Abstract
Parkinson’s disease (PD) is characterized by a progressive loss of dopaminergic neurons. Exercise has been reported to slow the clinical progression of PD. We evaluated the dopaminergic system of patients with mild and early PD before and after a six-month program of intense exercise. Using 18F-FE-PE2I PET imaging, we measured dopamine transporter (DAT) availability in the striatum and substantia nigra. Using NM-MRI, we evaluated the neuromelanin content in the substantia nigra. Exercise reversed the expected decrease in DAT availability into a significant increase in both the substantia nigra and putamen. Exercise also reversed the expected decrease in neuromelanin concentration in the substantia nigra into a significant increase. These findings suggest improved functionality in the remaining dopaminergic neurons after exercise. Further research is needed to validate our findings and to pinpoint the source of any true neuromodulatory and neuroprotective effects of exercise in PD in large clinical trials.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra (SN)1. A growing literature has demonstrated the benefits of exercise programs for controlling motor symptoms of PD2,3,4,5. The reported benefits vary according to the type, intensity, and duration of the exercise. Moderate-to-high-intensity exercise multiple times per week for prolonged periods (e.g., 6 months) has been shown to ameliorate the motor severity of PD in clinical trials6,7,8.
Rodent models of PD have shown that exercise-induced improvements in motor performance were accompanied by neuroprotective effects on the dopaminergic neurons in the SN9,10,11,12,13,14,15,16. These neuroprotective effects of exercise are thought to be mediated through neurotrophic, anti-inflammatory, and angiogenic factors. It has been suggested that the interplay between these factors facilitates rescuing of the dopaminergic neurons and increased signaling capacity of healthy dopaminergic neurons17,18. In humans, indirect clinical evidence suggests that exercise may be neuroprotective. Exercise studies in patients with PD support the mediator role of neurotrophic and anti-inflammatory factors in clinical improvement19,20,21. Low pro-inflammatory microglial activation has been proposed as a pathway linking physical activity to brain health based on postmortem examination of older adults without PD but with varying degrees of physical activity22. Postmortem nigral volumes and white matter integrity have been found to be positively correlated with physical activity in older adults without PD23. A positron emission tomography (PET) study using [11C]-raclopride in people with PD demonstrated that a single bout of vigorous cycling by habitual exercisers released significantly more dopamine in the caudate nucleus than the same activity performed by those who were sedentary24. These findings suggest that the benefits of exercise may be due to neuromodulatory effects, such as the preservation of the dopaminergic reserves and enhanced dopamine transmission. However, these putative effects have not been directly investigated in humans with PD in vivo.
Multimodal neuroimaging enables the visualization and quantification of multiple aspects of the dopaminergic system and its functioning in vivo. Imaging of the dopaminergic system has been used to track disease progression in PD. A recent study using the radioligand 18F-FE-PE2I (selective for the presynaptic dopamine transporter (DAT) protein) demonstrated reduced DAT availability in the SN and striatum in subjects with early PD over the course of 2 years, whereas healthy age-matched controls showed no significant changes25. Neuromelanin (NM) is a cytosolic neuronal pigment and an autophagic product synthesized via oxidation and polymerization of catecholamines such as dopamine. It accumulates slowly in dopaminergic neurons with age26 and loss of NM is a hallmark of PD pathology. NM-sensitive magnetic resonance imaging (MRI) can assay NM in the dopaminergic neurons of the SN. A negative correlation between motor symptom severity and NM-MRI measurements in the SN has been demonstrated in PD27,28. In independent PD cohorts, the annual rates of decline in SN volumes were estimated using NM-MRI, suggesting a role for NM as a biomarker for disease progression in the brains of people with PD28. Lastly, using iron-sensitive MRI techniques such as Quantitative Susceptibility Mapping (QSM), increased ferromagnetic depositions in the SN have been demonstrated in people with PD29,30.
In this proof-of-concept study, we imaged the effects of 6 months of high-intensity interval training on the dopaminergic system in patients with PD. Our primary outcome was the change in DAT availability in the SN and striatum, as measured with 18F-FE-PE2I. Our secondary outcome was the change in NM concentration in the SN. We also used QSM to account for potentially confounding effects of ferromagnetic depositions on NM measurements.
