Abstract
The assessment of cognitive deficits is pivotal for diagnosis and management in patients with parkinsonisms. Low levels of correspondence are observed between evaluations assessed with screening cognitive tests in comparison with those assessed with in-depth neuropsychological batteries. A new tool, we named CoMDA (Cognition in Movement Disorders Assessment), was composed by merging Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Frontal Assessment Battery (FAB). In total, 500 patients (400 with Parkinson’s disease, 41 with vascular parkinsonism, 31 with progressive supranuclear palsy, and 28 with multiple system atrophy) underwent CoMDA (level 1–L1) and in-depth neuropsychological battery (level 2–L2). Machine learning was developed to classify the CoMDA score and obtain an accurate prediction of the cognitive profile along three different classes: normal cognition (NC), mild cognitive impairment (MCI), and impaired cognition (IC). The classification accuracy of CoMDA, assessed by ROC analysis, was compared with MMSE, MoCA, and FAB. The area under the curve (AUC) of CoMDA was significantly higher than that of MMSE, MoCA and FAB (p < 0.0001, p = 0.028 and p = 0.0007, respectively). Among 15 different algorithmic methods, the Quadratic Discriminant Analysis algorithm (CoMDA-ML) showed higher overall-metrics performance levels in predictive performance. Considering L2 as a 3-level continuous feature, CoMDA-ML produces accurate and generalizable classifications: micro-average ROC curve, AUC = 0.81; and AUC = 0.85 for NC, 0.67 for MCI, and 0.83 for IC. CoMDA and COMDA-ML are reliable and time-sparing tools, accurate in classifying cognitive profile in parkinsonisms.
This study has been registered on ClinicalTrials.gov (NCT04858893).
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) and atypical parkinsonian syndromes (APS) define the whole group of parkinsonisms, which are the major subsets of hypokinetic movement disorders. Nowadays, they represent a challenge for public health worldwide, because of their growing incidence in the population. The defective mesostriatal dopaminergic transmission in PD impairs both movement expression and action performing. The clinical spectrum includes motor symptoms (rigidity, resting tremor, bradykinesia, gait disturbances, postural abnormalities, and balance dysfunctions) and nonmotor symptoms. Nonmotor symptoms include sleep disorders, autonomic and gastrointestinal dysfunctions, sensory disturbances, motivational abnormalities, and cognitive deficits1. These latter may range from mild cognitive impairment (MCI) to impaired cognition (IC), as far dementia. Nonmotor symptoms can occur across all stages of PD and are key determinants of quality of life1,2,3. Progressive supranuclear palsy (PSP) and multiple system atrophy (MSA) are the most representative forms of neurodegenerative APS, whose etiology is different from that of PD. Vascular parkinsonism (VP) is a form of APS, whose features are of vascular origin, despite the symptoms are widely overlapped with those of other APS. As well as in PD, nonmotor features in APS determine a huge impact on quality of life and prognosis4.
The characterization of the right neuropsychological profile is pivotal to define the differential diagnosis with other neurological diseases and among different kinds of parkinsonisms5,6,7. Furthermore, it is also crucial to optimize both the pharmacological and rehabilitation treatments and, more generally, to improve the disease management5,6. For distinguishing between normal cognition (NC), MCI and IC in PD, the MDS-task force proposed two levels of evaluation: an abbreviated assessment (level I—L1) and/or a comprehensive assessment (level II—L2). The first one requires the administration of screening tests, while the second one requires large, in-depth neuropsychological batteries with at least two tests in each of the five principal cognitive domains: attention/working memory, executive function, language, memory, and visuospatial function8. To reach L1 classification, numerous screening tests have been proposed. Among them, the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) are widely adopted for evaluating global cognition8,9,10,11,12,13,14. Requiring no more than 10–15 min for administration, L1 evaluation provides an undoubtable advantage in terms of time-sparing in comparison with L2, which includes time-consuming neuropsychological tests. However, the two-level evaluation differs from at least two important aspects. First of all, by referring to the today-available cognitive screening tools, L1 provides clinicians with a classification along only two levels: scores higher than the cutoff indicate NC, while scores lower than the cutoff indicate MCI and IC, without distinction among the two classes8. Conversely, for L2 evaluation, MDS task force indicates the criteria for distinguishing NC from MCI and the last one from IC15. The second criticism refers to the low level of correspondence between L1 and L2 classifications16. In fact, several previous studies observed a discrepancy between the diagnoses obtained at L1 and those obtained at L2. Marras et al.17 found that the reliability of MoCA and MMSE is poor, when MDS task-force criteria were considered to define cognitive profile in PD patients. Moreover, comparing PD with parkinsonisms, Santangelo et al.6 observed very high percentage of impaired performances on L2 evaluation only in PSP patients, despite their MoCA score was within the normality range (mean score 20.1, when cut-off for Italian population is 15.5). Because frontal and executive functions are the most affected in PD and APS, FAB could be considered a valid alternative. In this concern, Eschlbock et al.14 studying cognition in MSA noted an impairment in executive functions in 40% of patients (evaluated with FAB), despite their MMSE was 27.6, making questionable the sensitivity of MMSE in detecting executive dysfunctions. Bezdicec et al.18 observed a specific relation between FAB scores and gray-matter density in frontal lobe of PD patients, recommending this test as a valid instrument for PD–MCI L1 screening.
