Abstract
Primary care physicians (PCPs) play a crucial role in the diagnosis and management of chronic obstructive pulmonary disease (COPD). By working together with patients to target exertional breathlessness and increase physical activity, PCPs have an important role to play, early in the disease course, in improving patient outcomes in both the short and long term. In this article, we consider how physical activity affects disease progression from the PCP perspective. We discuss the role of pharmacological therapy, the importance of an holistic approach and the role of PCPs in assessing and promoting physical activity. The complexity and heterogeneity of COPD make it a challenging disease to treat. Patients’ avoidance of activity, and subsequent decline in capacity to perform it, further impacts the management of the disease. Improving patient tolerance of physical activity, increasing participation in daily activities and helping patients to remain active are clear goals of COPD management. These may require an holistic approach to management, including pulmonary rehabilitation and psychological programmes in parallel with bronchodilation therapy, in order to address both physiological and behavioural factors. PCPs have an important role to optimise therapy, set goals and communicate the importance of maintaining physical activity to their patients. In addition, optimal treatment that addresses activity-related breathlessness can help prevent the downward spiral of inactivity and get patients moving again, to improve their overall health and long-term prognosis.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic disease and a major health problem worldwide1. The hallmark of COPD is airflow limitation, and patients suffer with persistent respiratory symptoms, such as cough, sputum production and dyspnoea (also referred to as breathlessness1). This can lead to a reduction in physical activity in the early stages of COPD2.
Primary care physicians (PCPs), who play a crucial role in the diagnosis and management of COPD, face a number of challenges in the diagnosis of the disease. These include a lack of specific symptoms, the failure of patients to report symptoms, the presence of multiple chronic conditions, inadequate training of physicians, nurse practitioners and physician assistants, and a lack of access to spirometry3. Lack of patient awareness regarding the disease also contributes to delayed diagnosis; the early symptoms of COPD, such as breathlessness and reduced exercise tolerance, are often attributed by patients to aging4,5.
PCPs experience several challenges with patients who already have a confirmed diagnosis of COPD, including (a) non-adherence to prescribed therapies, which is common in patients with COPD due to the chronic nature of the condition and the use of multiple medications6; (b) inhaler selection, which can be challenging given the wide range of different inhalation devices available7; (c) poor inhaler technique, which is often responsible for sub-optimal treatment of COPD, making device training essential8,9; (d) different perceptions between patients and physicians as to how COPD affects daily living; (e) different perceptions between younger and older patients on the impact of the disease on their quality of life10; and (f) challenges with smoking cessation1.
One of the most common symptoms of COPD is breathlessness. Breathlessness is distressing and debilitating and has a severe impact on all aspects of patients’ lives, preventing them from participating in certain daily activities11,12. Reducing physical activity is a natural and socially accepted way of avoiding symptoms, and there are several factors that affect engagement in physical activity. These include low motivation, correlated with fear of breathlessness and comorbidity13, and sociodemographic factors, such as age, race and social support14. Limiting physical activity due to breathlessness, sometimes referred to as “exertional breathlessness”, leads to a downward spiral of muscle wastage and further reductions in activity, ultimately leading to poor patient outcomes such as reduced quality of life and premature mortality15,16,17. Exacerbations are also associated with worse breathlessness, a reduction in exercise capacity and muscle weakness18.
By working together with patients to target exertional breathlessness and increase physical activity, PCPs have an important role to play (especially early in the disease course) in improving patient outcomes in both the short and long term. Agreement between PCPs and patients on the importance of physical activity may improve management of COPD19. In this educational narrative review, we consider the PCP perspective on physical activity and how it affects disease progression. We discuss the role of pharmacological therapy, the importance of an holistic approach (including pulmonary rehabilitation and psychological programmes) and the role of PCPs in managing physical activity.
