Abstract
Parkinson disease (PD) has become one of the most rapidly growing causes of disability among the older population and social isolation is a major concern in the PD community. However, the relationship between social isolation and future risk of PD remains unclear. This study included 192,340 participants aged 60 or older who were free of dementia and PD at baseline from the UK Biobank study. Social isolation was measured using a composite score derived from three questions on number in household, frequency of friend/family visits, and leisure/social activities. Incident PD cases were identified through electronic health records. Multivariable-adjusted Cox regression models were used to compute the hazard ratio (HR) and 95% confidence interval (CI). Among the 192,340 participants (mean [standard deviation] age, 64.2 [2.9] years; 103,253 [53.7%] women), 89,075 (46.3%) participants were in the least isolated group and 26,161 (13.6%) were in the most isolated group. Over a median follow-up of 12.5 years, 2048 incident PD cases were documented. Compared to the least isolated group, the multivariable-adjusted HRs (95% CIs) for PD were 1.00 (0.91−1.10) for the moderately isolated group and 1.19 (1.05−1.36) for the most isolated group (P-trend = 0.04). The observed association was independent of the genetic susceptibility to PD and consistent in subgroup analyses. Social isolation was associated with a higher risk of PD regardless of genetic risk. Our findings highlighted the importance of developing screening and intervention strategies for social isolation among older adults to reduce the risk of PD.
Similar content being viewed by others
Introduction
Parkinson disease (PD) is a chronic progressive neurodegenerative disease characterized by symptoms such as tremor, rigidity, and bradykinesia, which is the second most common neurodegenerative disease1. In addition, PD has become one of the most rapidly growing causes of disability among the older population in the world, with a significant economic burden borne by both individuals and health systems2. Although symptomatic therapies can help patients to maintain good quality of life for several years, therapies to modify or reverse the disease do not exist. Therefore, to identify the modifiable risk factors is paramount to prevent or delay the development of PD.
Social isolation, defined as a lack of meaningful social connections3, is an increasing public health concern among older adults. In the United States, it is estimated that over 20% of the older adults do not have social connections with friends or family4. Experience of social isolation has been linked to a higher risk of depression, dementia, cardiovascular disease, and premature death5,6,7,8,9,10. Among patients with PD, social isolation was associated with worsened severity, decreased quality of life and elevated mortality rates11,12. However, the relationship between social isolation and the risk of developing PD remains unclear. We hypothesized that social isolation could be a risk factor of PD in the present study.
To address the research gaps, we examined the prospective association between different levels of social isolation and risk of PD, leveraging data from the UK Biobank study, which represents one of the largest community-based cohort studies. Additionally, as PD is influenced by both genetic and environment risk factors13,14, we also investigated whether the association between social isolation and PD could be modified by genetic determinants of PD.
Results
Baseline characteristics
Among the 192,340 participants included in the present analysis (53.7% women), the mean age [SD] was 64.2 [2.9] years. Of all the study participants, 26,161 (13.6%) were defined as the most isolated, 77,104 (40.1%) were in the moderately isolated category and 89,075 (46.3%) were in the least isolated category. Distributions of baseline characteristics of the study population according to social isolation status are presented in Table 1. Compared with the least isolated group, participants in the most isolated group were more likely to be men, deprived, non-White, current smokers, never drinkers, and sleep deprived. They also tended to have a higher prevalence of pre-existing hypertension, diabetes, cardiovascular disease, and chronic kidney disease.
Association of social isolation and risk of PD
During a median of 12.5 (IQR,11.7–13.2) years of follow-up, 2048 incident PD events were documented. Compared with the least isolated group, the HRs (95% CIs) of PD were 1.01 (0.92–1.11) for moderately isolated group and 1.23 (1.08–1.40) for most isolated group (P-trend = 0.01) after adjusting for the social demographic characteristics in Model 1. The corresponding estimates were slightly attenuated to 1.00 (0.91–1.10) and 1.19 (1.05–1.36) after the adjustment for lifestyle factors and comorbidities in Model 2 (P-trend = 0.04; Table 2).