Results
Subjects
Thirteen subjects were enrolled in the “Beat Parkinson’s Today” high-intensity interval training program. After the initial exercise trial period, two subjects dropped out, one due to scheduling conflicts and the other due to incomplete healing after a foot surgery. One additional subject was excluded from the NM-MRI analysis due to imaging artifacts and from the PET analysis due to inability to schedule the scan within the time window of the study and the inability of the subject to continue with exercise beyond the 6-month period for reasons unrelated to the study. The demographic and clinical data (n = 10), exercise data, and pre- and post-exercise motor function test results are summarized in Tables 1, 2, and 3, respectively.
On average, our PD cohort had mild bilateral disease, intact global cognition; and no significant anxiety, depression, apathy, or fatigue compared with the normative population data (see supplementary data). One subject was not taking any PD medication, and only six subjects were on L-dopa. Subjects fulfilled the class attendance requirements and reported high levels of motivation to exercise and high satisfaction with the program. Subjects reported only mild and transient side effects of exercise such as muscle soreness and fatigue. In about two-thirds of all classes, the target HR was achieved (i.e., 80% of the maximum HR), however, 90% of classes were rated as very intense by the subjects. Motor function tests showed similar or slightly improved scores from pre- to post-exercise.
Six subjects continued the exercise program beyond the six months period. Their average annual motor exam score (collected at 12.6 ± 2.3 months after the start of exercise in the “off” medication state) was 23.7 ± 6.6, improved from the baseline (26.7 ± 7.2) and unchanged from the 6-month (23.8 ± 7.9) scores.
Imaging
After the last exercise class, there was a period of 4.9 ± 3.0 days until the MRI scan and 10.1 ± 3.1 days until the PET scan.
PET
The pre- and post-exercise 18F-FE-PE2I BPND values were: Caudate: 0.93 ± 0.11 and 0.95 ± 0.09, putamen: 1.51 ± 0.18 and 1.61 ± 0.27, and SN: 0.43 ± 0.03 and 0.50 ± 0.03 (Fig. 1A).
A Average 18F-FE-PE2I DAT BPND images before and after six months of exercise. The red box including the midbrain and SN is enlarged. Note: The left side of the color bar (0.0–3.0) corresponds to the DAT BPND in the striatum and the right side (0.0–1.0) to the DAT BPND in the SN shown in the inset images. BPND is unitless. Orientation is axial. B 18F-FE-PE2I BPND in the SN pre- and post-exercise by study participant. Individual lines are red if an increase was observed, blue if a decrease was observed. The solid black line represents the mean of our cohort, the dashed black line represents the expected decrease from the pre-exercise average in the absence of intervention25.
For the caudate, the observed average of the individual change was a 20.16% increase (90% CI: −21.3–61.6%) was higher than the reported 3.90% decrease in BPND per six months for a similar PD cohort but did not reach significance (p = 0.160). For the putamen, the observed 4.32% increase (90% CI: −4.6–13.3%) was significantly higher than the reported 5.35% decrease in BPND per six months (p = 0.004). For the SN, the observed 19.95% increase (90% CI: 5.7–34.2%) was significantly higher than the reported 2.25% decrease in BPND per six months (p = 0.010) (Fig. 1B).
NM-MRI and QSM
The pre- and post-exercise CR values of the SNc were 18.46 ± 0.65 and 19.33 ± 0.71, respectively (Fig. 2A).
A Average NM-MRI pre- and post-exercise. Axial image on the left shows the acquired NM image and the red box locates the cut-out sections shown. Top row shows the raw T1-weighted NM image (T1w NM), the bottom row shows the corresponding CR values. B CR in the SNc pre- and post-exercise by study participant. Individual lines are red if an increase was observed, blue if a decrease was observed. The solid black line represents the mean of our cohort, the dashed black line represents the baseline average multiplied by the expected decrease without intervention31.
The observed 5.3% increase (90% CI: −5.1–34.0) in the SNc is significantly greater (p = 0.008) than the 3.15% decrease reported for a CR in a similar PD cohort over six months (Fig. 2B). The participant with lower DAT availability in the SN after exercise (blue line, Fig. 1B) is also one of the participants with lower CR post-exercise. No significant difference in the QSM value in SNc was observed (pre-exercise = 131.5, post-exercise = 126.0, paired t-test, p = 0.59), suggesting that the observed increases in NM were not confounded by the change in iron deposits.
Discussion
Six months of high-intensity exercise induced brain changes in patients with early and mild PD. We observed a consistent increase in available DAT sites in the SN. A more variable increase was observed in available DAT sites in the putamen. Using NM-MRI, we observed an increase in the NM signal in the SNc. These apparent increases in DAT and NM in the SN were significantly different from previously observed natural declines in comparable PD populations25,31.