Actually, while the weakness of MMSE and MoCA is the poor reliability, the weak point of FAB is to be too specific, thus losing the global vision in addressing patients’ cognition. In this study, we aim first at leveraging the screening capacity of MMSE, MoCA, and FAB by merging them into a single and composite measure, namely Cognition in Movement Disorders Assessment (CoMDA) (see Table 1). Further, we aim to overcome the limitations of L1 classification optimizing the screening-tool reliability of cognitive profile classification and reaching the 3-level class distribution. To reach these purposes, we referred to machine learning (ML). ML is a sub-area of artificial intelligence (AI) and excels in generating predictive models (both parametric and nonparametric) that learn effectively linear and nonlinear complex relationships among multivariate data patterns. ML has shown excellent accuracy and cross-generalization levels in diagnostic prediction19,20 and, therefore, such architectures are becoming increasingly important in the modern medical decision-making process21. The use of ML in healthcare has become common to carry out regression, classification, or unsupervised clustering tasks for various predictive models applied to a wide range of clinical uses22. Also, such predictive modeling capacity proved to outperform other more classical or deterministic solutions22. This is true, especially when the input variables (aka predictors) have nonlinear relations, abnormal distributions, or when they change over time. In fact, ML implementation is based on loss-function minimization methods, which allow to reduce the expected error in prediction. Another leveraging factor in referring ML models consists in their intrinsic capacity to improve the rate of accuracy and precision over time. Indeed, ML models improve their performances proportionally to resampling and retraining events. Such methods learn new patterns in the data and augment accuracy, precision, or sensitivity as their fine-tune parameters are exposed to learning based on new samples23.
Resuming, the aims of this study are (1) to define a new tool, named CoMDA, able to evaluate the global cognition with particular attention at frontal and executive functions; (2) to model and deploy ML algorithms, by implementing CoMDA-score as key predictor feature, to develop a powerful and fast screening tool with greater sensitivity in comparison with the available tools; and (3) to differentiate the patients’ performance along three classes of cognitive profile: “NC”, “MCI”, and “IC”.
Results
Baseline statistics and comparison substudy
The study population consisted in 400 PD, 41 VP, 31 PSP, and 28 MSA patients: Table 2 reports demographic variables of patients, grouped by disease.
The results of the method-comparison substudy are reported in e-Table 1 (Supplementary material) and confirmed that the CoMDA-derived MMSE, MoCA, and FAB scores are in excellent agreement with the values of the original source measures. The bias (i.e., systematic error) was nonsignificant for all scores and ranged from 1.2% (MoCA) to 0.4% (MMSE). According to the limits of agreement, one can be 95% confident that the error is less than 7% and 12% in the best case (MMSE) and in the worst case (FAB), respectively.
The CoMDA evaluation took 14.1 ± 1.1 min. This time was considerably longer than the time for assessing FAB and MMSE (4.1 ± 0.2 and 4.2 ± 0.4 min, respectively), but only about 44% more than MoCA (9.8 ± 0.6 minutes) and 28% less than the time needed for administering all three tests (18.0 ± 1.0 minutes).
Statistics between disease groups
In e-Table 2 (Supplementary material), the percentage of normal performances to screening tests and in-depth neuropsychological tests according to normative data for Italian population are reported, stratified by disease.
Table 3 reports the results of nonparametric analysis of variance for screening and in-depth neuropsychological test scores for all groups of patients. Global group effect was significant for all screening tests (p = 0.032 for MMSE, p < 0.0001 for FAB, MoCA, and CoMDA). Post hoc analysis revealed only borderline significant differences in MMSE and CoMDA between PD and MSA, and in MoCA between PD and VP. At variance, largely significant differences in FAB, MoCA, and CoMDA were found between PD and PSP.
Regarding in-depth evaluation, global group effect was not statistically significant for WCST and ROCF-C (p = 0.10 and p = 0.17, respectively), while it was significant for all remaining tests. Post hoc analysis revealed several differences between groups of patients (see Table 3). On the other hand, as e-Table 2 describes, WCST and ROC-C stand out for being the most frequently impaired, in all groups, confirming that the executive and visuospatial ones are the most impaired cognitive functions in PD and APS.
