Abstract
The transport paths of O during intergranular oxidation in binary Ni-Cr were investigated. To isolate the selective oxidation of Cr, oxidation was performed with a CO/CO2 gas mixture in which the oxygen partial pressure was kept under the NiO dissociation pressure. A combination of electron microscopy and atom probe tomography (APT) was used to study the nanometer-scale details of the passivation and penetrative intergranular oxidation processes at high-energy grain boundaries. Oxygen transport towards the terminating oxidation front is elucidated with dedicated usage of oxygen tracer exchange experiments. Secondary ion mass spectroscopy and APT support classical theories of internal oxidation, revealing preferred transport paths at the oxide/alloy interface with sub-nanometer resolution.
Similar content being viewed by others
Introduction
Various Ni-base alloys rely on the formation of protective oxide scales to enhance their durability in applications at elevated temperatures. On the other hand, exposure of similar alloys with an insufficient concentration of desirable oxide-forming elements (e.g., Cr, Al), can lead to fast internal oxidation with concomitant negative effects on the material performance and mechanical properties1,2,3. Even with sufficiently high concentrations of these alloying elements, severe localized attack can result from the local loss of initially protective behavior. In these cases, internal oxidation can be detrimental to material lifetimes in oxidizing atmospheres at intermediate and high temperatures4,5.
In 1959, an idealized treatment to predict the transition from internal oxidation towards the growth of a continuous protective layer6. Besides the free energy of formation, diffusivities of elements and the solubility of O are important parameters that predict the oxidation behavior of alloys6,7. Although various studies investigated and discussed internal oxidation phenomena in detail for decades, conclusive evidence of the exact oxygen transport paths is still missing. The usage of Rhines packs is a dedicated approach to isolate internal oxidation by reducing the oxygen partial pressure below the equilibrium dissociation pressure of the base metal and its pure oxides (e.g., Ni/NiO)8. Stott and co-workers followed this strategy to investigate the internal oxidation of binary Ni alloys with up to 5 wt.% of alloying elements9. Faster internal oxidation in diluted Ni-Al and Ni-Cr alloys was attributed to accelerated oxygen transport along the interfaces between alloy and internal oxide precipitate9,10. For binary Ni-Cr alloys exposed at low oxygen partial pressures, the morphology of oxidation attack ranges from continuous oxides and/or formation of discrete oxide precipitates11,12,13, depending on the parameters of exposure. Besides transport, a second crucial mechanism in Wagner’s theory of internal oxidation is the supersaturation of oxygen, which has been more difficult to verify and quantify experimentally. A recent atom probe tomography (APT) study by Poplawsky et al. took an important step in this direction by revealing the enrichment of oxygen on grain boundaries (GBs) in pure Ni below the oxidation front14. However, the measured O concentration was surprisingly low, which indicates the need to revise classical model descriptions.
For Ni-base alloys, diffusion along GBs and other interfaces represents a particularly important mechanism during oxide scale growth in the intermediate temperature range (32–600 °C)15,16,17. More generally, this temperature regime corresponds to type B or C diffusion18, in which matrix diffusion is essentially frozen out. Owing to the enhanced transport of both, oxygen and alloying elements, detrimental corrosion scenarios are known to originate and proceed preferentially along GBs and interfaces. For example, intergranular (IG) oxidation is oftentimes a precursor to the initiation of stress corrosion cracking (SCC)19,20,21. Similarly, heat treatment of Cr-containing alloys can lead to the severe local depletion of Cr that sensitizes the material to SCC by inhibiting protective oxide formation22,23.
In both internal and IG oxidation, a detailed understanding of elemental transport is critical to elucidating the controlling mechanisms of corrosion. Two-stage exposure to oxygen isotopes (first 16O2 and subsequently 18O2) is an effective approach to visualize the transport paths of oxygen. Since labeled (18O) isotopes are only present during the second step, their distribution directly informs the dominant oxygen transport pathways and reaction sites relative to the initially formed oxide (16O) through the growing oxide scale. The majority of classical approaches for two-stage oxidation experiments used secondary ion mass spectroscopy (SIMS)17,24,25,26 or secondary neutral mass spectroscopy (SNMS)27,28 to analyze the distribution of labeled oxygen. While powerful in interrogating the isotopic distribution as a function of depth, neither technique possesses sufficient lateral resolution to investigate the three-dimensional distribution of oxygen isotopes through the oxide layer(s). To do so, we have deployed APT after two-stage oxidation experiments to directly visualize the 3D O isotope distribution accompanying IG oxidation of diluted Ni-Cr alloys. Another important contribution demonstrated the usage of an 18O tracer together with high-resolution analysis to elucidate the propagation of oxide growth along GBs below crack tips29. Similar to the Rhines pack method, the present study uses a controlled oxygen potential via a mixture of CO/C18O2 gas to bias the system towards selective oxidation of Cr and IG oxidation. By monitoring the penetration and distribution of 18O from C18O2 using APT, we can directly observe short-circuit diffusion of 18O towards the oxidation front along the IG oxide/metal interface.