Physical activity and exercise capacity: predictors of poor outcomes in COPD
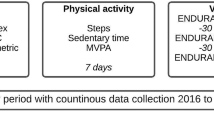
Individuals with COPD frequently limit their activity because of activity-related breathlessness, often early in the disease course2,20,21,22,23. During physical activity, as a patient’s breathing rate and tidal volume increases, the time for expiration shortens and the degree of gas trapping worsens. This is typically assessed by demonstrating a reduction in inspiratory capacity during exercise. This phenomenon is called “dynamic hyperinflation” and is a key cause of the feeling of breathlessness during physical activity24. This process begins in the early stages of COPD and worsens with disease progression12. As such, sedentary behaviour occurs across the spectrum of COPD severity, from mild to very severe, and has negative implications for patients’ prognosis2,25 (Fig. 1).
Physical activity refers to the range of bodily movements that a patient undertakes as part of their normal daily routine. Low physical activity is an independent predictor for exacerbations, hospital admissions and mortality in patients with COPD26,27,28,29. In fact, it is one of the strongest predictors of all-cause and disease-specific mortality in COPD30,31. In addition, maintaining physical activity has been noted to decrease depression and anxiety over time32. Exercise capacity is a related concept that refers to the maximum extent of physical movement that a patient is capable of undertaking, but it does not necessarily correlate with levels of physical activity. A reduction in exercise capacity is also a predictor of poor outcomes, such as mortality and decreased quality of life, in patients with COPD33,34.
Sustaining physical activity is an important component of preventative medicine. Even if the progression of COPD prevents patients from increasing their level of physical activity, preserving exercise capacity allows them to maintain activity levels for longer before they become limited by exertional breathlessness. Within clinical trials, symptoms and exercise capacity are often measured, but there are less data on physical activity for evaluation. It is key that PCPs are aware of their patients’ health status and level of deconditioning before taking measures to improve physical activity. To facilitate this, assessment frameworks can help to profile patients and guide their referral to exercise-based care programmes—including ones that involve physiotherapy or more specialised pulmonary rehabilitation35.
Tools to measure physical activity and exercise capacity
Regular measurement of physical activity in patients with COPD by PCPs, both initially and at follow-up, is vital in helping patient adherence to physical activity programmes36. Many different tools are available to PCPs to evaluate the functional status of their patients, including their ability to sustain physical activity and their overall exercise capacity. Some physical activity questionnaires, such as the International Physical Activity Questionnaire—Short Form (IPAQ-SF), are non-specific to COPD. The accuracy of these instruments in assessing physical activity is variable; for example, a review of studies that used the IPAQ-SF questionnaire showed only weak evidence as a predictor of relative or absolute physical activity37. The Physical Activity Scale for the Elderly is another widely used questionnaire, which has been shown to be a valid measure of physical activity, health and physical function in elderly patients in epidemiology studies38. Further activity questionnaires available include the Stanford Seven-day Physical Activity Recall Questionnaire39 and the Yale Physical Activity Survey40; however, these questionnaires may be more appropriate in a research environment than a primary care setting.
Specific tools to measure physical activity in COPD have also been produced, such as the London Chest Activity of Daily Living scale41 and the PROactive instrument42. The London Chest Activity of Daily Living scale41 is a questionnaire that assesses the impact of dyspnoea on activities of daily living in patients with severe COPD. However, it may be less practical for PCPs in their day-to-day practice due to the time it takes to complete and the difficulty of evaluating its results. Activity monitoring using wearable devices such as the PROactive instrument39 is not common in the primary care setting, but this may change with the evolution of digital healthcare. In addition, the Spanish Physical Activity Questionnaire in COPD has been specifically designed for an easy measurement of physical activity in patients with COPD during daily clinical practice43.
PCPs may also refer patients to a physiotherapist for assessment of functional impairments and determination of the most appropriate intervention to improve physical activity and exercise tolerance44. Physical activity can also be assessed objectively using pedometers. Pedometers can provide feedback on daily activities and allow patients to track their own progress45.
More practical approaches to measure functional performance in primary care include the British Medical Research Council dyspnoea questionnaire and the functional status domain of the Clinical COPD Questionnaire (CCQ)46. The British Medical Research Council dyspnoea questionnaire is a simple tool that grades the effect of breathlessness on daily activities47. However, its responsiveness to change is limited. The CCQ is another reliable yet quick and easy-to-use tool, consisting of ten items that cover symptoms and the functional and mental state of the patient48. The CCQ has been used in assessing interventions such as pulmonary rehabilitation, and is responsive to change, making it suitable for longitudinal use48.