Predictive discrimination analysis
Cross-tabulations of L1 cognitive impairment by patients’ groups (‘1’ ≥ cutoff, ‘0’ = < cutoff) intercepted by MMSE, MoCA, and FAB are reported in e-Table 3 (Supplementary material), while L2 cognitive impairment cross-tabulation by patients’ groups is reported in e-Table 4 (Supplementary material). There is a highly significant association between L2 and disease-group distribution (Chi-Square p < 0.0001).
The comparison of MMSE, MoCA, FAB and CoMDA scores in NC and MCI + IC as assessed by L2 is reported in e-Table 5 (Supplementary material). It can be seen that MMSE, MoCA, FAB, and CoMDA scores are significantly worse in MCI and IC than NC as assessed by L2.
ROC analysis was used to quantify how accurately CoMDA, MMSE, MoCA, and FAB scores can discriminate between NC, MCI, and IC as assessed by L2. Figure 1 shows the ROC curves obtained for the four cognitive-screening tools. The AUC ranged from 0.708 for MMSE, to 0.789 for MoCA, to 0.759 for FAB, and 0.814 for CoMDA. Notably, contrast analysis for ROC curves revealed that AUC for CoMDA was significantly higher than those for MMSE, MoCA, and FAB (p < 0.0001, p = 0.028, and p = 0.0007) (see Fig. 1).
Machine learning model: CoMDA-ML
A total of 15 different models were engineered and tested. They underwent a standard “10-fold cross validation technique” and e-table 6 reports the averaged metric performances for each estimator respectively. Cross-validation performance was based on a training set of 349 samples (~70% of total 500 patient-sample data) implementing a k-fold cross-validation procedure, k = 10. The trained algorithms were then scored on a final test/holdout set of 151 samples (remaining ~30% of sample data) to verify cross-generalization performance and the presence of undesirable model overfitting on unseen data (test/holdout set).
The predictive performance of the Quadratic Discriminant Analysis (QDA) algorithm showed higher overall-metrics performance levels across all algorithmic methods. Indeed, as shown in e-Table 6 (Supplementary material), the QDA algorithm resulted in the best-performing one across several competing estimators considered during the training validation process, outperforming other algorithms on the majority of the metrics.
This model maximized the bias-variance trade-offs, as it expresses optimal L2-prediction accuracy while maintaining appropriate cross-generalization performance.
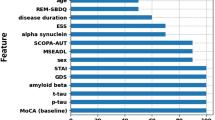
Out of the 7 candidate predictors (see the statistics section—Machine learning), only 4 were selected by the QDA algorithm to predict 3-level L2 classification: “CoMDA score”, “age”, “disease duration”, and “years of education”. The resulting ML model was named “CoMDA-ML”.
The final model implemented a weighting on “CoMDA score” separately with “age” and “education”. These additional weightings defined two new predictor variables in the algorithm: “age*CoMDA score” and “education*CoMDA score”.
Among all candidate and new predictors, CoMDA score is the more informative one. In fact, as it can be seen in e-table 7 and e-table 8, the Information Gain tests revealed that CoMDA score provides the highest Information Gain, or the best-information entropy reduction, both in the original data sample and in the training set, where age*CoMDA and education*CoMDA features have been defined in the algorithm.
These variables, used to train the QDA, improved sensibly the predictive power. The final hyperparameters configurations of the finalized CoMDA-ML model are the exposed algorithm of the QDA: a classifier with a quadratic decision boundary, generated by fitting class-conditional densities to the data and using Bayes’ rule24.
QDA is derived by a target-class conditional distribution of the form
and instantiation of Bayes’ rule for any given training sample
where a targeted class k maximizes the posterior distribution with the log of the posterior
with \(Cst = P\left( x \right)\).
(Source: Scikit Learn API, 2021—https://scikit-learn.org/stable/modules/lda_qda.html#lda-qda). The QDA predicted class is the one that maximizes this log-posterior (see also e-Table 9—Supplementary materials).
The L2 multilevel-classification results are given by standard micro- and macro-average ROC curves, as well as individual class (one-vs-others) ROC curve values shown in Fig. 2. There is favorable convergence between micro- and macro-average AUC and ROC curve values at 0.81 and 0.79, respectively. Likewise, adequate single L2-class (one-vs-others) AUC ROC values have been achieved. Such findings replicate the ROC curve profiles obtained by the preceding baseline statistical tests. It is to note that micro-averaging combines all true/false-positive instances (across classes) to compute a single global ROC curve. Instead, macro-averaging pools together multiple ROC curve statistics as computed by each L2 class separately. Micro-averaging weights according to class dimension and macro-averaging equally weights across classes. Both are relevant to a complete multilevel assessment.