Results
Morphology of oxide scales formed during exposure in CO-CO2 mixtures at 600 °C
Figure 1 displays SEM micrographs of representative surface regions of Ni-Cr model alloys after exposure to a CO-CO2 mixture at 600 °C. The same locations are characterized with SE and BSE contrast to demonstrate as many details as possible.
a, b SE micrographs of Ni-5Cr. c, d SE micrographs of Ni-20Cr. e, f BSE micrographs of identical regions (Ni-5Cr) as in top row. g, h BSE micrographs of identical regions (Ni-20Cr) as in top row. Specimens after two-stage exposures are indicated. Inset images show, respectively, a FIB-SEM cross-section view of IG attack (b) and a higher magnification of extruded Ni nodules (d) on the oxidized sample surface.
The focus lies on the appearance of GBs and directly adjacent regions. All specimens demonstrated comparable oxidation behaviors, with significant oxidation confined to regions close to GBs. Depending on the individual GB, the width of the surface oxide varies significantly. FIB cross-sections (inserts Fig. 1b) indicated that GBs that demonstrate a narrow dark contrast in the BSE micrographs were attacked by penetrative IG oxidation, while a wider stretch of surface oxide correlates with a protective oxide preventing penetrative oxidation along GBs. The latter revealed minor penetration of IG oxide phases, less than 50 nm below the original surface. Consequently, these GB with broader dark-appearing oxide caps were classified as protected GBs (e.g., Fig. 1a, h). After 1 h exposure, the width of these protective oxide caps is asymmetric, spreading further into one adjacent grain over the other. Lateral extensions of protective GB oxide phases appear less dependent on the grain orientation after 2 h exposure to CO/CO2. Additionally, it has to be noted that low-energy (twin) boundaries did not exhibit any oxidation. Consequently, no significant oxide on the surface of these characteristic interfaces could be recognized from to top view SEM micrographs (Fig. 1f). The grain interiors are covered by bright-appearing features. These nearly circular phases on the surface can be identified as Ni nodules that have already been observed in previous oxidation studies performed in atmospheres with particularly low \({p}_{{{{{\rm{O}}}}}_{2}}\)11. The classical oxidation theory associates the growth with metallic nodules on the surface of binary alloys with considerable internal oxidation and associated internal stress30. The density of these features increases with the time of exposure.
Site-specific TEM samples were prepared for a more detailed consideration of elemental distribution in oxidation-affected regions. Figure 2 displays high-angle annular dark-field (HAADF) micrographs of a Ni-5Cr GB that is protected by Cr2O3 and one GB that formed IG oxides after 1 h exposure at 600 °C. The protective oxide (Fig. 2) spreads laterally along the sample surface of both adjacent grains. The oxide layer covering the left grain is thicker than that formed on the opposite grain. To a minor extent, oxide penetrations (depth > 50 nm) at both termination sides are visible. However, the micrograph only displays the penetration of the protective oxide into the interior of the left grain. Above the oxide are extruded Ni-rich nodules that exhibit a bright contrast in HAADF imaging. The GB below the oxide cap appears unaltered in the HAADF image. Figure 2b–d demonstrates the morphology of a GB exhibiting IG oxidation. As suggested from the top view SEM images, no significant oxide formed across the exposed surface. Instead, a porous IG oxide extends ≈ 220 nm into the GB (Fig. 2b). Tilt series (shown in the supplementary material) demonstrated the interconnected nature of the porosity. The interface of the IG oxide with the left alloy grain is displayed in higher magnification in Fig. 2c, d. Fast Fourier transformed (FFT) of the atomically resolved HAADF is consistent with an M2O3 structure, and likely Cr2O3.