The weighted GRS was significantly associated with PD. With each unit increment of the weighted GRS, the HR (95% CI) of PD was 1.07 (1.05–1.08) with the adjustment for age and sex (Supplementary Table 3). In addition, for each additional unit increase in the social isolation score, the fully adjusted HR (95% CI) of PD was 1.08 (1.02–1.15) (Supplementary Table 4).
Secondary analyses and sensitivity analyses
In the stratified analysis by genetic susceptibility to PD, comparing the most isolated with the least isolated groups, the HR (95% CI) of PD was 1.28 (1.07–1.52) among individuals at high genetic risk of PD. In contrast, the association was significantly attenuated and not significant among the participants at low genetic risk of PD, although P for interaction did not reach statistical significance (P-interaction = 0.51; Table 3).
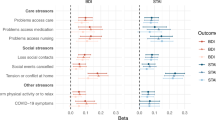
In the component analysis, frequency of friend/family visits was significantly associated with a higher risk of PD. Compared to individuals having friend/family visits about once a week to almost daily, the HR (95% CI) of PD was 1.12 (1.00–1.25) for those with no friends/family to about once a month (Supplementary Table 5). In addition, we did not find significant heterogeneity in the risk estimates of social isolation, and the stratified factors including age, sex, smoking status, BMI, history of hypertension, diabetes, and cardiovascular disease on the risk of PD (Ps-interaction > 0.05; Fig. 1).
HRs were adjusted for age at recruitment (continuous, years), sex (men, women), education (college or university degree, others), Townsend Deprivation Index (continuous), ethnicity (White, others), body mass index (continuous, kg/m2), alcohol intake (never or special occasions, monthly to weekly, daily), smoking status (never, past, current), sleep duration (≤6, 7–8, ≥9 hours/day), and history of hypertension, cardiovascular disease, diabetes, cancer, and chronic kidney disease (yes, no), except for the stratified factors. The hazard ratios are indicated by the circles and squares, and the 95% confidence intervals are shown by the error bars.
Our results remained robust when we excluded participants with less than two years of follow-up (Supplementary Table 6), or on the dataset with multiple imputation method (Supplementary Table 7), or with the additional adjustment for C-reactive protein, loneliness, and physical activity (Supplementary Table 8).
Discussion
Our data from this population-based study of 192,340 participants aged 60 years or older showed that the most social isolation was associated with a higher risk of developing PD. The observed association was independent of the social demographic factors, lifestyles, chronic conditions, and the genetic susceptibility to PD.
Our results complement the body of work on associations of social isolation with a broad spectrum of health outcomes. In the UK Biobank study and Million Women Study with 938,558 participants, social isolation was associated with a higher risk of coronary heart disease (HR: 1.86; 95% CI: 1.63–2.12) and stroke events (HR: 1.91; 95% CI: 1.48–2.46) that resulted in death without an associated hospital admission7. Social isolation was also associated with a higher risk of incident heart failure in the UK Biobank study (most vs. least social isolation, HR: 1.17; 95% CI: 1.11–1.23), and the observed association was independent of the genetic risk of heart failure6. Further, the National Health and Aging Trends Study representing 5,705,675 survey-weighted intensive care unit hospitalizations, assessed the social isolation using 6 questions corresponding to domains including social connectedness, contacts with family and friends, membership in a religious organization, and participation in other community groups. The study found that a higher score of social isolation was associated with a higher risk of post-intensive care unit disability and mortality9. In addition, social isolation was associated with 1.26-fold increased risk of developing all-cause dementia and individuals who were socially isolated had lower volumes of gray matter15.
The component analysis showed that the frequency of friend/family visits was significantly associated with the risk of developing PD, which suggests that among the metrics of social isolation, the frequency of friend/family visits may be an independent risk factor of PD. Similarly, a cross-sectional study also showed that in individuals with PD, those with more friends had 21% fewer symptoms of PD than those with no friends11. However, future studies with prospective design and more cases are needed to clarify the associations between items of social isolation and risk of development and progression of PD.