At the final test/holdout set CoMDA-ML resulted to provide accurately the 3-level multilevel classifications, having accuracy = 0.682, AUC = 0.81, sensitivity/recall = 0.682, precision = 0.681, F1 score = 0.676, Kappa = 0.508, and MCC = 0.513.
The model confusion matrix at test/holdout set is given in Fig. 3 and it shows positive distribution rate of true positives in the main diagonal with respect to false-positive and false-negative rates.
Finally, e-Table 10 (Supplementary material) shows 10 random samples in the test/holdout set where the last two columns report the predicted L2 class (Label) besides the actual L2 class (L2): model-prediction results effective at this test-deployment outset: it can be seen that CoMDA-ML provides a reliable prediction of L2 classification.
Discussion
The present study was aimed at creating CoMDA, a useful and time-sparing cognitive-screening tool thought to specifically evaluate the cognitive profile of PD and APS patients, and at developing CoMDA-ML to ameliorate the reliability of evaluation and to classify the patients’ performance along three classes of cognitive profile (“NC”, “MCI”, and “IC”), thus reducing the discrepancies between the classifications obtained with screening tests in comparison with in-depth neuropsychological batteries.
To the best of our knowledge, this is the first study in which AI is applied in cognitive-screening assessments to generate a prediction of the cognitive profile.
CoMDA has been built based on preexisting, frequently adopted screening tests, whose utilization is widely accepted, both in clinical and research settings. By merging the MMSE, MoCA, and FAB items, CoMDA guarantees a global evaluation, with particular attention on executive and frontal functions, specifically impaired in PD and APS patients. Furthermore, CoMDA-ML allows to improve the reliability of L1 evaluation and, finally, to classify the cognitive profile of PD and APS patients along three different classes: “NC”, “MCI”, and “IC”.
PD and APS share many signs and symptoms and present different rates of incidence in the population. These assumptions explain why our sample was not balanced among diseases, age, gender, and age at onset. As patients were continuously enrolled, the rate of PD and APS in our sample reflects the distribution of these pathological conditions in the general population25.
The results from the neuropsychological evaluation confirm the impairment of cognition in patients with PD and APS26,27. Fifty-nine percent of them get defective performances in one or more neuropsychological tests. We found that visuospatial deficits are the most common among PD and APS patients, followed by impairments in attention (specifically in interference-suppression capability), and executive functions, (mainly in mental flexibility). PSP patients manifest greater cognitive impairments than those suffering from other parkinsonisms. More than 50% of PSP patients showed pathological performances in WCST, TMT B–A, ROCF-c, ROCF-dr, and ST-E. Performances in ST-E and ROCF-c were defective in about 80% of PSP patients6,28. ROCF copy was the most impaired test among patients with MSA (67.9% of defective performances)29. The WCST test was defective in 42.7% of patients with PD30, thus confirming the executive dysfunction31. Therefore, deficits in executive functions remain the common neuropsychological denominator in all these pathological conditions. This finding may be prognostically valuable for identifying PD and APS patients at risk of dementia7,27,32,33,34. CoMDA is powerful in bringing together the intrinsic value of each single item belonging to the most used screening tests into a single instrument equipped with an effective elaboration processing, able to depict simultaneously both executive and visuospatial functions.
The results of the method-comparison substudy confirmed that the CoMDA-derived MMSE, MoCA, and FAB scores are in excellent agreement with the values of the original source measures. This is a point of strength of CoMDA, which can automatically provide the score of three well-known and widely adopted tests. By referring at nowadays-available normative data for these scores, we can observe that MMSE and FAB differ between patients and healthy controls and among the disease groups. Conversely, MoCA score did not differ between patients and healthy controls. This finding could be related to the low MoCA cutoff score identified in the normative data for the Italian population35.
MMSE, MoCA, and FAB scores showed a strong relation with L2, which is considered as the gold standard to diagnose cognitive impairment in patients with PD7,8,15. Comparing the classifications obtained with CoMDA with those of the other three screening tests, we objectivated a significant greater reliability of the first one. This result is really worth of attention, as misleading cognitive data could hinder or delay the right diagnosis in patients suffering from PD or APS. Reaching a good classification of cognitive profile is fundamental not only for diagnostic concerns, but also for several clinical and management-related aspects: (i) to select the best pharmacological treatments36, (ii) to direct the neurorehabilitation approaches2,3, and (iii) to optimize the home-based patient’s management37.
The present study highlights how combining psychometric tests and AI can be effective for defining the cognitive profile in PD and APS and for identifying patients at risk of dementia.