Overall, although there are a number of tools available to measure physical activity and exercise capacity in patients with COPD, simple objective measures and quick and reliable questionnaires to capture patients’ symptoms and functional state are the most suitable for use in primary care.
The role of bronchodilators on physical activity in COPD: review of data on activity-related endpoints with long-acting bronchodilators
One of the cornerstones of COPD management is effective pharmacological treatment to reduce symptoms and exacerbations as well as improving exercise tolerance and health status1. A systematic review and meta-analysis of 22 studies found that long-acting bronchodilators (either as monotherapy or in combination) increase exercise capacity in patients with COPD49. The authors of the review noted that this appears to be mainly due to an increase in inspiratory capacity rather than a modification of dynamic hyperinflation during exercise49. Several studies have demonstrated the benefits of long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) dual therapy in improving lung function and health-related quality of life, and in reducing symptoms, in patients with COPD50. Umeclidinium/vilanterol has been shown to reduce breathlessness versus placebo and its monocomponents51. Although randomised trial data evaluating exercise endurance showed that umeclidinium/vilanterol improves measures of lung function, hyperinflation and health status versus placebo, the effects on exercise endurance were variable52,53. Glycopyrronium/indacaterol has been shown to reduce hyperinflation and improve physical activity levels versus placebo, including increasing peak inspiratory capacity, activity-related energy expenditure and average number of steps per day54. It has also been demonstrated that aclidinium/formoterol improves breathlessness and overall night-time and early-morning symptom severity versus placebo and monocomponents55. Clinical trials have demonstrated that tiotropium/olodaterol improves breathlessness56,57 (including during exercise tests58), lung hyperinflation59 and inspiratory capacity58,60 compared with its monocomponents, and activity-related breathlessness and breathing discomfort versus placebo60. This combination has also been shown to increase exercise capacity during walking and cycling tests60,61 versus placebo and improve physical activity in treatment-naive patients, as indicated by reductions in breathlessness and in the amount of time spent in a sedentary position59,62,63,64,65. Notably, in a multicentre, multinational clinical trial, improvements from baseline in exercise endurance time for both cycling (19%) and walking (20%) were observed in patients with COPD following 6 weeks of treatment with tiotropium/olodaterol66. Overall, in clinical trials, long-acting bronchodilators are associated with improvements in exercise-related endpoints, with dual bronchodilation generally better than monotherapy.
Real-world studies
In addition, several real-world studies suggest that bronchodilators have a beneficial effect on physical activity. In terms of monotherapy, the ON-AIR real-world evidence study evaluated the effects of the LAMA aclidinium bromide on quality of life, symptom severity and daily activity impairment in patients with COPD67. Aclidinium therapy improved quality of life, as demonstrated by reductions in mean COPD Assessment Test™ score, and reduced the severity of night-time and early-morning symptoms67. At least moderate impairment in performance of daily activities due to COPD symptoms was reported by 59.5% of patients at enrolment, improving to 38.7% after 12 weeks of aclidinium treatment67. Tiotropium improves physical functioning, with 61.5% of patients achieving an improvement of ≥10 points in the Physical Function subdomain after 6 weeks of treatment68. Furthermore, dual bronchodilation with tiotropium/olodaterol has been shown to improve physical function in patients with COPD62,63,64. This combination has also been shown to improve patients’ general condition and ability to manage their daily routines within 6 weeks of treatment63,64. In addition, a further open-label, non-interventional study demonstrated improvements in clinical health status, measured using the CCQ, in patients with COPD taking tiotropium/olodaterol in routine clinical practice69.
The main role of bronchodilators on physical activity in COPD: what do the guidelines say?