Although the exact mechanism underlying the relationship between social isolation and risk of PD remains to be further elucidated, we speculated several potential mechanisms to explain the observed association. First, social isolation may contribute to PD development through dysregulations of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system16,17. Further, social isolation was associated with increased systemic and neuro-inflammation and oxidative stress, which play a critical role in the development and progression of PD18,19,20. In addition, social disconnection can induce mitochondrial dysfunction, and increasing evidence has highlighted the mitochondrial dysfunction as a critical driver of PD pathology21,22,23. Finally, social isolation can modulate social inequality and further restrict people from seeking social support and health care resources, which may lead to poor management of risk factors and prodromal symptoms of PD24. Nevertheless, more studies are warranted to further illuminate the potential mechanisms through which social isolation plays a role in the development of PD.
PD is a rising public health issue. The number of people who are living with PD and the resulting disability and deaths are increasing faster than any other neurodegenerative disorders25. It is well-known that clinical PD is preceded by a long prodromal period before the onset of the classic motor features26. Therefore, it is paramount to identify the risk factors and eliminate/management the risk factors for PD prevention. Social isolation is a major issue among older adults. The National Health and Aging Trends Study showed that 24% of the adults aged 65 or older reported to be socially isolated in 20114. A latest meta-analysis including 30 investigations during the COVID-19 reported that the prevalence of social isolation was 31.2% among older adults27. Our findings, if confirmed, may contribute towards the scientific basis for the development of public health strategies that targeted on eliminating and improving social isolation status for PD prevention among the older adults.
The strengths of this study include its prospective study design, large sample size, long-term follow-up, and meticulous adjustment for a wide range of potential confounding factors. However, our results should be interpreted in the context of several potential limitations. First, the self-reported data on social isolation was subject to measurement error and some misclassification was inevitable. However, given the prospective study design, such misclassification could have underestimated the true associations between social isolation and risk of PD. In addition, the questions used to derive social isolation in the UK Biobank have not been validated; however, social isolation in the UK Biobank has been associated with multiple health outcomes in previous studies6,7,10,28. Second, some early staged cases of PD may not be captured because the incident PD cases were ascertained only through electronic health records. This approach may lead to misclassification of cases. Third, the self-reported and one-time assessment of covariates may result in misclassification bias. Fourth, majority of the study population was White, thus limiting the generalizability of our findings to other ethnic groups. Finally, due to the nature of observational study design, residual confounding cannot be completely ruled out.
Methods
Study population
The UK Biobank is a large population-based prospective cohort study including more than 500,000 participants (aged 37–73 years) from 22 assessment centers across the UK from Mar 2006 to Dec 2010. Participants provided information on socio-demographics, habitual diet, lifestyle factors, and medical history through touch-screen questionnaires and face-to-face interviews at recruitment. After interview, participants underwent a standardized portfolio of physical measurements including anthropometric data, blood pressure indices, etc. Blood, urine, and saliva samples were also collected among all the participants at baseline. Details of the study were presented previously29,30. The UK Biobank study was approved by the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland and the North West Multicenter Research Ethics Committee. All participants gave written informed consent.
A total of 192,340 participants were included in the current analysis after excluding individuals with a previous diagnosis of PD and dementia (n = 839), those aged < 60 years old (n = 284,945), or those with missing values on social isolation items (n = 24,284). The flowchart for the selection of the study population is presented in Supplementary Figure 1.
Assessment of social isolation
Social isolation was assessed by asking three questions: (1) “Including yourself, how many people live in your household? Include those who usually live in the house such as students living away from home during term time, and partners in the armed forces or in professions such as pilots” Participants received 1 point if they answered living alone; (2) “How often do you visit friends or family or have them visit you?” Participants were assigned 1 point if they answered less than one friend or family visit per month; and (3) “Which of the following (leisure or social activities) do you engage in once a week or more often? You may select more than one” Participants reported not participating in any social activities received 1 point. The final score of social isolation ranged 0–3 and was further classified into least isolation (0 point); moderate isolation (1 point); and most isolation (≥2 points).