The DAT protein is critical in maintaining intracellular dopamine storage32. Regulation of the DAT protein homeostasis is particularly complex in PD. In early disease stages, dopamine turnover has been found to be increased. Significantly lower levels of DAT mRNA expression in SN neurons have been found in post-mortem brain tissue in people with PD compared to controls33,34. Yet, higher levels of DAT mRNA expression have also been observed in the remaining SN dopaminergic neurons in PD. These changes in DAT homeostasis have been attributed to neuronal dysfunction or compensatory changes in dopaminergic signaling in the remaining neurons. Recently, more than 44% annual reductions in DAT availability in the SN were observed with 18F-FE-PE2I by Delva et al. in a comparable cohort with early and mild PD25. We found that six months of intense exercise induced a significant increase in DAT availability in the SN in 90% of our participants. Given that there were no signs of motor disease progression (i.e., no worsening of MDS-UPDRS-III scores) and no change in the total levodopa daily dose, the increase in DAT availability likely reflects improved functionality in the remaining dopaminergic neurons post-exercise. These findings are also in line with the neuroprotective effects of aerobic exercise on the SN dopaminergic neurons in rodent models of PD9,10,11,12,13,14,15,16.
We found smaller and less consistent increases in DAT availability in the putamen and caudate than in the SN. Post-mortem studies have demonstrated a near total loss of nigrostriatal terminals in the dorsal putamen at 4 years post-diagnosis35. Our cohort, restricted to patients with less than 4 years of disease, was followed during a period in which a considerable loss of nigrostriatal terminals would have been expected25. The absence of a clear decrease in the striatal DAT availability in our cohort may be seen as evidence of a protective effect of exercise on the nigrostriatal terminals, especially in the putamen.
Neuromelanin accumulates linearly with age in dopaminergic neurons36 but decreases up to 60% in the first years of PD37. Neuromelanin is thought to play a dual role: (1) when confined to the intracellular space, it can protect the cell against free radicals; (2) when released from dying dopaminergic neurons, it can cause more toxicity by inducing neuroinflammation28,36. We observed an increase in NM signal in the SNc which was a significant reversal of the expected decrease in six months reported by Xing et al. in a comparable cohort, but with a longer disease duration (4.9 ± 1.8 years in Xing et al. versus 2.0 ± 0.8 years in our cohort)31. Considering that the fastest decline in the nigral NM concentration takes place early in the disease course, we think that the NM signal increase in our cohort is striking. NM-MRI cannot differentiate whether the observed increase in NM concentration in the SNc is intra- or extracellular, but given our clinical and PET findings, the increase in NM signal can reasonably be seen as reflecting greater metabolic/synthetic activity in the remaining dopaminergic neurons.
Our cohort included high-functioning patients with mild PD who were motivated to exercise and to comply with the program. Subjects reached HRs of 70% of their maximum and higher in more than 85% of classes, which is consistent with their rating 90% of their classes as very intense. This suggests that subjects exercised at more than moderate-intensity level (typically, 60–65% of the maximum HR) in most classes. Participants reached the target HR of 80% of their theoretical maximum in two-thirds of classes. The discrepancy between the ratings and HR data may be due to mechanisms such as cardiac sympathetic denervation which is common in PD38 and would restrict increases in HR in response to an exercise challenge. Importantly, we did not find any significant motor progression or increase in levodopa equivalent daily dose. Together, these points suggest that our exercise program resulted in clinical benefit despite not always exceeding the prescribed HR threshold. Finally, the 1-year MDS-UPDRS-III scores of a subset of subjects who continued the program were comparable to their 6-month scores suggesting sustained clinical benefit. Similar delays in motor progression have been reported in randomized controlled trials of 6-months of high intensity exercise6,7. Our results suggest extension of this benefit over 1 year.
Two main limitations exist in our study. The first is the absence of a control group. A matched PD control group in a mild exercise program would have controlled for the potential confound of positive feedback received by participation in any group activity. It would have allowed for direct measurement of the natural disease progression at hand. Moreover, we only enrolled patients with mild and early PD and tried to minimize attrition by including an exercise trial period before enrollment. While these measures limit the generalizability of our results and may have introduced a selection bias, we think that they were necessary measures to ensure post-exercise signal detection in the brain in this proof-of-concept study. The second limitation is the small sample size. Smaller samples can lead to over- and underestimation of true effects due to the risk of relatively high proportion of extreme cases. But an increase of DAT availability in SN in 9/10 participants is unlikely to have occurred by chance. Related to the small sample size issue, we did not include sex or side of symptom onset as covariates in our analyses. An additional point of concern might be the scheduled break of 1-2 weeks between the end of the exercise sessions and imaging sessions. We included this break as a wash-out period, during which the known acute increase in dopamine concentration after exercise would be expected to disappear and only the longer lasting changes would remain. While we cannot be certain that this break was of appropriate duration, we think that any potential carryover or withdrawal effect from exercise on the imaging data was balanced out because the break duration between the exercise and imaging sessions was approximately the same at baseline and 6 months.