Long-acting bronchodilators are central to the treatment of COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 strategy report recommends starting with a LAMA or LABA as first-line maintenance therapy for most patients with COPD1. For highly symptomatic patients, LAMA plus LABA dual therapy should be considered at initiation of pharmacological treatment, whereas LABA plus inhaled corticosteroid (ICS) should be considered in patients with previous exacerbations (≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalisation) and with blood eosinophil levels ≥300 cells/µL1. Indeed, many patients do remain symptomatic on LABA or LAMA monotherapy70. In line with this, the American Thoracic Society (ATS) strongly recommends LAMA plus LABA dual therapy over LABA or LAMA monotherapy in patients who experience breathlessness or exercise intolerance, based on improved critical outcomes with dual therapy71. Similarly, the United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines recommend (a) LAMA plus LABA (no ICS) for patients without asthmatic features/features suggesting steroid responsiveness who remain breathless or have exacerbations despite smoking cessation treatment, (b) optimised non-pharmacological management and (c) use of a short-acting bronchodilator72.
The GOLD, ATS and NICE recommendations1,71,72 reflect the growing awareness of the importance to treat patients effectively from the initiation of treatment while also individualising COPD therapy according to the needs of the patient. Considering this guidance, PCPs should be aiming to optimise both non-pharmacological management and pharmacological therapy at an early stage of the disease1,71,72,73, which should increase physical activity and lead to improved prognosis for patients with COPD.
Importance of an holistic approach, including pharmacological treatment, pulmonary rehabilitation and psychological programmes
Increasing physical activity and reducing discomfort during physical activity requires an holistic approach. In addition to the pharmacological approaches discussed above, this should include smoking cessation programmes, pulmonary rehabilitation and psychological programmes74,75. Non-pharmacological treatment should consider all aspects of the disease, including mental, physical and emotional health, as well as social implications. Pulmonary rehabilitation is a comprehensive, evidence-based, low-cost treatment intervention that has been proven to improve breathlessness, inactivity and quality of life74,75. It is recommended for those patients who have persistent symptoms of COPD and reduced physical activity and who have otherwise not improved through optimal medical management76. Exercise training offers benefits that are complementary to the effects gained from pharmacotherapy. Together, pharmacotherapy and rehabilitation enhance health-related quality of life and exercise tolerance and reduce exacerbation rates in patients with COPD77. In addition, improving physical activity and exercise tolerance calls for a multidisciplinary approach that is patient-centred. Involving physiotherapy within a patient’s treatment plan has shown positive effects on physical tolerance and levels of dyspnoea78. However, access to pulmonary rehabilitation can also be an issue, suggesting the need for restructuring of healthcare resources for COPD in the primary care setting79 (Fig. 2).
In addition to the improvements provided by pulmonary rehabilitation and exercise programmes, psychological programmes support the change in behaviour that is needed for patients to increase their activity80. For pulmonary rehabilitation and psychological programmes to be successful, PCPs should first ensure that pharmacological management is optimised according to treatment guidelines as an initial step1,71,72. In one study, a combination of self-management behaviour modification (SMBM) and tiotropium/olodaterol, with or without exercise training, significantly improved exercise endurance time and breathlessness during daily life in patients with COPD compared with SMBM plus placebo81. Physical inactivity in COPD can be affected not only by lung function but also by extra-pulmonary comorbidities82. Therefore, effective management of comorbidities is also crucial to help maintain patients’ activity levels.
Role of PCPs
PCPs have a key role in the diagnosis and management of patients with COPD83,84. Encouragement of smoking cessation is a primary goal; it is also important to ensure that influenza vaccinations are kept up to date84. Since the vast majority of patients with COPD may suffer from breathlessness on exertion85, regular treatment with dual bronchodilators is important to relieve symptoms and improve exercise capacity71. PCPs should also support their patients in maintaining or increasing physical activity, which is essential for patient quality of life, ensuring patients understand that their breathlessness, though it may feel debilitating, can be treated. PCPs should encourage their patients to attend pulmonary rehabilitation and should try to help in overcoming any barriers in their participation. Additionally, PCPs need to ensure education on inhaler technique is given at regular intervals during the patient’s follow-up to maintain an optimal treatment strategy. To increase inhaler adherence, it is important to discuss and assess this regularly with the patient.
As PCPs may be responsible for measuring and monitoring patients’ functional status, it is important to consider what is feasible in the primary care setting46. There are several tools that are available, each with their own benefits and drawbacks. Although the 6-min walking test is the most reliable test to measure exercise capacity in COPD, it is not very practical for the primary care setting46. The physical activity questionnaires presented in the previous section can also be useful, especially the more COPD-specific ones. Indeed, sustained improvements in health status (measured using the CCQ) and exercise capacity have been shown in the primary care setting over 2 years in patients with COPD, emphasising the importance of regular monitoring and follow-up by PCPs to optimise outcomes86.