The application of predictive analytics through CoMDA-ML for 3-level cognitive-profile classification allowed to reach a reliable level to distinguish among NC, MCI, and IC.
Notably, the crucial condition to reach these two aims is the application of the AI methods. The use of ML in healthcare has become common to carry out regression, classification, or unsupervised clustering tasks for various predictive models. ML implementation is based on loss-function minimization methods, which allow to reduce the expected error in prediction. Notably, the finalized 6-predictor set (i.e., CoMDA, age, education, disease duration, age*CoMDA and education*CoMDA) was not directly anticipated or given by specific hypotheses. The deep-learning process promoted the exclusion of 3 candidate predictors (“gender”, “years of education”, and “L1 score”) and the setting of two additional ones. These last have been obtained by running ML experimentations, with feature-engineering processes, based on the available information, which were the baseline predictors. Interestingly, the two new-built features, used to train the QDA, improved the predictive power, but only together with the other original features.
The present study presents such limitations. Despite the studied population came from all over Italy, this is a single-center study, so that the generalizability of the results is reduced. Further, it could be very interesting to build the same tool by adopting different psychometrics batteries.
Since the rising number of people suffering from PD and APS represents a critical challenge for the public health worldwide, the effort of engaging applicative AI research in this field is really actual. Diagnostic approaches like CoMDA, designed to be equally time-saving and markedly reliable, could maximize our diagnostic capacity and the disease management, thus representing one of the most important steps to follow in the upcoming future.
Methods
This study is part of a larger prospective, observational, analytical, single-center, cohort study, devised to create a database of clinical, functional, motor, neuropsychological and neurophysiological variables in patients affected by PD and APS coming from all over Italy. The whole study aims at optimally characterizing the profile of these patients in order to better define their treatment into the neurorehabilitative setting.
The protocol was conducted at the Department of Parkinson’s Disease and Movement Disorders Rehabilitation of the “Moriggia-Pelascini” Hospital (Gravedona ed Uniti, Italy) between January 2017 and December 2019. The study design and protocol were approved by the local Ethics Committee (“Comitato Etico Interaziendale delle Province di Lecco, Como e Sondrio”) and were in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1967). The study was also registered on ClinicalTrials.gov (NCT04858893).
Subjects
Six-hundred sixty-one patients were consecutively enrolled by neurologists with experience in movement disorders.
Patients were included in the present study if they met one of the following criteria: (a) diagnosis of idiopathic PD according to the MDS clinical diagnostic criteria38; (b) diagnosis of PSP according to the MDS clinical diagnostic criteria39; (c) diagnosis of MSA according to the second diagnostic consensus statement40 and (d) diagnosis of VP according to Zijlmans et al.41.
Exclusion criteria were (a) any focal brain lesion detected with brain-imaging studies; (b) psychiatric disorders, psychosis (evaluated with Neuropsychiatric Inventory), and/or delirium; (c) previous diagnosis of dementia; (d) neurological diseases other than PD or APS; (e) other medical conditions negatively affecting the cognitive status; (f) disturbing resting and/or action tremor, corresponding to scores 2–4 in the specific items of MDS Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) III, such as to affect the psychometric evaluation; (g) disturbing dyskinesia, corresponding to scores 2–4 in the specific items of MDS-UPDRS IV, such as to affect the psychometric evaluation; (h) auditory and/or visual dysfunctions impairing the patient’s ability to perform cognitive tests.
Accordingly, 161 patients were excluded from the study: 76 had previous diagnosis of dementia, 13 presented sensorial deficits (2 with visual impairment, 11 with hearing impairment), 51 suffered from psychiatric disorders and 21 presented disturbing tremor and/or dyskinesia. This led to a final study population of 500 patients. According to the clinical diagnostic criteria38,39,40,41, patients were classified as follows: 400 with PD, 41 with VP, 31 with PSP, and 28 with MSA.
A complete explanation of the study protocol was provided, and written informed consent was obtained from all participants before their participation in the study.
Neuropsychological evaluation
The in-depth neuropsychological evaluation was administered by expert neuropsychologists, blinded to patients’ diagnosis. All patients were tested during the morning, in two consecutive days, in a laboratory setting, with constant artificial lighting condition and in the absence of auditory interferences. PD patients were evaluated in medication “on” state.
-
1.
First evaluation: CoMDA
“CoMDA” stands for “Cognition in Movement Disorders Assessment” and combines MMSE, MoCA and FAB individual measures into a single tool. More specifically, CoMDA consists of all items of the three tests, without repetition for items that appear in more than one of them (e.g., this occurs for the 6 items evaluating orientation, which are both in MMSE and MoCA). CoMDA is thought to maximize the diagnostic-capacity power to screen patients with PD and APS. In our assumption, CoMDA was adopted to define and categorize these patients into three classes: NC, MCI, and IC.