In summary, this proof-of-concept study provides in vivo evidence that sustained periods of intense exercise can induce brain changes in individuals with mild and early PD. Two different biomarkers for the health of the dopamine system were increased in the SN following six months of exercise demonstrating the neuromodulatory effects of exercise on the dopaminergic system. Moreover, the increases were both significantly different from, and reversals of, the expected natural decline. These same markers did not decline in the striatum as would also have been expected during the natural course of disease progression. Our results not only support the inclusion of high-intensity exercise early in the treatment plans of PD patients, but also suggest a role for exercise as an effective non-invasive neuromodulatory therapy. Future randomized controlled trials will be needed to optimize exercise regimens. Our observations could also have far-reaching implications for neuroprotective effects of exercise in PD, but further work is needed to validate them and elucidate the underlying mechanisms.
Methods
Subjects
This study was designed as a within-subject proof-of-concept. We recruited subjects with mild PD defined according to the Movement Disorders Society (MDS) diagnostic criteria39 through the Yale Movement Disorders Clinic and via ‘Beat Parkinson’s Today’ gyms that cater to PD exercisers. Subjects were excluded if they had: Hoehn & Yahr disease stage >240, who were >4 years after diagnosis35, had a neurological or psychiatric disorder (other than PD and comorbid depression or anxiety), a medical condition that might affect the central nervous system, history of alcohol or illicit drug abuse, head injury resulting in loss of consciousness, dementia (Montreal Cognitive Assessment (MoCA) score <21)41, contraindications for MRI or PET, or high baseline exercise levels (i.e., baseline exercise equals or exceeds the Beat Parkinson’s Today intensity and frequency).
Eligible subjects participated in 5-6 exercise classes for a 2-week trial period. Subjects who could perform the exercises and were committed to continue were enrolled in the 6-month program by giving written informed consent in accordance with the procedures approved by the Yale Human Investigation Committee.
Clinical evaluations
At the start of the exercise period, clinical evaluations were performed including the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire42 to determine baseline moderate-intensity physical activity levels, neurological and movement exams using the MDS-Unified PD Rating Scale (MDS-UPDRS)43, motor function tests, cognitive evaluation using the MoCA test41, and self-evaluation surveys for anxiety44, depression45, apathy46, fatigue47, and quality of life48. All assessments except CHAMPS were repeated at a post-exercise clinical visit. The motor exams were performed in the medication “off” state (i.e., 12-h washout after the last dose) and videotaped for scoring by a masked movement disorders neurologist (A.P.) (except for rigidity, which was scored by a movement disorders neurologist (S.T.) during the exam). We also performed a motor exam in a subset of subjects after one year. The motor function tests of gross movement speed included the two-minute endurance walking, timed up-and-go, five times sit-to-stand, 360° turning, and climbing one flight of stairs.
During the 6-month exercise period, weekly surveys were filled out by subjects reporting their experience with the program and by trainers reporting their observations about subjects during each class. The trainers and research team addressed issues regarding the subjects and logistics (exercise performance, protocol adherence, injuries, proper use of chest straps, etc.) at monthly meetings.
‘Beat Parkinson’s Today’ exercise program
The Beat Parkinson’s Today exercise program was developed for people with PD mirroring the ParkFit exercise program49. Beat Parkinson’s Today offers high-intensity interval training and boxing, both of which have been shown to benefit people with PD50,51. The high-intensity interval training circuits are designed to improve aerobic capacity, muscle strength and endurance, gait and balance, physical function, and flexibility. Every workout is adjusted to an individual’s needs and ability (e.g., using chairs if kneeling down is difficult). The exercises are performed in small groups in a supportive environment fostering a community bond and compliance. A typical workout includes warm-up (5 min), exercise (30 min) with two consecutive circuits (composed of strength, cardio, and power exercises) each performed twice with 30 s rest periods between rounds, boxing (15 min), and cool-down (10 min). Trainers provided individual feedback to participants and logged class attendance to verify that each subject completed the required number of classes (72 in total). Due to the Covid-19 pandemic, subjects participated in live online Beat Parkinson’s Today classes in the period between January 2021 to August 2022. The online classes were taught by Beat Parkinson’s Today trainers in small groups (5–8 attendees). Trainers monitored the participants in real-time for safety and compliance and provided immediate feedback. From here on, for simplicity, we refer to the Beat Parkinson’s Today exercise regimen as “exercise”.