PCPs should discuss personal physical activity goals with their patients, as well as different approaches to meet these goals. Studies have shown that doing activities that are enjoyable promotes physical activity in daily life, suggesting that activities that boost motivation may help patients with COPD to stay active13. Strategies may include identifying which activities patients can undertake in the home and discussing options for individuals without access to exercise equipment (e.g. use of water bottles instead of weights). For example, home-based breathing exercises, such as diaphragmatic breathing, yoga breathing, breathing gymnastics and singing, can have significant effects on pulmonary function, respiratory muscle strength, exercise capacity, breathlessness and health-related quality of life in patients with COPD87. The ATS/European Respiratory Society official statement on pulmonary rehabilitation reinforces that home-based exercises have been proven to effectively reduce dyspnoea and increase exercise performance in patients with COPD88. In addition, some patients may be considered for referral to multicomponent rehabilitation programmes. The patient should be made aware of anything their local communities are setting up, such as programmes with physiotherapists and walking groups, to incorporate social interaction into their rehabilitation.
Conclusions
The complexity and heterogeneity of COPD make it a challenging disease to treat, and patients’ avoidance of activity, and subsequent decline in capacity to perform, further impact the management of the disease. Improving patient tolerance of physical activity, increasing participation in daily activities and helping patients to remain active are clear goals of COPD management. These require an holistic approach to management, including smoking cessation, pulmonary rehabilitation and psychological programmes in parallel with bronchodilator therapy, in order to address both physiological and behavioural factors. PCPs have an important role to play in optimising therapy, setting goals and relaying the importance of maintaining physical activity to their patients. In addition, optimal treatment that addresses activity-related breathlessness can help prevent the downward spiral of inactivity and get patients moving again to improve their overall health and long-term prognosis.
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report). https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (2020).
Troosters, T. et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir. Med. 104, 1005–1011 (2010).
Yawn, B. P. & Wollan, P. C. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int. J. Chronic Obstr. Pulm. Dis. 3, 311–317 (2008).
Fromer, L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int. J. Gen. Med. 4, 729–739 (2011).
Labaki, W. W. & Han, M. K. Improving detection of early chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 15, S243–S248 (2018).
Restrepo, R. D. et al. Medication adherence issues in patients treated for COPD. Int. J. Chronic Obstr. Pulm. Dis. 3, 371–384 (2008).
Dhand, R., Cavanaugh, T. & Skolnik, N. Considerations for optimal inhaler device selection in chronic obstructive pulmonary disease. Clevel. Clin. J. Med. 85, S19–S27 (2018).
Yawn, B. P., Colice, G. L. & Hodder, R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int. J. Chronic Obstr. Pulm. Dis. 7, 495–502 (2012).
Kocks, J. W. H. et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim. Care Respir. Med. 28, 43 (2018).
Dekhuijzen, R., Hass, N., Liu, J. & Dreher, M. Daily impact of COPD in younger and older adults: global online survey results from over 1300 patients. COPD https://doi.org/10.1080/15412555.2020.1788526 (2020).
Hutchinson, A., Barclay-Klingle, N., Galvin, K. & Johnson, M. J. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur. Respir. J. 51, 1701477 (2018).
Hanania, N. A. & O’Donnell, D. E. Activity-related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. Int. J. Chronic Obstr. Pulm. Dis. 14, 1127–1138 (2019).
Sritharan, S. S. et al. Barriers toward physical activity in COPD: a quantitative cross-sectional, questionnaire-based study. COPD https://doi.org/10.1080/15412555.2021.1922371 (2021).
Dragnich, A. G. et al. Sociodemographic characteristics and physical activity in patients with COPD: a 3-month cohort study. COPD https://doi.org/10.1080/15412555.2021.1920902 (2021).
Ramon, M. A. et al. The dyspnoea-inactivity vicious circle in COPD: development and external validation of a conceptual model. Eur. Respir. J. 52, 1800079 (2018).
