Abstract
Chronic comorbid conditions are common in adults with asthma, and some may influence a patient’s asthma exacerbation risk. We explored associations between eighteen chronic comorbid conditions and asthma exacerbation occurrence in adults with asthma in a cross-sectional study nested within a cohort study using data from the two-yearly US National Health and Nutrition Examination Survey (NHANES) program. Data of 2387 adults with self-reported doctor-diagnosed current asthma from the 2007 to 2018 NHANES surveys were selected. Investigated chronic comorbidities were: angina pectoris; congestive heart failure; coronary heart disease; depression; diabetes mellitus; soft and hard drug use; gastroesophageal reflux; gout; history of heart attack; history of stroke; hypercholesterolemia; hypertension; kidney failure; liver conditions; obesity; rheumatoid arthritis; and thyroid problems. Outcome was defined as asthma exacerbation category: no, moderate, or severe exacerbation(s) in the past year. Ordinal logistic regression analysis with correction for potential confounders was used to estimate odds ratios (OR) for moderate or severe exacerbations. Observed associations with increased severe asthma exacerbation occurrence were: obesity (OR = 1.67; 95% confidence interval 1.24, 2.26), and rheumatoid arthritis (OR = 1.55; 1.04, 2.30). History of stroke (OR = 1.95; 1.22, 3.11) and rheumatoid arthritis (OR = 1.33; 1.00, 1.75) showed associations with increased moderate exacerbation occurrence. Age-stratified analysis showed soft drug use, obesity, depression, thyroid problems, and rheumatoid arthritis to be associated with moderate and/or severe exacerbation occurrence in one or more 10-year age strata. In conclusion, several chronic comorbid conditions were associated with asthma exacerbation occurrence, which confirms but also complements previous studies. Our observations contribute to understanding exacerbation risk estimation and, ultimately, personalized asthma management.
Similar content being viewed by others
Introduction
Asthma is a common chronic airways disease, with a prevalence of 8.4% among adults in the United States (US) in 20181. The typical chronic airway inflammation is characterized by symptoms of airflow limitation that vary over time and in intensity. Patients with asthma may experience episodic flare-ups (acute exacerbations) of their asthma, which can be life-threatening2. Poor asthma control involves symptoms that can affect activities of daily living and increase the risk of undesirable outcomes, including exacerbations2. Uncontrolled asthma is a considerable cause of asthma morbidity, mortality, and healthcare costs3.
Presence of other chronic morbidities is common in adults with asthma4. In primary care an overall presence of one or more comorbid conditions in 72.8% in female and 65.3% in male asthma patients has been observed5. The current literature provides ambiguous results regarding possible associations between specific chronic comorbidities and increased risk of asthma exacerbations. Multiple studies found overweight and obesity to be associated with increased asthma exacerbation risk6,7,8. Another often studied chronic comorbid condition is gastroesophageal reflux disease (GERD), which is more common in severe asthma compared to mild-to-moderate asthma9. Several studies have reported an association between GERD and worse asthma control6,7,8, although a systematic review found no consistent benefit on asthma symptoms following treatment for gastroesophageal reflux10. Recent studies have shown a higher incidence of cardiovascular diseases (i.e., acute myocardial infarction and ischemic stroke) in patients with asthma11,12 and that lack of asthma control is associated with increased risk of stroke13,14. An association between rheumatoid arthritis and risk of asthma exacerbations has been reported15, whereas depression has shown to be relatively common in exacerbation-prone asthma16. This is explained by brain centers’ functional response to psychological disease and stress and the subsequent stress hormones production from the neuroendocrine system, leading to increased asthma exacerbation risk17.
More insight in the prevalence of chronic comorbid conditions in patients with asthma and, especially, a better understanding of their influence on asthma exacerbation occurrence and thus asthma control may contribute to patient-centered decision making, chronic disease management, and better asthma-related outcomes. Therefore, in this study we investigated (i) the prevalence of eighteen different chronic comorbid conditions and (ii) explored their associations with asthma exacerbation occurrence in a US general population sample of adults with a self-reported diagnosis of asthma. As no specific a priori hypotheses to be tested were formulated, the study should be considered to be hypotheses-generating in nature.