For comparison, representative regions of Ni-20Cr specimens after exposure at 600 °C in CO-CO2 were analyzed in the TEM. The micrographs in Fig. 3 display a protected GB after 1 h exposure and IG oxidation in a sample after 2 h total exposure during tracer exchange experiments. Generally, the Cr concentration of 20 at.% Cr leads to a higher number of protected than unprotected GBs, although the response is qualitatively quite comparable with the 5 at.% Cr oxidation. However, the displayed surface oxide is thicker compared to the one found on Ni-5Cr specimens, while the IG oxidation is shallower. Porosity developed in the region directly above the GB. Additionally, diffusion-induced grain boundary migration (DIGM) can be recognized in the HAADF image (Fig. 3a). The protective oxide layer on the Ni-20Cr sample (Fig. 3a) developed during the same length of exposure as the one displayed for Ni-5Cr (Fig. 2a). The internal oxidation front (IOF) penetrated ≈60 nm into the alloy. Both, the protective as well as the IG oxide, reveal considerable porosity, and a comparably thick surface oxide formed on top of the displayed IG oxidized GB (Fig. 2b). The increase of oxidation time caused a thicker surface oxide over a comparably wide section. The IOF terminates ≈ 140 nm below the surface. For each cross-section, a region directly below the pore in the IG oxide is displayed with higher magnification in Fig. 3c, d. Oxide at the termination of the protective cap penetrated both grains and reached a comparable depth as the IG oxide section along the GB. The surface of the Ni-20Cr sample is also covered with Ni nodules that nearly reach the region directly above the GB.
Additional STEM-EDX data were collected for representative regions of Ni-5Cr and Ni-20Cr after 1 h oxidation, as indicated in the HAADF micrographs (Figs. 2b and 3a). The resulting elemental distribution maps of O, Ni, and Cr are displayed in Fig. 4.
During IG oxidation of Ni-5Cr, the majority of Cr is supplied along the GB. High intensities of O do not exhibit considerable Ni enrichment, which excludes the formation of Ni-Cr spinel oxides. Both Ni maps confirm Ni nodules extrusion above the oxide layers, as speculated about previously in Fig. 1). Directly below the IOF, the Cr-depleted zone that surrounds the GB of the Ni-5Cr sample exhibits a width of ≈ 20 nm. This is broader than in the protected GB of the Ni-20Cr sample (≈ 12 nm) at the same depth. Additionally, Cr depletion adjacent to the lateral sections of the GB is recognizable in both grains of the Ni-20Cr sample. This finding indicates that lattice diffusion of Cr occurs at the considered temperature, however to a limited extent. The elemental distribution adjacent to the selectively oxidized GB of Ni-20Cr (Fig. 3b) during tracer exchange experiments in the CO-CO2 mixture was also investigated by means of STEM-EDX. Detailed results can be found elsewhere31. Again, Cr depletion is confined to the GB. However, the grain interiors adjacent to the oxide do not indicate any significant depletion in Cr. The growth of Cr2O3 has to be sustained by a Cr flux along the GB. In both cases, Ni is enriched along the GB as a consequence of Cr consumption during oxidation. With the demonstrated conventional electron microscopic analysis we confirm that the current experimental conditions evoke selective IG oxidation of Cr at select GBs in both Ni-Cr alloy compositions. In the next section, we further investigate these behaviors using isotopic tracers in the two-stage oxidation method.
Oxygen tracer exchange in CO/CO2 mixture at 600 ∘C
Two-stage isotopic tracer exchange experiments with labeled C18O2 were conducted at 600 °C to visualize O diffusion paths during IG oxidation. ToF-SIMS depth profiles were first recorded to document the average depth profile of the 18O incorporation. Figure 5 shows the measured intensity for relevant ion signal intensities versus sputter time for Ni-20Cr after 1 h with no 18O introduction and a total of 2 h in the two-stage 16O/18O oxidation. Since the morphological investigation indicated an inhomogeneous depth of oxide penetration, the sputtering time was not calibrated. Therefore, no specific (sputter) depth for the elucidated model alloys is given in the figure. For the applied \({{{{{\rm{Bi}}}}}_{3}}^{++}\) beam characteristics (50 keV acceleration voltage and 77 nA beam current), the sputter rate on a pure Ni sample can be approximated with 0.20 +/– 0.01 nm/s.
The selection of conclusive components of the SIMS signals can be quite tedious for complex oxides that developed in gaseous atmospheres. TEM-EDX confirmed Cr2O3 as the only relevant oxide phase that needs to be considered after exposure to binary Ni-Cr alloys in CO-CO2 mixtures. Intensity (depth) profiles of 52Cr16O− and 52Cr18O− (68 and 70 Da) are shown along with peaks for the two most-abundant oxygen ions (16O− and 18O−) at M/e values of 16 and 18 Da. Owing to the significantly lower isotopic abundance of 54Cr (0.024) compared to 52Cr (0.838), the peak at 70 Da can be attributed to labeled 52Cr18O− with sufficiently high confidence. As Ni did not exhibit meaningful oxidation under these low \({p}_{{{{{\rm{O}}}}}_{2}}\) conditions, the Ni− signal (58 Da) can be used to represent the substrate. The increase of the Ni signal indicates the transition to mostly oxygen-free sample regions below the IOF of the included GBs. This was similarly suggested in previous work that used ToF-SIMS to characterize oxide layer growth on Ni-Cr alloys26.