Weighted genetic risk score construction
Details of the design of genotyping, quality control and imputation procedures were described previously31. In the gene and environmental interaction analysis section, we further excluded the participants who did not have European ancestry, exhibited discrepancies between genetic sex and self-reported sex, and those had excessive heterozygosity or high levels of missingness.
We selected 44 single nucleotide polymorphisms (SNPs) associated with PD at genome-wide significance (p < 5 × 10−8) among individuals of European ancestry32, which is present in Supplementary Table 1. All the 44 SNPs were available in the UK Biobank. Individual SNPs were coded as 0, 1, or 2 based on the number of risk alleles. A weighted genetic risk score (GRS) for each participant was calculated by the widely used formula: weighted GRS = (β1 × SNP1 + β2 × SNP2 + β3 × SNP3 + … +βn × SNPn) / (average of the β coefficients). The weighted GRS followed a normal distribution, ranging from 13.8 to 47.9 as shown in Supplementary Figure 2, and a higher score indicated a higher genetic predisposition to PD. The GRS score was categorized into low (<median) and high (≥median) groups for further analysis.
Ascertainment of incident PD
Incident PD cases were identified using the algorithm method developed by the UK Biobank, with a positive predictive values of 91%33. These cases are determined by combining coded data from multiple sources, including participants’ self-reported medical conditions, operations, and medications collected during data collection at baseline. Additionally, data from hospital admissions and death registries are also linked to provide a comprehensive record in PD cases. Details of the definition are present in Supplementary Table 2. Each participant’s person-year was calculated from the date of recruitment to the date reported for PD diagnosis, death, loss to follow-up, or end of the follow-up (10th Oct 2021), whichever occurred first.
Assessment of the covariates
Data on age, sex, ethnicity, education, sleep duration, smoking status, and alcohol intake were acquired at baseline through a touchscreen questionnaire. Townsend deprivation index (TDI) is a composite measure of area-level socio-economic deprivation, with higher scores indicating greater deprivation34. Body mass index (BMI) was calculated by dividing body weight in kilograms by the square of height in meters. Total physical activity including walking and moderate and vigorous intensity activities, in metabolic equivalent minutes per week (MET-min/week) was computed from the International Physical Activity Questionnaire (IPAQ). Physical activity levels <10 min/day was recoded to 035. Pre-existing hypertension was defined based on self-reported physician diagnosis, use of anti-hypertensive medications, essential hypertension cases based on ICD-10 codes (I10), or blood pressure measurements of 140/90 mm Hg or higher. Diabetes was defined by self-reported diagnosis, use of insulin or hypoglycemic drugs, diabetes-related ICD-10 codes (E10-14), fasting glucose levels of ≥7.0 mmol/L, random glucose levels of ≥11.1 mmol/L, or HbA1c levels of ≥48 mmol/mol (6.5%). Prevalent cardiovascular disease cases including ischemic heart disease, stroke, and heart failure were identified using ICD-10 codes (I20-I25, I50, I60-I64). Prevalent cancer cases were self-reported, and prevalent chronic kidney disease cases were defined as estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2. Loneliness was assessed using the 2 questions from the revised loneliness index: 1) “Do you often feel lonely?” and 2) “How often are you able to confide in someone close to you?”36
Statistical analysis
Missing values for continuous variables (0.09% for TDI, and 0.5% for BMI) were imputed using median values, and missing values for categorical variables (0.06% for alcohol intake, 0.5% for smoking, 1.2% for education, and 0.6% for sleep duration) were imputed using the indicator approach. The differences in baseline characteristics by social isolation were examined using the chi-squared test for categorical variables and ANOVA test for continuous variables.