Results
Study population characteristics
Table 1 shows the baseline characteristics of the study population (n = 2387), which consisted for the larger part of females (65.1%) and non-Hispanic whites (40.9%). Among the study population 1344 subjects (56.3%) reported not to have had asthma exacerbations in the past year, 281 (11.8%) reported at least one severe exacerbation, and 762 (31.9%) reported one or more moderate but no severe exacerbations. Statistically significant differences between the three asthma exacerbation categories were observed for sex, race, PIR, and most categories of asthma medication (i.e., SABA, LABA, ICS, OCS, leukotriene modifiers, anticholinergics, and antihistamines). The proportion of subjects using anti-inflammatory medication (i.e., ICS, OCS and leukotriene modifiers) and bronchodilators (i.e., SABA, LABA, and anticholinergics) increased with the severity of the asthma exacerbation category. Age, time since asthma diagnosis, health insurance coverage, and smoking status were not statistically significant different between the exacerbation categories.
Distribution of chronic comorbid conditions in different age categories
Table 2 shows the prevalence of eighteen different chronic comorbid conditions in the total study population and in the three asthma exacerbation categories. Comorbid conditions with an overall prevalence >10% in the study population were obesity (49.6%), depression (34.1%), hypertension (30.3%), hypercholesterolemia (23.9%), diabetes mellitus (15.6%), rheumatoid arthritis (13.8%), and soft drug use (11.4%). Figure 1 shows the prevalence distribution of the chronic comorbid conditions in the different age categories. Prevalence of most conditions increased with age, except for soft drug use which showed a decreasing trend with age. Depression showed a high prevalence in all age categories, with rates around 30%. Cardiovascular conditions showed an increasing trend with age, with the highest prevalence in the ≥71 years age category for hypertension, hypercholesterolemia, congestive heart failure, coronary heart disease, and heart attack. The age category 61–70 years showed the highest prevalence of angina pectoris and stroke. Figure 2 shows the frequency distribution of the cumulative number of chronic comorbid conditions in the different age groups, which gradually increased with age. The majority of the study subjects in the age categories up to 50 years had two or less other chronic conditions, while in the two oldest age categories approximately 65% of subjects suffered from three or more.
Chronic comorbidities associated with asthma exacerbation occurrence
Table 3 shows the results of the univariable and multivariable ordinal regression analyses for the associations between the respective chronic comorbid conditions and asthma exacerbation category. The multivariable analyses showed the following chronic comorbid conditions to be associated with an increased odds of having severe asthma exacerbations: obesity (OR = 1.67, 95% CI 1.24 to 2.26) and rheumatoid arthritis (OR = 1.55, 95% CI 1.04 to 2.30). Conditions associated with increased odds of moderate asthma exacerbations were history of stroke (OR = 1.95, 95% CI 1.22 to 3.11) and rheumatoid arthritis (OR = 1.33, 95% CI 1.00 to 1.75).
Table 4 shows the odds ratios from the univariable and multivariable ordinal regression analyses split in the different age categories. In the youngest age category (18–30 years) soft drug use was associated with a decreased odds of severe exacerbations (OR = 0.40, 95% CI 0.17, 0.97). In subjects aged 31–40 years obesity (OR = 2.01, 95% CI 1.15 to 3.53) and depression (OR = 2.25, 95% CI 1.32, 3.81) were associated with increased odds of moderate exacerbations. In subjects aged 41 to 50 years obesity was associated with increased odds of severe asthma exacerbations (OR = 3.07, 95% CI 1.31 to 7.19), whereas in those aged 51 to 60 years or 61 to 70 years this was true for thyroid problems (OR = 4.49, 95% CI 1.69 to 11.95) and rheumatoid arthritis (OR = 2.39, 95% CI 1.04 to 5.47), respectively. In subjects aged 71 years or older no comorbid conditions were found to be associated with moderate or severe exacerbations.
Discussion
In this retrospective cohort study based on the NHANES program we explored associations between chronic comorbid conditions and the occurrence of moderate and severe exacerbations in adult subjects with a self-reported diagnosis of asthma drawn from the US general population. We found that obesity and rheumatoid arthritis were statistically significant associated with the odds of severe asthma exacerbations and that rheumatoid arthritis was associated with the odds of moderate asthma exacerbations, as was a history of stroke. Stratification of the study sample in age categories revealed specific associations between certain comorbid conditions and asthma exacerbation occurrence. For example, soft drug use was especially high in younger subjects (i.e., those aged between 18 and 30 years) and associated with a reduced occurrence of severe exacerbations, while depression was associated with an increased occurrence of moderate asthma exacerbations in subjects aged 31–40 years.