Heart-rate chest-strap
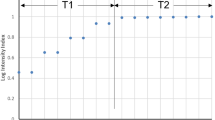
Participants wore chest straps with a Polar H7 heart rate (HR) monitor (Polar Electro Oy, Kempele, Finland) starting five minutes before, and continuing throughout every exercise session. The target HR for each participant was defined as 80% of his/her maximum HR. Maximum HR was determined according to the formula: Maximum HR = 220-age. Beat-to-beat interval data were collected with the EliteHRV app and filtered using a Butterworth filter to minimize high frequency artifacts. Average HRs during the 20 min containing the highest mean HR during the exercise session were analyzed to confirm that participants had achieved and maintained their HR within the targeted range (Supplementary Fig. 1).
Fitbit
Participants were also outfitted with a Fitbit Charge 4 (Fitbit, San Francisco, USA) wristwatch, which collected data for the duration of the study. Fitbit’s proprietary algorithm was used to estimate participants’ resting HR. Reported resting HRs were taken from the first 7 days of study participation.
Imaging
MRI and 18F-FE-PE2I PET scans were collected on separate days at baseline and after six months of exercise in the medication “off” state. Subjects were instructed to pause the exercise classes 1-2 weeks before the baseline and post-exercise PET scans to ensure that the measurements were not confounded by immediate exercise effects.
PET
Dynamic PET scans were acquired on the High Resolution Research Tomograph scanner (Siemens/CTI, Knoxville, TN, USA) for 60 min starting with [18F]-FE-PE2I bolus injection over 1 min. After reconstruction and motion correction52, individual images were registered to MNI space by registering the PET image to the high-resolution MPRAGE image of the same subject and visit using SPM1253. The MPRAGE images were spatially normalized to MNI space54 and the estimated warping parameters were subsequently applied to the corresponding PET images. The registration and normalization quality of the MPRAGE and PET scans was checked visually. Three bilateral regions of interest were identified in each PET image; the putamen and caudate from the AAL template in the MNI space55, and a hand-drawn SN mask which was used in our previous studies56,57,58. For every region, a time-activity curve was extracted and modeled using the simplified reference tissue model (SRTM) with the cerebellum as reference region to estimate the regional binding potential (BPND) and relative input parameter (R1). A voxel-level approach, still using the cerebellum curve as reference, was applied to normalized images to create parametric BPND maps59.
MRI
Scans were collected in a 3.0 Tesla Siemens Prisma scanner using a 32-channel head coil. The following were collected: (1) high-resolution T1-weighted MPRAGE images (176 slices, voxel size: 1 mm3, FoV: 250 mm, matrix: 256 ×256, TR: 1900 ms, TE: 2.52 ms, TI: 900 ms, flip angle: 9°, scan time: 4 min 32 s), (2) 6-7 NM scans (magnetization transfer gradient echo sequences, FOV: 220 mm, 11 slices without gap aligned to the AC-PC line and the top slice 3 mm above the roof of the midbrain, voxel size: 0.4 ×0.4 ×2.5 mm, TR: 468 ms, TE: 3.7 ms, flip angle: 40°, scan time per scan: 2 min 53 s), and (3) high-resolution gradient echo sequences for QSM (FOV: 256 mm, aligned to the AC-PC line, voxel size: 0.5 ×0.5 ×1 mm, TR: 47 ms, TE1/∆TE: 6.10/4.02 ms, flip angle: 15°, scan time: 4 min 38 s).
The NM data were processed using an automated voxel-wise analysis pipeline that has been shown to be reliable and reproducible60. The FMRIB Software Library 6.0 (FSL)61 was used for motion correction and averaging, SPM12 was used for registration of the NM scans with the high-resolution MPRAGE anatomical scans53, and Advanced Normalization Tools (ANTS) for the spatial normalization of the MPRAGE scans to the standard MNI brain template62. The estimated warping parameters were then applied to the NM scans. The quality of the normalization of the NM scans to the MNI template for each subject was checked visually using SPM12. The SN pars compacta (SNc) mask in the MNI space defined by Pauli et al.63 was used as the region of interest. A hand-drawn in-house crus cerebri mask in the MNI space was used as the reference region. Voxel-level contrast ratio (CR) was calculated at voxel j according to Eq. (1):
The CR for the entire SNc was calculated as the median value over all voxels.