We used multivariable Cox proportional hazards regression models to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between social isolation and risk of PD. Schoenfeld residuals method showed the assumption of proportional hazards was not violated. In Model 1, we adjusted for age (continuous, years), sex (men, women), ethnicity (White, non-White), TDI (continuous), and education (college and above, others). In Model 2, we further adjusted for smoking status (never, former, current), alcohol intake (never or special occasions/occasionally, monthly to weekly, daily), BMI (continuous, kg/m2), sleep duration (≤6, 7–8, ≥9 hours/day), and history of hypertension, cardiovascular disease, diabetes, cancer, and chronic kidney disease (yes, no). We chose variables for adjustment beforehand, considering their availability and general understanding of PD’s risk factors37,38.
Further, we stratified the analysis by the GRS of PD (low vs. high) and tested the interaction between social isolation and the GRS using the likelihood ratio test by including an interaction term in the multivariable-adjusted model. In the models with GRS, genotyping batch, and the first 10 principal component of ancestry were additionally adjusted based on Model 2. We also tested the association between the social isolation score and risk of PD. Further, we stratified the analyses by age (<65, ≥65 years), sex (men, women), BMI (<25, ≥25 kg/m2), smoking status (never, ever smoking), history of hypertension (yes, no), history of diabetes (yes, no), and history of cardiovascular disease (yes, no). The interactions of social isolation and the stratified factors on the risk of PD were also tested. In addition, we investigated the associations of individual social isolation items with risk of PD with mutual adjustment for the other social isolation items.
We performed several sensitivity analyses to test the robustness of our findings. First, to minimize the potential confounding effect of preclinical PD on the observed associations, we repeated the analyses after excluding cases that occurred within the first two years of follow-up. Second, we performed the analysis using the multiple imputation by chained equations with 5 imputations. Finally, to determine whether the observed association between social isolation and risk of PD could be explained by systemic inflammation, loneliness, or physical activity, we further adjusted for circulating C-reactive protein (continuous, mg/L), loneliness, and total MET-mins per week based on Model 2.
All analyses were performed in Stata statistical software, release 15.1 (StataCorp LP, College Station, Texas), and P-value < 0.05 was considered as statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The UK Biobank data can be retrieved by applying to the UK Biobank, www.ukbiobank.ac.uk/. This research has been conducted using the UK Biobank Resource under Application Number 96083.
Code availability
Codes are available from the corresponding author upon reasonable request.
References
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. Neurol. 18, 459–480 (2019).
Valtorta, N. & Hanratty, B. Loneliness, isolation and the health of older adults: do we need a new research agenda? J. R. Soc. Med. 105, 518–522 (2012).
Cudjoe, T. K. M. et al. The Epidemiology of Social Isolation: National Health and Aging Trends Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 75, 107–113 (2020).
Ward, M., May, P., Normand, C., Kenny, R. A. & Nolan, A. Mortality risk associated with combinations of loneliness and social isolation. Findings from The Irish Longitudinal Study on Ageing (TILDA). Age Ageing 50, 1329–1335 (2021).
Liang, Y. Y. et al. Association of Social Isolation and Loneliness With Incident Heart Failure in a Population-Based Cohort Study. JACC Heart Fail. 11, 334–344 (2023).
Smith, R. W. et al. Social isolation and risk of heart disease and stroke: analysis of two large UK prospective studies. Lancet Public Health 6, e232–e239 (2021).
Lee, S. L. et al. The association between loneliness and depressive symptoms among adults aged 50 years and older: a 12-year population-based cohort study. Lancet Psychiatry 8, 48–57 (2021).
Falvey, J. R. et al. Association of Social Isolation With Disability Burden and 1-Year Mortality Among Older Adults With Critical Illness. JAMA Intern. Med. 181, 1433–1439 (2021).
Elovainio, M. et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health 2, e260–e266 (2017).
Subramanian, I., Farahnik, J. & Mischley, L. K. Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. NPJ Parkinsons Dis. 6, 28 (2020).
D’Iorio, A. et al. A longitudinal study on the effects of COVID-19 pandemic on non-motor symptoms in Parkinson’s disease. Neurol. Sci. 43, 4605–4609 (2022).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Homayoun, H. Parkinson Disease. Ann. Intern. Med. 169, Itc33–itc48 (2018).
Shen, C. et al. Associations of Social Isolation and Loneliness With Later Dementia. Neurology 99, e164–e175 (2022).