A link between obesity and risk of asthma exacerbations has been reported in several previous studies6,7,8,18. Our observed association between rheumatoid arthritis and (moderate as well as severe) asthma exacerbations is consistent with a recent study from Luo et al.15. A possible mechanism could be that patients with rheumatoid arthritis have a dysregulated immune system and often receive treatment with immunosuppressive medication, which may lead to a higher risk of acute respiratory infections (ARI)19, a known trigger for asthma exacerbations2. The observed association in our study of a history of stroke and increased asthma exacerbation occurrence is again in line with previous studies13,14,18. Underlying mechanisms may be that asthma patients with a history of stroke are not only more prone to ARI leading to asthma exacerbation, but also to systemic vascular inflammation with platelet activation, inhibition of fibrinolysis, and elevation of C-reactive protein levels resulting in cardiovascular events20. Besides, increased stroke risk may also be the result of increased atrial fibrillation risk in uncontrolled asthma11, as a result of asthma exacerbation management with β2-agonists, discontinuation of β-blockers and discontinuation of aspirin in patients with aspirin-exacerbated respiratory disease14. In our study, a history of stroke was associated with moderate exacerbations, but not with severe exacerbations. The limited number of subjects in the severe exacerbations category and thus a Type 2 error may explain this.
In the age category of 31–40 years, we found that symptoms of depression were associated with moderate asthma exacerbations. A meta-analysis based on prospective cohort studies strongly suggests that depression significantly increased the risk of asthma exacerbation16. However, we did not observe this association in the total study population nor in the other age categories. Possible explanations for this are that we used a questionnaire-based (PHQ-9) definition for depressive symptoms in the past two weeks instead of a clinical diagnosis of depression, or that previous studies have mainly included asthma patients in this particular age range. Conceivable mechanisms underlying the interaction between depression and asthma exacerbation are that depression may accompany behavior changes in terms of less help-seeking and non-adherence to medication but may also contribute to risk behaviors resulting in smoking, physical inactivity, and obesity16. Furthermore, cerebral changes in depression play a possible role in asthma symptoms21 and long-term stress stimulation may influence airway inflammation and asthma severity17.We also observed a statistically significant association between thyroid problems and severe asthma exacerbations in subjects aged between 51 and 60 years. This observation is difficult to interpret since we cannot determine, based on the data available, what the specific underlying thyroid problems were. Nonetheless, a recent study has reported a close relationship between thyroid hormone levels and severity of asthma in older adults22, providing a possible explanation for the observed association in our study.
In our study current soft drug use in the 18 to 30 years age group showed a statistically significant association with a decreased occurrence of severe asthma exacerbations. This is in contrast with a 2017 review, which found no or insufficient evidence for a statistical association between cannabis smoking and asthma exacerbation23. One possible explanation for our deviating finding could be that younger subjects who have sufficiently controlled asthma may be more likely to smoke cannabis or marijuana than those whose asthma is less well controlled. Another explanation might be that we did not include the most prevalent allergic disease in the younger population (allergic rhinitis and atopic dermatitis) in the study.
A clear strength of our study is that we investigated a large sample of 2387 subjects with doctor-diagnosed asthma in a study population that is presumably representative for the asthma population in the United States. The data we used is from the well-established NHANES program, which routinely collects data every two years using validated measures and questionnaire administration by trained medical personnel24. The data collection in NHANES is rather extensive and robust, which guarantees high-quality data regarding participants’ asthma exacerbations, comorbid conditions, smoking habits, and other risk factors for asthma exacerbations (e.g., PIR and asthma preventer medication use). We also consider the differentiation between moderate and severe exacerbations and the age-stratified analysis of chronic comorbid conditions in relation to asthma exacerbation occurrence strengths of our study, as this provides a more detailed insight compared to pooling all exacerbations and all age groups together.