After 1 h oxidation in CO-CO2, the Ni-20Cr sample did not exhibit significant surface oxide apart from the protective Cr2O3 cap that covered certain GBs. Consequently, a maximum in the Ni− signal is visible after ≈ 58 s of sputtering in Fig. 5a. The intensity maximum of CrO− lies at ≈ 30 s of sputtering. Increasing the oxidation time to 2 h resulted in considerably thicker surface oxides as demonstrated with STEM-EDX. Consequently, the intensity maxima of Cr16O− (≈ 52 s) and Ni (≈ 141 s) were detected after longer sputtering times. Even more interesting is the depth distribution of labeled 18O in the ToF-SIMS data. Figure 5b indicates clear maxima of 18O− and Cr18O− signals after ≈ 95 s of sputtering. Since only the atmosphere of the second stage of exposure contained a significant 18O content, this shows that O has diffused through the pre-formed 16O oxide to the deeper IOF. Additionally, the lateral distribution of 16O− and 18O− was analyzed in more detail. The insets in Fig. 5b show the intensity distribution of the 16 Da (blue) and 18 Da (red) peaks between 60 and 500 s sputtering. Significant 18O− signal is confined along GBs, showing 18O penetration to the leading IG oxidation front.
The deliberate deviation from the natural isotopic abundance of oxygen during the second stage of the oxidation experiments added complexity to the interpretation of APT mass spectra. A custom treatment of the APT mass spectra was therefore needed. Figure 6 shows typical mass spectra that were collected after 1 h exposure to natural CO-CO2 and after 2 h tracer exchange experiments with labeled (CO-C18O2) oxygen. Intensities for both datasets were normalized to the 16O peak.
For both datasets (Fig. 6), the peak at 16 Da can unambiguously be allocated to 16O+. The mass spectra after exposure to isotopically labeled C18O2 exhibit a significantly increased peak intensity at 18 Da. The relative peak intensities at 17 Da are nearly identical and can therefore be allocated to 16O1H+. In contrast, the peak at 18 Da after two-stage exposure exceeds the 16O1H++ natural abundant oxide by two orders of magnitude. This is an explicit indication of the presence of labeled oxygen in the dataset. Additionally, it was stated in the literature, that a significantly higher 18 Da peak without considerable contribution at 17 Da excludes the formation of \({{{{{\rm{O}}}}}_{2}}^{++}\)32. Since combinations of Cr isotopes with both 16O and 18O have to be examined the treatment becomes complex. Peaks are indicated that correspond to contributions from CrO++ ions with the distinctions of all isotopes are indicated in Fig. 6. Since combinations of Cr isotopes with both 16O and 18O have to be examined the treatment becomes complex. For more details, the reader is referred to the supplementary section of the paper. After considering contributions from the most-abundant Cr and O isotopes the expected exclusive growth of Cr2O3 in the investigated APT data can be confirmed. Furthermore, the applied procedure for peak identification delivers relevant and accurate information about the distribution of natural and labeled contributions in the Cr2O3 region.
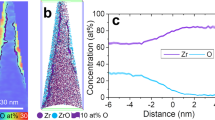
After reconstruction, the distribution of Cr16O and Cr18O along with Ni is represented as 3D ion maps. Figure 7a displays the oxide alloy interface of Ni-5Cr after two-stage exposure in the CO-CO2 mixture. For a more detailed analysis of the interface between oxide and alloy, a 6 at.% O isoconcentration surface was computed. To determine an accurate surface, the dataset was decomposed and all O contributions (16/18O) were considered. Towards the bottom of the reconstruction, the oxygen signal is dominated by contributions from Cr18O (green dots), whereas the upper region is highly enriched in Cr18O (red dots). Therefore, the displayed sample volume contains oxide that grew during the first stage (16O-rich) and oxide that formed during the second stage with a considerable 18O from the atmosphere. To elucidate the concentration changes across the oxide/alloy interface, local proximity histograms were calculated (Fig. 7b, c). The isoconcentration surface was subdivided manually into one section that separates the alloy and Cr16O-rich region and a second section between alloy and oxide that grew during exposure to CO-C18O2. The conducted workflows to compute local proximity histograms from a curved interface were adapted from Felfer et al.33,34. The surface was composed of a mesh of individual vertices (307 in Fig. 7) to calculate the “local" proximity histogram, only vertices adjacent to the region of interest are considered. For more details, the reader is referred to the original publications. To gain a visual impression of the lateral distribution of considered vertices (and triangulated faces), the isoconcentration surface is displayed along with the underlying mesh. The 93 vertices that were used to calculate proxigrams for the 18O-rich regions are displayed in the same color (red) as Cr18O ions, whereas the remaining vertices are drawn in the color (green) of Cr16O ions.
