. Functional Mechanisms of Episodic Memory Impairment in Schizophrenia. Can. J. Psychiatry 52, 693–701 (2007).
Orellana, G. & Slachevsky, A. Executive functioning in schizophrenia. Front. Psychiatry 4, 35–35 (2013).
Egan, M. F. et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol. Psychiatry 50, 98–107 (2001).
Sitskoorn, M. M., Aleman, A., Ebisch, S. J. H., Appels, M. C. M. & Kahn, R. S. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr. Res 71, 285–295 (2004).
Keshavan, M. et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. 3, 62 (2010).
Harave, V. S. et al. Neurocognitive Impairments in Unaffected First-degree Relatives of Schizophrenia. Indian J. Psychol. Med. 39, 250–253 (2017).
Gur, R. E. et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr. Bull. 33, 49–68 (2007).
Toulopoulou, T., Rabe-Hesketh, S., King, H., Murray, R. M. & Morris, R. G. Episodic memory in schizophrenic patients and their relatives. Schizophr. Res. 63, 261–271 (2003).
Barch, D. M., Cohen, R. & Csernansky, J. G. Altered Cognitive Development in the Siblings of Individuals With Schizophrenia. Clin. Psychol. Sci. 2, 138–151 (2014).
Allen, D. N., Goldstein, G. & Warnick, E. A consideration of neuropsychologically normal schizophrenia. Journal of the Int. Neuropsychol. Soc. 9, 56–63 (2003).
Cosgrove, D. et al. Cognitive Characterization of Schizophrenia Risk Variants Involved in Synaptic Transmission: Evidence of CACNA1C’s Role in Working Memory. Neuropsychopharmacology 42, 2612–2622 (2017).
Foley, J. et al. Pattern of memory compromise in chronic geriatric schizophrenia, frontotemporal dementia and normal geriatric controls. Acta Neuropsychiatr 20, 9–19 (2008).
Fornito, A., Yücel, M., Patti, J., Wood, S. J. & Pantelis, C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr. Res. 108, 104–113 (2009).
Honea, R., Crow, T. J., Passingham, D. & Mackay, C. E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 162, 2233–2245 (2005).
Zhang, Y. et al. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl. Psychiatry 6, e982–e982 (2016).
Liu, N. et al. Characteristics of gray matter alterations in never-treated and treated chronic schizophrenia patients. Transl. Psychiatry 10, 136 (2020).
Gaser, C., Nenadic, I., Buchsbaum, B. R., Hazlett, E. A. & Buchsbaum, M. S. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am. J. Psychiatry 161, 154–156 (2004).
Horga, G. et al. Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 261, 467–476 (2011).
Cannon, T. D. et al. Regional Gray Matter, White Matter, and Cerebrospinal Fluid Distributions in Schizophrenic Patients, Their Siblings, and Controls. Arch. General Psychiatry 55, 1084–1091 (1998).
Mattai, A. A. et al. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry 50, 697–704 (2011).
Sugranyes, G. et al. Gray Matter Volume Decrease Distinguishes Schizophrenia From Bipolar Offspring During Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 54, 677–684.e672 (2015).
Harms, M. P. et al. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. Br. J. Psychiatry 196, 150–157 (2010).
Xiao, Y., Zhang, W., Lui, S., Yao, L. & Gong, Q. Similar and different gray matter deficits in schizophrenia patients and their unaffected biological relatives. Front. Psychiatry 4, 150–150 (2013).
Rajarethinam, R., Sahni, S., Rosenberg, D. R. & Keshavan, M. S. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J. Psychiatry 161, 1121–1124 (2004).
Moran, M. E., Hulshoff Pol, H. & Gogtay, N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain 136, 3215–3226 (2013).
Gogtay, N. & Thompson, P. M. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 72, 6–15 (2010).
Kurtgoz Zorlu, P., Bilgic, O., Sahin, T. & Zorlu, N. Associations of psychiatric symptoms and neurocognition with clinical insight in schizophrenia. Düşünen Adam: J. Psychiatry Neurological Sci. 30, 79–85 (2017).