On the other hand, the main limitation is that this cross-sectional study is depending on self-report of participants, which may have caused self-reporting bias to occur. For instance, 228 participants answered affirmatively on the question about once having received a diagnosis of chronic bronchitis, but that the diagnosis is not active anymore. These participants were not excluded from the study, but incorrect affirmative answers may have led to some subjects who also suffered from COPD (i.e., asthma-COPD overlap) to be included in the study sample. Ideally all NHANES participants would have had an extensive respiratory assessment to establish whether or not asthma (and/or COPD) was present25. Besides, the most reflective definition for moderate and severe asthma exacerbations could not be derived from the data: an episode of progressive increase in asthma symptoms that required a change in treatment2, with ideally health-record derived additional information about the medical management and the in- or outpatient setting during this episode. Thirty-one participants denied having had an episode of asthma worsening in the previous year, but at the time same indicated to have visited an emergency room due to their asthma. We categorized these subjects in the severe asthma exacerbation category, but misclassification may have occurred as the actual validity of the NHANES survey questions on occurrence of exacerbations (i.e., ‘During the past 12 months, have you had an episode of asthma or an asthma attack?’ and ‘During the past 12 months, have you had to visit an emergency room (ER) or urgent care center because of asthma?’) may be limited and phrasing these questions as asthma being ‘worse’ or ‘out of control’ instead may be a better option26.
No information to verify the chronic nature of certain comorbid conditions (e.g., gout and depression) could be extracted from the data, leading to the possible inclusion of single episodes of the conditions instead of truly chronic conditions. The use of a PHQ-9-based definition for depressive symptoms as a surrogate for clinical depression is also a limitation, as a questionnaire-based definition and an actual clinical diagnosis of depression do not necessarily coincide27. Finally, a notable observation was that ICS were used by a relatively small proportion of the study sample (13.6%), which is quite a bit lower than the 30.8% in the CDC’s nationwide BRFSS Asthma Call-back Survey in active asthma28. Although questionnaire-based self-reports of asthma diagnoses are widely used in population surveys29,30, the relatively low rate of ICS use does raise some concern about the validity of participants’ asthma diagnoses in the NHANES survey data. On the other hand inadequate asthma therapy among adults has been demonstrated to be an important issue in the US31 and elsewhere32. The high use of SABA combined with the low use of ICS by subjects in the moderate and severe exacerbations categories fits the observed increased exacerbation occurrence33.
It would have been interesting to determine whether allergic rhinitis, chronic sinusitis, atopic dermatitis and obstructive sleep apnoea are also associated with asthma exacerbations, but data on these conditions is not available in the NHANES database. A final limitation is that there was no measure to determine asthma control available, like—for instance—the Asthma Control Test (ACT)34.
In conclusion, in this study obesity and rheumatoid arthritis were associated with the occurrence of severe asthma exacerbations, whereas rheumatoid arthritis and a history of stroke were associated with the occurrence of moderate asthma exacerbations. Age-stratified analysis showed soft drug use, obesity, depression, thyroid problems, and rheumatoid arthritis to be associated with moderate and/or severe exacerbation occurrence in one or more 10-year age strata. These findings confirm but also complement the current body of knowledge about the role that specific chronic comorbid conditions may have in exacerbation-prone asthma. Health professionals involved in asthma management should be aware of other chronic conditions their asthma patients may have and incorporate this information in their exacerbation risk estimation, chronic disease management, and personalized asthma care.
Methods
Study design and study population
We performed a cross-sectional study nested in a cohort study based on the 2007–2018 data from the two-yearly National Health and Nutrition Examination Survey (NHANES), a program of studies from the US National Center for Health Statistics designed to assess the health and nutritional status of adults and children in the United States24. The survey comprises an extensive interview questionnaire on demographic and health-related issues and a range of physical examinations (physician’s exam, height, weight, and other body measures; blood pressure measurement; bone density measurement; liver ultrasound; lab tests on blood and urine, among other clinical assessments). The questionnaire and measurements are administered by trained medical personnel. Random selection of participants aims to produce a representative study sample that reflects the US population24.
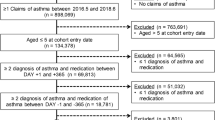
From the consecutive NHANES datasets we selected individuals with a self-reported diagnosis of asthma using the question: ‘Has a doctor ever told you that you have asthma?’ (n = 8590; Fig. 3). From this group we selected subjects aged 18 years or older with an affirmative response to the question ‘Do you still have asthma?’. Subjects who reported to have received a healthcare provider-diagnosis of chronic obstructive pulmonary disease (COPD) or emphysema or reported that they still had chronic bronchitis were excluded because these respiratory conditions are also accompanied by exacerbations. The final study population consisted of 2387 adults with a self-reported asthma diagnosis.