For QSM scans, we followed the same motion correction, registration, and spatial normalization steps as for the NM scans. We used the same SNc template to extract the QSM values63. We used the automated QSM processing pipeline as described in Spincemaille et al.64. The total field was computed from the phase images by least-squares fitting, followed by background field removal using the projection onto dipole fields method to obtain the tissue field65. An R2* map was computed from the magnitude images using ARLO algorithm66, from which a ventricular cerebrospinal fluid (CSF) mask was created by thresholding. Finally, the morphology-enabled dipole inversion with automatic uniform CSF zero referencing (MEDI + 0) algorithm was used to reconstruct the susceptibility maps (in ppb) from the tissue field67,68. The QSM values were extracted from the SNc mask.
Statistical analysis
The sample size was chosen based on reported annual decline in NM and reported positive effect sizes after exercise in PET69,70,71. Based on these reported estimates, a sample of 11 subjects would provide 80% power to detect a 5.6% increase in NM and a 10% increase in PET. Data analyses were conducted using JMP pro 16 (SAS Institute, Cary, USA). PET and NM-MRI data were analyzed using non-inferiority tests which compared the average of the observed individual change, defined as (post–pre)/pre * 100, to the reported percentage decreases in comparable PD cohorts. The observed change in DAT BPND over 6 months was compared to half the annual decline reported in Delva et al.25 (n = 27, age = 60.4 ± 9.7 years, H&Y = 2.0 ± 0.0, disease duration in years = 3.1 ± 1.0) and the observed change in NM CR over 6 months was compared to half the annual decline reported in Xing et al.31 (n = 46, age = 67.3 ± 8.7 years, H&Y = 2.0 ± 0.7, disease duration in years = 4.9 ± 1.8) (see supplementary material for details). Exploratory correlations between outcomes and demographics are in the supplementary material. Demographics are reported as median (range). Statistical outcomes are reported with their standard error and significance is defined as p < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author request.
References
Braak, H. & Braak, E. Pathoanatomy of Parkinson’s disease. J. Neurol. 247, II3–II10 (2000).
Bloem, B. R., de Vries, N. M. & Ebersbach, G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov. Disord. 30, 1504–1520 (2015).
Reynolds, G. O., Otto, M. W., Ellis, T. D. & Cronin-Golomb, A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson’s Disease. Mov. Disord. 31, 23–38 (2016).
Ahlskog, J. E. Aerobic exercise: evidence for a direct brain effect to slow parkinson disease progression. Mayo Clin. Proc. 93, 360–372 (2018). Elsevier.
Li, X., Gao, Z., Yu, H., Gu, Y. & Yang, G. Effect of long-term exercise therapy on motor symptoms in Parkinson disease patients: a systematic review and meta-analysis of randomized controlled trials. American. J. Phys. Med. Rehabilit. 101, 905–912 (2022).
Schenkman, M. et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 75, 219–226 (2018).
van der Kolk, N. M. et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 18, 998–1008 (2019).
Mak, M. K. & Wong-Yu, I. S. Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: a randomized controlled trial. J. Parkinsons Dis. 11, 1431–1441 (2021).
Tillerson, J. L., Caudle, W., Reveron, M. & Miller, G. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119, 899–911 (2003).
Petzinger, G. M. et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 27, 5291–5300 (2007).
Gerecke, K. M., Jiao, Y., Pani, A., Pagala, V. & Smeyne, R. J. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 1341, 72–83 (2010).
Tajiri, N. et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 1310, 200–207 (2010).
Svensson, M., Lexell, J. & Deierborg, T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: what we can learn from animal models in clinical settings. Neurorehabilit. Neural Repair. 29, 577–589 (2015).
Real, C. C. et al. Evaluation of exercise-induced modulation of glial activation and dopaminergic damage in a rat model of Parkinson’s disease using [(11)C]PBR28 and [(18)F]FDOPA PET. J. Cereb. Blood Flow. Metab. 39, 989–1004 (2019).
Binda, K. H. et al. Exercise protects synaptic density in a rat model of Parkinson’s disease. Exp. Neurol. 342, 113741 (2021).
Ferreira, A. F. F., Binda, K. H. & Real, C. C. The effects of treadmill exercise in animal models of Parkinson’s disease: A systematic review. Neurosci. Biobehav Rev. 131, 1056–1075 (2021).