Casaburi, R. Activity promotion: a paradigm shift for chronic obstructive pulmonary disease therapeutics. Proc. Am. Thorac. Soc. 8, 334–337 (2011).
O’Donnell, D. E., Hamilton, A. L. & Webb, K. A. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J. Appl. Physiol. 101, 1025–1035 (2006).
Alahmari, A. D. et al. Physical activity and exercise capacity in patients with moderate COPD exacerbations. Eur. Respir. J. 48, 340–349 (2016).
Small, M. et al. Physician-patient concordance in pharmacological management of patients with COPD. COPD 12, 473–483 (2015).
Van Helvoort, H. A., Willems, L. M., Dekhuijzen, P. R., Van Hees, H. W. & Heijdra, Y. F. Respiratory constraints during activities in daily life and the impact on health status in patients with early-stage COPD: a cross-sectional study. NPJ Prim. Care Respir. Med. 26, 1–7 (2016).
Van Remoortel, H. et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax 68, 962–963 (2013).
Sanchez-Martinez, M. P. et al. Patterns and predictors of low physical activity in patients with stable COPD: a longitudinal study. Ther. Adv. Respir. Dis. 14, 1753466620909772 (2020).
Watz, H., Waschki, B., Meyer, T. & Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 33, 262–272 (2009).
Garcia-Rio, F. et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am. J. Respir. Crit. Care Med. 180, 506–512 (2009).
Waschki, B. et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 192, 295–306 (2015).
Fan, V. S., Ramsey, S. D., Make, B. J. & Martinez, F. J. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD 4, 29–39 (2007).
Pitta, F. et al. Physical activity and hospitalization for exacerbation of COPD. Chest 129, 536–544 (2006).
Garcia-Aymerich, J., Lange, P., Benet, M., Schnohr, P. & Antó, J. M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 61, 772–778 (2006).
Moy, M. L., Gould, M. K., Liu, I.-L. A., Lee, J. S. & Nguyen, H. Q. Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res. 2, 00062–02015 (2016).
Waschki, B. et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 140, 331–342 (2011).
ZuWallack, R. & Esteban, C. Understanding the impact of physical activity in COPD outcomes: moving forward. Eur. Respir. J. 44, 1107–1109 (2014).
Tselebis, A. et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr. Dis. Treat. 12, 297–328 (2016).
Oga, T., Nishimura, K., Tsukino, M., Sato, S. & Hajiro, T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am. J. Respir. Crit. Care Med. 167, 544–549 (2003).
Zeng, G.-S. et al. The relationship between steps of 6MWT and COPD severity: a cross-sectional study. Int. J. Chronic Obstr. Pulm. Dis. 14, 141–148 (2019).
Spruit, M. A. et al. Profiling of patients with COPD for adequate referral to exercise-based care: the Dutch model. Sports Med. 50, 1421–1429 (2020).
Liao, S.-Y., Benzo, R., Ries, A. L. & Soler, X. Physical activity monitoring in patients with chronic obstructive pulmonary disease. Chronic Obstr. Pulm. Dis. 1, 155–165 (2014).
Lee, P. H., Macfarlane, D. J., Lam, T. H. & Stewart, S. M. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Act. 8, 115 (2011).
Washburn, R. A., McAuley, E., Katula, J., Mihalko, S. L. & Boileau, R. A. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J. Clin. Epidemiol. 52, 643–651 (1999).
Garfield, B. E. et al. Stanford Seven-Day Physical Activity Recall questionnaire in COPD. Eur. Respir. J. 40, 356–362 (2012).
Kruskall, L. J., Campbell, W. W. & Evans, W. J. The Yale Physical Activity Survey for older adults: predictions in the energy expenditure due to physical activity. J. Am. Dietetic Assoc. 104, 1251–1257 (2004).
Garrod, R., Bestall, J. C., Paul, E. A., Wedzicha, J. A. & Jones, P. W. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL). Respir. Med. 94, 589–596 (2000).
Gimeno-Santos, E. et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 46, 988–1000 (2015).
Soler-Cataluña, J. J. et al. Creation of the SAQ-COPD questionnaire to determine physical activity in COPD patients in clinical practice. Arch. Bronconeumol. 54, 467–475 (2018).