CoMDA scores result by linear non-weighted combination (additive model) of the non-redundant MMSE, MoCA and FAB items (see Table 1).
CoMDA allows four different scores in L1: the first three ones are “partial” scores, which are obtained by scoring and summarizing all items of each single test (MMSE: 0–30, MoCA: 0–30, and FAB: 0–18) adjusted (weighted) on the Italian population data as by previous research42,43,44. The fourth one is the “total” score (CoMDA score), which is obtained by summarizing the first three “partial” scores. CoMDA score ranges from 0 (worst performance) to 78 (best performance).
-
2.
Psychometric test battery
Furthermore, patients underwent a large battery of neuropsychological tests for evaluating several cognitive domains (see Table 4), according to the indications provided by Goldman et al.15. The majority of studies for obtaining normative values conducted on the Italian population45,46,47,48,49,50 adopted a statistical procedure, which provides regression-based norms and a system of scores on an ordinal scale, named Equivalent Scores (ES). It ranges from class 0 (scores equal or higher than the outer tolerance limit of 5%) to class 4 (scores lower than the median value of the whole sample); 1, 2, and 3 classes were obtained by dividing into three equal parts the area of distribution between 0 and 4. This method makes it possible to judge the scores obtained by the person under examination with respect to those of normal subjects, taking into account of the influence of variables related to age, education, and gender.
Hence, the whole patient’s performance was classified based on the termed L2, by adapting the indications provided by Litvan et al.8 to the described system of scores, along three consecutive classes: 2 = NC (all ES > 0 or one ES = 0); 1 = MCI (two ES = 0, in tests evaluating the same cognitive domain or two ES = 0, in tests evaluating two different cognitive domains); 0 = IC (more than two ES = 0).
Baseline statistics and machine learning
Descriptive statistics and method-comparison substudy
Basic descriptive statistics for continuous variables were reported as mean ± SD. Descriptive statistics for categorical variables were reported as N (percent frequency).
To assess whether MMSE, MoCA, and FAB scores, derived from CoMDA values, fit the scores computed in the standard way, we set up a method-comparison substudy. A group of 20 patients underwent two assessment sessions, in random order: one session included the administration of MMSE, MoCA, and FAB; the other one included the administration of the CoMDA. The agreement among “standard MMSE, MoCA and FAB scores” and “CoMDA-derived MMSE, MoCA and FAB scores” was assessed by Bland–Altman analysis, computing the bias (systematic difference) and the 95% limits of agreement (the range within which 95% of the differences are expected to lie). The Pearson correlation coefficient was also computed.
The time needed to administer CoMDA was also registered and compared both to the time needed for each standard test and the sum of the times required for the three single tests.
Inferential statistics
Non parametric Kruskal–Wallis test and the Chi-square test were carried out for between-group comparisons for continuous and categorical variables respectively. Non parametric Mann–Whitney U-test was applied for single pairwise between-group comparisons. Post hoc comparisons with Dunn–Sidak adjustments were applied for paired multiple-comparison tests.
Predictive discrimination analysis
The area under the curve (AUC) of receiver operating characteristic (ROC) curves was computed to assess the ability of all available cognitive screening tools to discriminate between two classes: NC versus MCI or IC, as assessed by the L2 classification (L2 = 0 vs. L2 = 1 + L2 = 2). A value of 0.5 indicates no predictive discrimination, while a value of 1 indicates perfect separation of patients with and without cognitive impairment. The AUC for the CoMDA, MMSE, MoCA, and FAB tools was compared by the Hanley–McNeil test.
A p-value < 0.05 was considered statistically significant. All analyses were carried out using the SAS/STAT statistical package, release 9.4 (SAS Institute Inc., Cary, NC, U.S.A.).
Machine learning
The ML solutions were engineered to finalize L2 classification on the total sample of 500 patients. All ML models availed of a baseline pool of 7 prescreening candidate predictors: “CoMDA score”, “gender”, “age”, “disease”, “disease duration”, “years of education”, and “L1 score”. A large set of ML architectures (i.e., algorithms, parameters and hyperparameter combinations) were concurrently tested to obtain the final model, which is the best in fitting algorithmic configuration to correctly predict L2 classification from the available data.
Models were validated via k-fold cross-validation operated on a training partition set out of the 500 available samples. This procedure implies to split the available dataset into k non-overlapping folds. Each of the k folds could be used as a held-back test set, while all other folds collectively are used as a training dataset. A test/holdout set was used to measure unbiased cross-generalization performance level of the ML solution on unseen data. A random-shuffling train-test split was carried out to avoid any potential selection bias. This procedure was performed on the original data sample before any model-training operation.