Zigmond, M. J. et al. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 15, S42–S45 (2009).
Zigmond, M. J., Cameron, J. L., Hoffer, B. J. & Smeyne, R. J. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat. Disord. 18, S147–S150 (2012).
Frazzitta, G. et al. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabilit. Neural Repair. 28, 163–168 (2014).
Zoladz, J. et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol. Pharm. 65, 441–448 (2014).
Marusiak, J. et al. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 47, 372–375 (2015).
Casaletto, K. B. et al. Microglial correlates of late life physical activity: Relationship with synaptic and cognitive aging in older adults. J. Neurosci. 42, 288–298 (2022).
Buchman, A. S. et al. Brain pathology is related to total daily physical activity in older adults. Neurology 90, e1911–e1919 (2018).
Sacheli, M. A. et al. Habitual exercisers versus sedentary subjects with Parkinson’s Disease: Multimodal PET and fMRI study. Mov. Disord. 33, 1945–1950 (2018).
Delva, A., Van Laere, K. & Vandenberghe, W. Longitudinal Positron Emission Tomography Imaging of Presynaptic Terminals in Early Parkinson’s Disease. Mov. Disord. 37, 1883–1892 (2022).
Sulzer, D. et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis. 4, 11 (2018).
Kawaguchi, H. et al. Principal component analysis of multimodal neuromelanin MRI and dopamine transporter PET data provides a specific metric for the nigral dopaminergic neuronal density. PLoS One 11, e0151191 (2016).
Biondetti, E. et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 144, 3114–3125 (2021).
Pyatigorskaya, N. et al. Iron imaging as a diagnostic tool for Parkinson’s disease: a systematic review and meta-analysis. Front. Neurol. 11, 366 (2020).
Du, G. et al. Dynamics of Nigral iron accumulation in Parkinson’s disease: from diagnosis to late stage. Mov. Disord. 37, 1654–1662 (2022).
Xing, Y. et al. Neuromelanin-MRI to Quantify and Track Nigral Depigmentation in Parkinson’s Disease: A Multicenter Longitudinal Study Using Template-Based Standardized Analysis. Mov. Disord. 37, 1028–1039 (2022).
Bu, M., Farrer, M. J. & Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. npj Parkinsons Dis. 7, 22 (2021).
Uhl, G. R., Walther, D., Mash, D., Faucheux, B. & Javoy-Agid, F. Dopamine transporter messenger RNA in Parkinson’s disease and control substantia nigra neurons. Ann. Neurol. 35, 494–498 (1994).
Harrington, K. A., Augood, S. J., Kingsbury, A. E., Foster, O. J. F. & Emson, P. C. Dopamine transporter (DAT) and synaptic vesicle amine transporter (VMAT2) gene expression in the substantia nigra of control and Parkinson’s disease. Mol. Brain Res. 36, 157–162 (1996).
Kordower, J. H. et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136, 2419–2431 (2013).
Zucca, F. A. et al. Neuromelanin of the Human Substantia Nigra: An Update. Neurotox. Res. 25, 13–23 (2014).
Zecca, L. et al. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 510, 216–220 (2002).
Goldstein, D. S. et al. Cardiac sympathetic denervation in Parkinson disease. Ann. Intern Med. 133, 338–347 (2000).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology 17, 427–442 (1967).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatrics Soc. 53, 695–699 (2005).
Stewart, A. L. et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 33, 1126–1141 (2001).
Goetz, C. G. et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 23, 2129–2170 (2008).
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R. & Jacobs, G. A. Manual for the State-Trait Anxiety Inventory. (Palo Alto, CA: Consulting Psychologists Press, 1983).
Beck, A. T., Steer, R. A., Ball, R. & Ranieri, W. Comparison of Beck depression inventories – IA and II in psychiatric outpatients. J. Pers. Assess. 67, 588–597 (1996).
Starkstein, S. E. et al. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 4, 134–139 (1992).
Brown, R., Dittner, A., Findley, L. & Wessely, S. The Parkinson fatigue scale. Parkinsonism Relat. Disord. 11, 49–55 (2005).
Jenkinson, C., Stradling, J. & Petersen, S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J. Sleep. Res. 6, 199–204 (1997).
Speelman, A. D., van Nimwegen, M., Bloem, B. R. & Munneke, M. Evaluation of implementation of the ParkFit program: A multifaceted intervention aimed to promote physical activity in patients with Parkinson’s disease. Physiotherapy 100, 134–141 (2014).