All NHANES survey cycles are approved by the National Center for Health Statistics Research Ethics Review Board. The protocols of the 2007 until 2018 surveys are available at https://www.cdc.gov/nchs/nhanes/irba98.htm. All participants provided informed consent.
Asthma exacerbation categories
The study population was divided into three ‘asthma exacerbation categories’, based on two questions: (i) ‘During the past 12 months, have you had an episode of asthma or an asthma attack?’ and (ii) ‘During the past 12 months, have you had to visit an emergency room (ER) or urgent care center because of asthma?’. Study subjects with negative replies to both questions were classified as ‘no asthma exacerbation’, a positive answer to question (i) and a negative answer to question (ii) as ‘moderate asthma exacerbation’, and a positive answer to question (ii) as ‘severe asthma exacerbation’ (i.e., independent of the reply to question (i)). These three asthma exacerbation categories constituted the single response variable for the statistical analysis.
Chronic comorbidities
The NHANES data allowed us to look at eighteen chronic comorbid conditions: angina pectoris; congestive heart failure; coronary heart disease; diabetes mellitus; gastroesophageal reflux-like symptoms (GERS); gout; history of heart attacks; history of stroke; hypercholesterolemia; hypertension; kidney failure; liver disease; obesity; rheumatoid arthritis; and thyroid problems (see Supplementary Table 1 for operationalization). We defined these conditions as being chronic based on their nonreversible nature and/or the increased lifetime risk of occurrence of recurrent episodes. Because depression, soft drug (i.e., marijuana or hashish) use and hard drug (cocaine, heroin, or methamphetamine) use can be recurrent we decided to consider these as chronic conditions too.
Depression was assessed using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) validated Patient Health Questionnaire-9 (PHQ-9), a screening instrument for frequency of depressive symptoms over the past two weeks. Depression was defined as ‘mild depression’ (PHQ-9 score 5 to 9), or ‘moderate to severe depression’ (PHQ-9 score ≥10)35.
Gastroesophageal reflux-like symptoms (GERS) were defined using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes from study subjects’ prescription medication use in the last month prior to the survey: ‘heartburn’ (R12), ‘gastro-esophageal reflux disease’ (K21), ‘gastric ulcer’ (K25), ‘peptic ulcer, site unspecified’ (K27), and ‘functional dyspepsia’ (K30). These data were available for three survey cycles (2013–2014, 2015–2016, and 2017–2018).
Obesity was defined as Body Mass Index (BMI) ≥ 30 kg/m2.
Demographic, smoking, and asthma treatment data
We also extracted the following information from the NHANES survey data for the included study subjects: sex; age at the time of screening; race; time since asthma diagnosis; health insurance coverage; poverty income ratio; asthma-related medication; and cigarette smoking status, the latter categorized as current, former, and never smoker. A subject was considered a current smoker when (s)he reported active smoking in the year prior to the survey and having smoked at least 100 cigarettes in his/her lifetime. Poverty to income ratio (PIR), an indicator of socioeconomic status, divides family income by the poverty threshold. PIR was categorized as income at or below the poverty line ( ≤ 1) or above the poverty line ( >1)36.
We identified the following asthma-related prescription medication use in the past month: inhaled corticosteroids (ICS); short-acting β2-agonists (SABA); long-acting β2-agonists (LABA); anticholinergics; antihistamines; leukotriene modifiers; oral corticosteroids (OCS); and other (i.e., cromolyn, epinephrine, immunomodulator medication, phosphodiesterase-4 (PDE4) inhibitors, and unspecified anti-asthmatic combinations, -respiratory agents, and -respiratory inhalant products).
Statistical analysis
We compared baseline characteristics between the ‘no asthma exacerbation’, ‘moderate asthma exacerbation’, and ‘severe asthma exacerbation’ categories using one-way ANOVA for normally distributed, and Kruskal-Wallis tests for nonnormally distributed continuous variables. Chi-square and Fisher’s exact tests were used to compare categorical variables. Variables with counts below eight in one or more exacerbation categories were not statistically tested. No correction for multiple comparisons was applied. We calculated prevalence rates for the eighteen chronic comorbid conditions studied and the occurrence of the total number of chronic comorbid conditions across age categories.