To know the most important predictor among the 7 available, the value of Information Gain, obtained in prediction, has been quantified. This value reflects a measure of “entropy reduction” or “information relevance” of predictors in the dataset of reference51,52.
Finally, cross-algorithm performances were assessed by widely adopted standard prediction metrics: accuracy, AUC, recall, precision, F1, kappa and MCC.
All machine-learning experiments were carried out by coding in Python 3.8 (Python Software Foundation, 9450 SW Gemini Dr., ECM# 90772, Beaverton, OR 97008, USA) with full use of PyCaret 2.3.3 library (PyCaret.org. PyCaret) and Jupiter Notebook (Python editors). The finalized version of ML algorithm had hyperparameters fine-tuned via “Optuna” mathematical method53.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All source codes and models are available for consultation by the authors’ permission.
References
Chaudhuri, K. R. & Schapira, A. H. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474 (2009).
Ferrazzoli, D. et al. Basal ganglia and beyond: the interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci. Biobehav. Rev. 90, 294–308 (2018).
Ferrazzoli, D. et al. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J. Neurol. Neurosurg. Psychiatry 89, 828–835 (2018).
Bhatia, K. P. & Stamelou, M. Nonmotor features in atypical Parkinsonism. Int. Rev. Neurobiol. 134, 1285–1301 (2017).
Martinez-Horta, S., Horta-Barba, A. & Kulisevsky, J. Cognitive and behavioral assessment in Parkinson’s disease. Expert Rev. Neurother. 19, 613–622 (2019).
Santangelo, G. et al. Comparative cognitive and neuropsychiatric profiles between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J. Neurol. 265, 2602–2613 (2018).
Emre, M. et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 22, 1689–1707 (2007). quiz 1837.
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356 (2012).
Gill, D. J., Freshman, A., Blender, J. A. & Ravina, B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov. Disord. 23, 1043–1046 (2008).
Hoops, S. et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73, 1738–1745 (2009).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Zadikoff, C. et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov. Disord. 23, 297–299 (2008).
Fiorenzato, E. et al. Montreal Cognitive Assessment (MoCA) and mini-mental state examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J. Neural Transm. 123, 1435–1442 (2016).
Eschlböck, S. et al. Cognition in multiple system atrophy: a single-center cohort study. Ann. Clin. Transl. Neurol. 7, 219–228 (2020).
Goldman, J. G. et al. Diagnosing PD-MCI by MDS Task Force criteria: how many and which neuropsychological tests? Mov. Disord. 30, 402–406 (2015).
Rosenblum, S. et al. The Montreal Cognitive Assessment: is it suitable for identifying mild cognitive impairment in Parkinson’s disease? Mov. Disord. Clin. Pract. 7, 648–655 (2020).
Marras, C. et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov. Disord. 28, 626–633 (2013).
Bezdicek, O. et al. Frontal assessment battery in Parkinson’s disease: validity and morphological correlates. J. Int. Neuropsychol. Soc. 23, 675–684 (2017).
Brunetti, A., Carnimeo, L., Trotta, G. F. & Bevilacqua, V. Computer-assisted frameworks for classification of liver, breast and blood neoplasias via neural networks: a survey based on medical images. Neurocomputing 335, 274–298 (2019).
Li, X. et al. Using machine learning to predict stroke-associated pneumonia in Chinese acute ischaemic stroke patients. Eur. J. Neurol. 27, 1656–1663 (2020).
Jayatilake, S. & Ganegoda, G. U. Involvement of machine learning tools in healthcare decision making. J. Health. Eng. 2021, 6679512 (2021).
Sidey-Gibbons, J. A. M. & Sidey-Gibbons, C. J. Machine learning in medicine: a practical introduction. BMC Med. Res. Methodol. 19, 64 (2019).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Pedregosa, F. et al. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
de Lau, L. M. et al. Incidence of parkinsonism and Parkinson disease in a general population: the rotterdam study. Neurology 63, 1240–1244 (2004).
Goldman, J. G. et al. Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis. 4, 19 (2018).
Bak, T. H. et al. Cognitive bedside assessment in atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 76, 420–422 (2005).
Boxer, A. L. et al. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 16, 552–563 (2017).
Gerstenecker, A. The neuropsychology (broadly conceived) of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Arch. Clin. Neuropsychol. 32, 861–875 (2017).
Ortelli, P. et al. How Cognition and motivation “Freeze” the motor behavior in Parkinson’s disease. Front. Neurosci. 13, 1302 (2019).
Lange, F., Brückner, C., Knebel, A., Seer, C. & Kopp, B. Executive dysfunction in Parkinson’s disease: a meta-analysis on the Wisconsin Card Sorting Test literature. Neurosci. Biobehav Rev. 93, 38–56 (2018).