Combs, S. A. et al. Community-based group exercise for persons with Parkinson disease: a randomized controlled trial. NeuroRehabilitation 32, 117–124 (2013).
Fernandes, B. et al. High-intensity interval versus moderate-intensity continuous training in individuals with Parkinson’s disease: hemodynamic and functional adaptation. J. Phys. Act. Health 17, 85–91 (2020).
Carson R. E., Barker W. C., Liow J-S, Johnson C. A. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. In 2003 IEEE Nuclear Science Symposium Conference Record (IEEE Cat No 03CH37515) 3281–3285 (IEEE, 2003).
Penny W. D., Friston K. J., Ashburner J. T., Kiebel S. J., Nichols T. E. Statistical parametric mapping: the analysis of functional brain images (Elsevier, 2011).
Mazziotta, J. C., Toga, A. W., Evans, A., Fox, P. & Lancaster, J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2, 89–101 (1995).
Tzourio-Mazoyer, N. et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 15, 273–289 (2002).
Matuskey, D. et al. A preliminary study of dopamine D2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [(11)C](+)PHNO. Drug Alcohol Depend. 154, 167–173 (2015).
Gaiser, E. C. et al. Elevated Dopamine D2/3 Receptor Availability in Obese Individuals: A PET Imaging Study with [11C](+)PHNO. Neuropsychopharmacology 41, 3042–3050 (2016).
Gallezot, J. D. et al. Parametric Imaging and Test-Retest Variability of ¹¹C-(+)-PHNO Binding to D2/D3 Dopamine Receptors in Humans on the High-Resolution Research Tomograph PET Scanner. J. Nucl. Med. Off. Publ., Soc. Nucl. Med. 55, 960–966 (2014).
Gunn, R. N., Lammertsma, A. A., Hume, S. P. & Cunningham, V. J. Parametric Imaging of Ligand-Receptor Binding in PET Using a Simplified Reference Region Model. NeuroImage 6, 279–287 (1997).
Wengler, K., He, X., Abi-Dargham, A. & Horga, G. Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. Neuroimage 208, 116457 (2020).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Avants, B. B., Tustison, N. & Song, G. Advanced normalization tools (ANTS). Insight J. 2, 1–35 (2009).
Pauli, W. M., Nili, A. N. & Tyszka, J. M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 5, 1–13 (2018).
Spincemaille, P. et al. Clinical integration of automated processing for brain quantitative susceptibility mapping: multi‐site reproducibility and single‐site robustness. J. Neuroimaging 29, 689–698 (2019).
Liu, J. et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 59, 2560–2568 (2012).
Pei, M. et al. Algorithm for fast monoexponential fitting based on Auto-Regression on Linear Operations (ARLO) of data. Magn. Reson Med. 73, 843–850 (2015).
De Rochefort, L. et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 63, 194–206 (2010).
Liu, Z., Spincemaille, P., Yao, Y., Zhang, Y. & Wang, Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn. Reson Med. 79, 2795–2803 (2018).
Fisher, B. E. et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 24, 509–514 (2013).
Lai, J.-H. et al. Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson’s disease. Brain Res. 1720, 146301 (2019).
Matsuura, K. et al. A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci. Lett. 633, 112–117 (2016).
Acknowledgements
This works was supported by the NINDS/NIH grant R21 NS118764.
Author information
Authors and Affiliations
Contributions
Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the first draft, B. Editing the manuscript, C. Review and Critique. B. de Laat: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B, 3C. J. Hoye: 1C, 2A, 2B, 2C, 3B, 3C. G. Stanley: 1B, 1C. M. Hespeler: 1B. 1C. J. Ligi: 1B, 1C. V. Mohan: 2A, 2B, 2C, 3B, 3C. D.W. Wooten: 2A, 2B, 2C, 3B, 3C. X. Zhang: 2A, 2B, 2C, 3B, 3C. T.D. Nguyen: 2A, 2B, 2C, 3B, 3C. J. Key: 2A, 2B, 2C. G. Colonna: 2A, 2B, 2C. Y. Huang: 1C. N. Nabulsi: 1C. A. Patel: 1C. D. Matuskey: 1C, 3B, 3C. E.D. Morris: 1A, 1B, 1C, 2A, 2C, 3B, 3C. S. Tinaz: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B, 3C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Laat, B., Hoye, J., Stanley, G. et al. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson’s disease. npj Parkinsons Dis. 10, 34 (2024). https://doi.org/10.1038/s41531-024-00641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00641-1