To analyze associations between each separate comorbid condition and asthma exacerbation categories (the response variable) we used univariable and multivariable ordinal logistic regression models to estimate odds ratios for the moderate exacerbations category relative to the no exacerbations category, and for the severe exacerbations category relative to the no exacerbations category. For the multivariable analysis we started with a full model with all available potential confounders. Backward elimination was used to drop covariates with p > 0.1 from the ordinal logistic regression model. We consider the final (i.e., reduced) multivariable models to be the main results of the analysis. All statistical analyses were performed using SPSS statistical software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp). A two-sided p-value below 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data supporting the results reported in the article can be found at https://wwwn.cdc.gov/nchs/nhanes/. The SPSS syntax files used to analyze to dataset are available on request to the corresponding author.
References
Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (Date accessed: June 12, 2023).
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2023 Update). Available from: www.ginasthma.org.
Lee, L. K., Ramakrishnan, K., Safioti, G., Ariely, R. & Schatz, M. Asthma control is associated with economic outcomes, work productivity and health-related quality of life in patients with asthma. BMJ Open Respir. Res. 7, e000534 (2020).
Cazzola, M., Rogliani, P., Ora, J., Calzetta, L. & Matera, M. G. Asthma and comorbidities: recent advances. Pol. Arch. Intern Med. 132, 16250 (2022).
Veenendaal, M. et al. Age- and sex-specific prevalence of chronic comorbidity in adult patients with asthma: a real-life study. NPJ Prim. Care Respir. Med. 29, 14 (2019).
Denlinger, L. C. et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am. J. Respir. Crit. Care Med. 195, 302–313. (2017).
Nyenhuis, S. M., Akkoyun, E., Liu, L., Schatz, M. & Casale, T. B. Real-world assessment of asthma control and severity in children, adolescents, and adults with asthma: relationships to care settings and comorbidities. J. Allergy Clin. Immunol. Pr. 8, 989–996.e1 (2020).
Althoff, M. D., Ghincea, A., Wood, L. G., Holguin, F. & Sharma, S. Asthma and three colinear comorbidities: obesity, OSA, and GERD. J. Allergy Clin. Immunol. Pr. 9, 3877–3884 (2021).
Shaw, D. E. et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur. Respir. J. 46, 1308–1321 (2015).
Chan, W. W., Chiou, E., Obstein, K. L., Tignor, A. S. & Whitlock, T. L. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch. Intern. Med. 171, 620–629 (2011).
Cepelis, A. et al. Associations of asthma and asthma control with atrial fibrillation risk: results from the nord-trondelag health study (HUNT). JAMA Cardiol. 3, 721–728 (2018).
Carter, P. et al. Association of cardiovascular disease with respiratory disease. J. Am. Coll. Cardiol. 73, 2166–2177 (2019).
Cepelis, A. et al. Asthma, asthma control and risk of ischemic stroke: the HUNT study. Respir. Med. 2, 100013 (2020).
Raita, Y. et al. Risk of acute myocardial infarction and ischemic stroke in patients with asthma exacerbation: a population-based, self-controlled case series study. J. Allergy Clin. Immunol. Pr. 8, 188–194.e8 (2020).
Luo, Y. et al. Rheumatoid arthritis is associated with increased in-hospital mortality in asthma exacerbations: a nationwide study. Clin. Rheumatol. 37, 1971–1976 (2018).
Zhang, L. et al. Co-morbid psychological dysfunction is associated with a higher risk of asthma exacerbations: a systematic review and meta-analysis. J. Thorac. Dis. 8, 1257–1268 (2016).
Vafaee, F., Shirzad, S., Shamsi, F. & Boskabady, M. H. Neuroscience and treatment of asthma, new therapeutic strategies and future aspects. Life Sci. 292, 120175 (2022).
Tomisa, G., Horvath, A., Santa, B., Keglevich, A. & Tamasi, L. Epidemiology of comorbidities and their association with asthma control. Allergy Asthma Clin. Immunol. 17, 95 (2021).
Subesinghe, S., Rutherford, A. I., Byng-Maddick, R., Leanne Hyrich, K. & Benjamin Galloway, J. Recurrent serious infections in patients with rheumatoid arthritis-results from the British Society for Rheumatology Biologics Register. Rheumatol. (Oxf.) 57, 651–655 (2018).