Foltynie, T., Brayne, C. E., Robbins, T. W. & Barker, R. A. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 127, 550–560 (2004).
Marinus, J. et al. Assessment of cognition in Parkinson’s disease. Neurology 61, 1222–1228 (2003).
Pagonabarraga, J. et al. Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov. Disord. 23, 998–1005 (2008).
Bosco, A. et al. Italians do it worse. Montreal Cognitive Assessment (MoCA) optimal cut-off scores for people with probable Alzheimer’s disease and with probable cognitive impairment. Aging Clin. Exp. Res. 29, 1113–1120 (2017).
Cools, R., Altamirano, L. & D’Esposito, M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia 44, 1663–1673 (2006).
van Balkom, T. D. et al. COGTIPS: a double-blind randomized active controlled trial protocol to study the effect of home-based, online cognitive training on cognition and brain networks in Parkinson’s disease. BMC Neurol. 19, 179 (2019).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 32, 853–864 (2017).
Gilman, S. et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676 (2008).
Zijlmans, J. C., Daniel, S. E., Hughes, A. J., Révész, T. & Lees, A. J. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov. Disord. 19, 630–640 (2004).
Measso, G. et al. The mini‐mental state examination: normative study of an Italian random sample. Dev. Neuropsychol. 9, 77–85 (1993).
Santangelo, G. et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 36, 585–591 (2015).
Appollonio, I. et al. The frontal assessment battery (FAB): normative values in an Italian population sample. Neurol. Sci. 26, 108–116 (2005).
Novelli, G., Papagno, C., Capitani, E. & Laiacona, M. Tre test clinici di memoria verbale a lungo termine: Taratura su soggetti normali. / Three clinical tests for the assessment of verbal long-term memory function: Norms from 320 normal subjects. Archivio di Psicologia, Neurologia e Psichiatria, 278–296 (1970).
Giovagnoli, A. R. et al. Trail making test: normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 17, 305–309 (1996).
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F. & Venneri, A. Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol. Sci. 22, 443–447 (2002).
Caffarra, P., Vezzadini, G., Francesca, D., Zonato, F. & Venneri, A. A short version of the Stroop test: Normative data in an Italian population sample. Nuova Riv. di Neurologia 12, 111–115 (2002).
Laiacona, M., Inzaghi, M. G., De Tanti, A. & Capitani, E. Wisconsin card sorting test: a new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol. Sci. 21, 279–291 (2000).
Spinnler, H. T. G. Standardizzazione e taratura italiana di test neuropsicologici: gruppo italiano per lo studio neuropsicologico dell’invecchiamento. (Masson Italia Periodici, 1987).
Battiti, R. Using mutual information for selecting features in supervised neural net learning. IEEE Trans. Neural Netw. 5, 537–550 (1994).
Brown, G., Pocock, A., Zhao, M.-J. & Luján, M. Conditional likelihood maximisation: a unifying framework for information theoretic feature selection. J. Mach. Learn. Res. 13, 27–66 (2012).
Akiba, T., Sano, S., Yanase, T., Ohta, T. & Koyama, M. in Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining 2623–2631 (Association for Computing Machinery, 2019).
Author information
Authors and Affiliations
Contributions
P.O., D.F., A.G., V.C., and M.Z. → data acquisition, analysis and interpretation, and manuscript draft. V.V., G.F., D.V., L.R., R.N., I.R., and LeSa → data acquisition and paper revision for intellectual content. LuSe, V.V., and R.N. → data acquisition and interpretation, paper revision for intellectual content. R.M. and D.B. → data analysis and paper revision for intellectual content. LuSe, G.F., D.B., and R.M. → data acquisition and manuscript revision for intellectual content. P.O., A.G., V.C., M.Z., and M.G. → patients’ enrollment, manuscript revision for intellectual content. G.F., LeSa, LuSe, and V.V. → data interpretation and manuscript revision for intellectual content. P.O., D.F., D.B. and R.M. → study design, patients’ enrollment, interpretation of data, and paper revision for intellectual content. P.O., D.F., R.M., D.B., LuSe, and V.V. → study design, patient enrollment, data interpretation, paper draft, and revision.
Corresponding author
Ethics declarations
Competing interests
The suthors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ortelli, P., Ferrazzoli, D., Versace, V. et al. Optimization of cognitive assessment in Parkinsonisms by applying artificial intelligence to a comprehensive screening test. npj Parkinsons Dis. 8, 42 (2022). https://doi.org/10.1038/s41531-022-00304-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00304-z
This article is cited by
-
Artificial Intelligence for Cognitive Health Assessment: State-of-the-Art, Open Challenges and Future Directions
Cognitive Computation (2023)