Garrod, R. & Lasserson, T. Role of physiotherapy in the management of chronic lung diseases: an overview of systematic reviews. Respir. Med. 101, 2429–2436 (2007).
Mendoza, L. et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur. Respir. J. 45, 347–354 (2015).
Kocks, J. W., Asijee, G. M., Tsiligianni, I. G., Kerstjens, H. A. & van der Molen, T. Functional status measurement in COPD: a review of available methods and their feasibility in primary care. Prim. Care Respir. J. 20, 269–275 (2011).
Bestall, J. C. et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 54, 581–586 (1999).
Kon, S. S. et al. The Clinical COPD Questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax 69, 793–798 (2014).
Di Marco, F. et al. Long-acting bronchodilators improve exercise capacity in COPD patients: a systematic review and meta-analysis. Respir. Res. 19, 18 (2018).
Malerba, M. et al. Single inhaler LABA/LAMA for COPD. Front. Pharmacol. 10, 390 (2019).
Celli, B. et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 145, 981–991 (2014).
Riley, J. H. et al. Effects of umeclidinium/vilanterol on exercise endurance in COPD: a randomised study. ERJ Open Res. 4, 00073–02017 (2018).
Maltais, F. et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. Ther. Adv. Respir. Dis. 8, 169–181 (2014).
Watz, H., Mailänder, C., Baier, M. & Kirsten, A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (the MOVE Study). BMC Pulm. Med. 16, 95 (2016).
Bateman, E. D. et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir. Res. 16, 92 (2015).
Buhl, R. et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur. Respir. J. 45, 969–979 (2015).
Singh, D. et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir. Med. 109, 1312–1319 (2015).
Maltais, F. et al. Dual bronchodilation with tiotropium/olodaterol further reduces activity-related breathlessness versus tiotropium alone in COPD. Eur. Respir. J. 53, 1802049 (2019).
Beeh, K. M. et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm. Pharmacol. Therapeutics 32, 53–59 (2015).
O’Donnell, D. E. et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur. Respir. J. 49, 1601348 (2017).
Maltais, F. et al. Effect of 12 weeks of once-daily tiotropium/olodaterol on exercise endurance during constant work-rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 12, 1–13 (2018).
Sauer, R. et al. Impact of tiotropium + olodaterol on physical functioning in COPD: results of an open-label observational study. Int. J. Chronic Obstr. Pulm. Dis. 11, 891–898 (2016).
Steinmetz, K. O. et al. Assessment of physical functioning and handling of tiotropium/olodaterol Respimat® in patients with COPD in a real-world clinical setting. Int. J. Chronic Obstr. Pulm. Dis. 14, 1441–1453 (2019).
Valipour, A. et al. Improvement of self-reported physical functioning with tiotropium/olodaterol in Central and Eastern European COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 14, 2343–2354 (2019).
Takahashi, K. et al. Tiotropium/olodaterol improves pulmonary function and physical activity in patients with treatment-naïve COPD. Am. J. Respir. Crit. Care Med. 201, A2552 (2020).
Maltais, F., O’Donnell, D. E., Hamilton, A., Zhao, Y. & Casaburi, R. Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial. Ther. Adv. Respir. Dis. 14, 1753466620926858 (2020).
Kostikas, K. et al. A real-world observational study examining the impact of aclidinium bromide therapy on the quality of life, symptoms, and activity impairment of patients with chronic obstructive pulmonary disease: the Greek ON-AIR study. Int. J. Chronic Obstr. Pulm. Dis. 15, 515–526 (2020).
Rau-Berger, H., Mitfessel, H. & Glaab, T. Tiotropium Respimat® improves physical functioning in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 5, 367–373 (2010).
Valipour, A. et al. Late Breaking Abstract - Real-world evidence of dual bronchodilator therapy using Clinical COPD Questionnaire in 4700 COPD patients. Eur. Respir. J. 56, 5231 (2020).
Price, D. et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int. J. Chronic Obstr. Pulm. Dis. 9, 889–904 (2014).
Nici, L. et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 201, e56–e69 (2020).