Cacioppo, J. T., Cacioppo, S., Capitanio, J. P. & Cole, S. W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767 (2015).
Du, X. & Pang, T. Y. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front. Psychiatry 6, 32 (2015).
Pajares, M., Rojo, A. I., Manda, G., Boscá, L. & Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 9, 1687 (2020).
Koyama, Y. et al. Interplay between social isolation and loneliness and chronic systemic inflammation during the COVID-19 pandemic in Japan: Results from U-CORONA study. Brain Behav. Immun. 94, 51–59 (2021).
Al Omran, A. J. et al. Social isolation induces neuroinflammation and microglia overactivation, while dihydromyricetin prevents and improves them. J. Neuroinflamm. 19, 2 (2022).
Lawrence, G., Holley, C. L. & Schroder, K. Parkinson’s disease: connecting mitochondria to inflammasomes. Trends Immunol. 43, 877–885 (2022).
Eldeeb, M. A., Thomas, R. A., Ragheb, M. A., Fallahi, A. & Fon, E. A. Mitochondrial quality control in health and in Parkinson’s disease. Physiol. Rev. 102, 1721–1755 (2022).
Al Omran, A. J. et al. Dihydromyricetin ameliorates social isolation-induced anxiety by modulating mitochondrial function, antioxidant enzymes, and BDNF. Neurobiol. Stress 21, 100499 (2022).
Bzdok, D. & Dunbar, R. I. M. Social isolation and the brain in the pandemic era. Nat. Hum. Behav. 6, 1333–1343 (2022).
The Lancet, N. Parkinson’s disease needs an urgent public health response. Lancet Neurol. 21, 759 (2022).
Mahlknecht, P., Seppi, K. & Poewe, W. The Concept of Prodromal Parkinson’s Disease. J. Parkinsons Dis. 5, 681–697 (2015).
Su, Y. et al. Prevalence of loneliness and social isolation among older adults during the COVID-19 pandemic: A systematic review and meta-analysis. Int. Psychogeriatr. 35, 229–241 (2023).
Elovainio, M. et al. Association of social isolation and loneliness with risk of incident hospital-treated infections: an analysis of data from the UK Biobank and Finnish Health and Social Support studies. Lancet Public Health 8, e109–e118 (2023).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Caleyachetty, R. et al. United Kingdom Biobank (UK Biobank): JACC Focus Seminar 6/8. J. Am. Coll. Cardiol. 78, 56–65 (2021).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Chang, D. et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 49, 1511–1516 (2017).
Hughes, A. J., Daniel, S. E., Ben-Shlomo, Y. & Lees, A. J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125, 861–870 (2002).
Townsend, P., Phillimore, P. & Beattie, A. Health and deprivation: inequality and the North, (Routledge, 1988).
Guo, W., Fensom, G. K., Reeves, G. K. & Key, T. J. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br. J. Cancer 122, 726–732 (2020).
Hughes, M. E., Waite, L. J., Hawkley, L. C. & Cacioppo, J. T. A Short Scale for Measuring Loneliness in Large Surveys: Results From Two Population-Based Studies. Res. Aging 26, 655–672 (2004).
de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535 (2006).
Gao, C., Liu, J., Tan, Y. & Chen, S. Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl. Neurodegener. 9, 12 (2020).
Acknowledgements
We are grateful to all the participants of UK Biobank and all the people involved in building the UK Biobank study. This study was supported by the Startup grant at Fudan University (JIF201047Y, JIF201015Y and JIF201018Y).
Author information
Authors and Affiliations
Contributions
T.G. and X.G. designed the study; T.G. and Y.L. performed the statistical analysis; T.G. wrote the paper with critical input from all authors. All authors approved the manuscript. T.G. and X.G. have primary responsibility for final content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geng, T., Li, Y., Peng, Y. et al. Social isolation and the risk of Parkinson disease in the UK biobank study. npj Parkinsons Dis. 10, 79 (2024). https://doi.org/10.1038/s41531-024-00700-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00700-7