Xu, M., Xu, J. & Yang, X. Asthma and risk of cardiovascular disease or all-cause mortality: a meta-analysis. Ann. Saudi Med. 37, 99–105 (2017).
Wang, L. et al. Cerebral anatomical changes in female asthma patients with and without depression compared to healthy controls and patients with depression. J. Asthma 51, 927–933 (2014).
Bingyan, Z. & Dong, W. Impact of thyroid hormones on asthma in older adults. J. Int. Med. Res. 47, 4114–4125 (2019).
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan Available from: https://www.ncbi.nlm.nih.gov/books/NBK423845/, https://doi.org/10.17226/24625
Centers for Disease Control and Prevention. About National Health and Nutrition Examination Survey 2017, September 15. [Cited June 12, 2023]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
Bouwens, J. D. M., Bischoff, E. W. M. A., In ‘t Veen, J. C. C. M. & Schermer, T. R. Diagnostic differentiation between asthma and COPD in primary care using lung function testing. NPJ Prim. Care Respir. Med 32, 32 (2022).
Australian Centre for Asthma Monitoring. Survey questions for monitoring national asthma indicators. 2007. URL: https://www.aihw.gov.au/getmedia/b4cf5413-133a-446f-a4ad-a839f695da42/sqfmnai.pdf.aspx?inline=true.
Gotlib, I. H., Lewinsohn, P. M. & Seeley, J. R. Symptoms versus a diagnosis of depression: differences in psychosocial functioning. J. Consult Clin. Psychol. 63, 90–100 (1995).
Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. BRFSS Prevalence & Trends Data [online]. 2015. [Accessed June 12, 2023]. https://www.cdc.gov/brfss/brfssprevalence/.
Oh, H., Stickley, A., Singh, F. & Koyanagi, A. Self-reported asthma diagnosis and mental health: findings from the collaborative psychiatric epidemiology surveys. Psychiatry Res. 271, 721–725 (2019).
Molarius, A. & Hasselgren, M. Socioeconomic status, lifestyle factors and asthma prevalence: results from a population-based study in Sweden. Scand. J. Public Health 51, 561–569 (2023).
Ofoma, U. R., Lehman, E. & Sciamanna, C. Undertreated and uncontrolled asthma among US adults: findings from a national sample. Am. J. Respir. Crit. Care Med. 185, A5690 (2012).
Nolte, H., Nepper-Christensen, S. & Backer, V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir. Med. 100, 354–362 (2006).
Lugogo, N. et al. Real-world patterns and implications of short-acting beta(2)-agonist use in patients with asthma in the United States. Ann. Allergy Asthma Immunol. 126, 681–689.e1 (2021).
Nathan, R. A. et al. Development of the asthma control test: a survey for assessing asthma control. J. Allergy Clin. Immunol. 113, 59–65 (2004).
Patel, P. O., Patel, M. R. & Baptist, A. P. Depression and asthma outcomes in older adults: results from the national health and nutrition examination survey. J. Allergy Clin. Immunol. Pr. 5, 1691–1697.e1 (2017).
Greenblatt, R. E. et al. Factors associated with exacerbations among adults with asthma according to electronic health record data. Asthma Res. Pr. 5, 1 (2019).
Acknowledgements
We are grateful to the NHANES participants and staff members for providing and collecting the data. The current study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The National Health and Nutrition Examination Survey (NHANES) is funded by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention. Various US federal agencies help fund the survey. We thank Reinier Akkermans of the Department of Primary and Community Care of the Radboud University Medical Center for his statistical advice.
Author information
Authors and Affiliations
Contributions
T.S. designed the study. E.B. downloaded and prepared the NHANES survey data for further analysis. E.B. and T.S. analyzed the data. E.B., H.L., L.v.d.B., and T.S. interpreted the results of the analyses. E.B. and T.S. were the major contributors in writing the manuscript, H.L. and L.v.d.B. commented on a draft version of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baljet, E., Luijks, H., van den Bemt, L. et al. Chronic comorbid conditions and asthma exacerbation occurrence in a general population sample. npj Prim. Care Respir. Med. 33, 29 (2023). https://doi.org/10.1038/s41533-023-00350-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-023-00350-x