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. https://www.nice.org.uk/guidance/ng115/resources/chronic-obstructive-pulmonary-disease-in-over-16s-diagnosis-and-management-pdf-66141600098245 (2018).
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: non-pharmacological management and use of inhaled therapies. https://www.nice.org.uk/guidance/ng115/resources/visual-summary-treatment-algorithm-pdf-6604261741, (2019).
Anastasaki, M. et al. Establishing a pulmonary rehabilitation programme in primary care in Greece: a FRESH AIR implementation study. Chron. Respir. Dis. 16, 1479973119882939 (2019).
Simony, C., Riber, C., Bodtger, U. & Birkelund, R. Striving for confidence and satisfaction in everyday life with chronic obstructive pulmonary disease: rationale and content of the tele-rehabilitation programme > C☺PD-Life>>. Int. J. Environ. Res. Public Health 16, 3320 (2019).
Román, M. et al. Efficacy of pulmonary rehabilitation in patients with moderate chronic obstructive pulmonary disease: a randomized controlled trial. BMC Fam. Pract. 14, 21 (2013).
Troosters, T., Gosselink, R., Janssens, W. & Decramer, M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur. Respir. Rev. 19, 24–29 (2010).
Dimitrova, A., Izov, N., Maznev, I., Vasileva, D. & Nikolova, M. Physiotherapy in patients with chronic obstructive pulmonary disease. Open Access Maced. J. Med. Sci. 5, 720–723 (2017).
Arne, M., Emtner, M., Lisspers, K., Wadell, K. & Stallberg, B. Availability of pulmonary rehabilitation in primary care for patients with COPD: a cross-sectional study in Sweden. Eur. Clin. Respir. J. 3, 31601 (2016).
Bourbeau, J. et al. Behaviour-change intervention in a multicentre, randomised, placebo-controlled COPD study: methodological considerations and implementation. BMJ Open 6, e010109 (2016).
Troosters, T. et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 198, 1021–1032 (2018).
Mantoani, L. C., Dell’Era, S., MacNee, W. & Rabinovich, R. A. Physical activity in patients with COPD: the impact of comorbidities. Expert Rev. Respir. Med. 11, 685–698 (2017).
Levy, M. L. et al. International Primary Care Respiratory Group (IPCRG) guidelines: diagnosis of respiratory diseases in primary care. Prim. Care Respir. J. 15, 20–34 (2006).
Bellamy, D. et al. International Primary Care Respiratory Group (IPCRG) guidelines: management of chronic obstructive pulmonary disease (COPD). Prim. Care Respir. J. 15, 48–57 (2006).
Worth, H. et al. The ‘real-life’ COPD patient in Germany: the DACCORD study. Respir. Med. 111, 64–71 (2016).
Kruis, A. L. et al. Sustained effects of integrated COPD management on health status and exercise capacity in primary care patients. Int. J. Chronic Obstr. Pulm. Dis. 5, 407–413 (2010).
Lu, Y. et al. Effects of home-based breathing exercises in subjects with COPD. Respir. Care 65, 377–387 (2020).
Spruit, M. A. et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 188, e13–e64 (2013).
Acknowledgements
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Francesca Lomas of MediTech Media (Manchester, UK), based on a draft provided by the authors, their feedback and under their conceptual direction.
Author information
Authors and Affiliations
Contributions
M.R.-R. and J.W.H.K. developed the outline, made substantial contributions in drafting the article and critically revised the content. M.R.-R. and J.W.H.K. provided final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
M.R.-R. reports grants and personal fees from AstraZeneca and GSK, and personal fees from Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Pfizer, Teva, Trudell and Bial outside the submitted work. J.W.H.K. reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim and GSK, grants and personal fees from Chiesi Pharmaceuticals and Teva, grants from Mundipharma, and personal fees from MSD and Covis Pharma outside the submitted work. He also holds 72.5% of shares in the General Practitioners Research Institute.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Román-Rodríguez, M., Kocks, J.W.H. Targeting exertional breathlessness to improve physical activity: the role of primary care. npj Prim. Care Respir. Med. 31, 41 (2021). https://doi.org/10.1038/s41533-021-00254-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-021-00254-8