Sapara, A. et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr. Res. 89, 22–34 (2007).
Brandt, C. L. et al. Assessing brain structural associations with working-memory related brain patterns in schizophrenia and healthy controls using linked independent component analysis. Neuroimage Clin. 9, 253–263 (2015).
Hartberg, C. B. et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 182, 123–133 (2010).
Kogan, A., Alpert, K., Ambite, J. L., Marcus, D. S. & Wang, L. Northwestern University schizophrenia data sharing for SchizConnect: A longitudinal dataset for large-scale integration. Neuroimage 124, 1196–1201 (2016).
Andreasen, N. C. Scale for the assessment of positive symptoms (SAPS). (University of Iowa Iowa City, 1984).
Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. Br J. Psychiatry 155, 49–52 (1989).
Czepielewski, L. S., Wang, L., Gama, C. S. & Barch, D. M. The Relationship of Intellectual Functioning and Cognitive Performance to Brain Structure in Schizophrenia. Schizophr. Bull. 43, 355–364 (2017).
Wang, L. et al. SchizConnect: Mediating neuroimaging databases on schizophrenia and related disorders for large-scale integration. Neuroimage 124, 1155–1167 (2016).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980 (2006).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. A sulcal depth-based anatomical parcellation of the cerebral cortex. Neuroimage 47, https://doi.org/10.1016/S1053-8119(09)71561-7 (2009).
Fink HA, H. L. & MacDonald R. Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality, Appendix D, Neuropsychological Test Descriptions, https://www.ncbi.nlm.nih.gov/books/NBK285344/ (2017).
Tulsky, D. S. Clinical interpretation of the WAIS III and WMS III. (Academic Press, 2003).
Heaton, R., Chelune, G., Talley, J., Kay, G. & Curtiss, G. Wisconsin Card Sorting Test Manual. (Psychological Assessment Resources, 1993).
Dehaene, S. & Changeux, J. P. The Wisconsin Card Sorting Test: theoretical analysis and modeling in a neuronal network. Cereb. Cortex 1, 62–79 (1991).
Wechsler, D. Wechsler Adult Intelligence Scale. 3 edn, (Psychological Corporation, 1997).
Crowe, S. F., Bittner, R. M., Raggl, R. & Senior, G. The Effect of Item Type on Performance of the Matrix Reasoning Subtest of the WAIS-III in Traumatically Brain Injured and Non Brain-injured Control Participants. Brain Impairment. 4, 146–154 (2003).
Silva, M. A. Development of the wais-iii: A brief overview, history, and description. Graduate J. Couns. Psychol. 1, 1–19 (2008).
Wang, L. et al. Northwestern University Schizophrenia Data and Software Tool (NUSDAST). Front. Neuroinformat. 7, 25 (2013).
Scahill, R. I. et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives Neurol. 60, 989–994 (2003).
Cropley, V. L. et al. Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am. J. Psychiatry 174, 286–295 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stati. Soc. Series B (Methodol.) 57, 289–300 (1995).
Kaushik, M. & Mathur, B. Comparative Study of K-Means and Hierarchical Clustering. Techniques. Int. J Software Hardware Res. Eng. 2, 93–98 (2014).
Acknowledgements
The data for subjects were obtained from the publicly available Northwestern University Schizophrenia Data and Software Tool (NUSDAST) database and downloaded from http://schizconnect.org website. As such, the investigators within NUSDAST contributed to the design and implementation of NUSDAST and/or provided data but did not participate in analysis or writing of this report. We thank the NUSDAST for publicly releasing the dataset. Data collection was funded by NIMH grant R01 MH056584 and Conte Center for the Neuroscience of Mental Disorders (P50 MH071616).
Author information
Authors and Affiliations
Contributions
E.A: Writing- Original draft preparation, Formal analysis, Methodology, Conceptualization. S.L.E.: Writing- Original draft preparation, Formal analysis, Methodology, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alkan, E., Evans, S.L. Clustering of cognitive subtypes in schizophrenia patients and their siblings: relationship with regional brain volumes. Schizophr 8, 50 (2022). https://doi.org/10.1038/s41537-022-00242-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00242-y