The proximity histogram in Fig. 7b indicates a locally homogeneous distribution of 16O and 18O through the oxide, at an 18O concentration of 45 at.%. Combining the contributions of 16O and 18O, the net oxygen concentration is approximately 60 at.%, which is consistent with the expected value for chromia. The high 18O contribution also is consistent with an interpretation that this region formed during the second stage of the exposure. Both proximity histograms indicate only minor depletion of Cr (≈ 2 at.%) in the alloy region directly adjacent to the alloy/oxide interface. The second proximity histogram shown in Fig. 7c reveals a more complicated behavior where 18O enrichment exists for ≈ 8 nm directly against the oxide/metal interface. This zone with increased contribution of labeled oxygen is hard to locate in the ion distribution map.
APT analysis of the internal alloy/oxide interface of a second sample volume is shown in Fig. 8. The targeted region was near the IOF. In this dataset, the entire IG oxide volume is homogeneously enriched in 18O. A proximity histogram from the 6 at.% O isoconcentration surface (Fig. 8b) shows no apparent enrichment of 18O at the oxide/metal interface. The Cr/O ratio (≈ 2:3) matches the expected ratio for Cr2O3. A weak depletion of Cr over approximately 6 nm can be seen in the alloy. However, the significant concentration of Cr directly at the interface (≈ 2 at.%) indicates a negligible contribution of Cr transport from the alloy to sustain oxide growth.
Further APT analysis on IG oxides in Ni-20Cr after two-stage exposure was conducted to verify if comparable O transport paths are present in alloys with a higher concentration of the alloying element. Particularly interesting is the interface between alloy and oxide phases that grew during exposure in CO-CO2 atmospheres with natural O abundance (e.g., Fig. 7c) since the enrichment of labeled oxygen suggests an important role during inward O transport. Figure 9 displays a representative APT analysis of an equivalent sample volume in the Ni-20Cr model alloy.
The IG oxide can be subdivided into two parts. One section contains a considerable 16O signal and therefore developed within the first 40 min of exposure (natural O abundance). A narrow region of newly formed oxide is located directly adjacent to the interface. As stated above, Cr2O3 with significantly enriched 18O content can only grow during the second stage of the experiment. This is an additional verification for preferential inward O transport along the interface between alloy and oxide.
The proximity histogram, displayed in Fig. 9b, quantitatively confirms the visual impression from the ion map. The newly formed 18O-rich oxide layer extends for 8–10 nm between the alloy and \({{{{{\rm{Cr}}}}}_{2}}^{16}\)O3. The proximity histogram reveals a clear depletion of Cr in the adjacent alloy. The Cr-depleted zone spreads over approximately 15 nm into the base alloy. Interestingly, the Cr concentration directly at the interface remains at a level of 2 at.%. This minimum in Cr concentration is comparable to the values measured for alloy/oxide interfaces in APT data from Ni-5Cr samples.
3D APT data from a different specimen that includes the IG oxide section directly above the IOF is shown in Fig. 10b. The displayed terminating portion is expected to be 10 nm above the IOF. Contributions of CrO+/++ ions that originate from the most-abundant oxygen isotopes, 16O and 18O, are displayed with different colors in the ion map. Two 2D concentration maps of the Cr distribution are presented from different depth slices of the reconstruction in Fig. 10a, d.
APT data from oxide termination adjacent to a Cr-depleted GB. Cr concentration maps (a, d) were calculated with a bin width of 2.5 nm and perpendicular to the direction of the analysis. The arrows in the reconstruction (b) indicate the respective positions in the data set as well as 1D concentration profiles (c).
The terminal IG Cr2O3 is adjacent to a grain boundary and is 18–20 nm thick. A 1D concentration profile through the oxide section (Fig. 10c) indicates a nearly identical 16O/18O ratio in labeled oxides that formed during the second stage in Ni-5Cr (Fig. 8b) and in Ni-20Cr. Once more, the constant high amount of labeled oxygen indicates that the displayed Cr2O3 section grew during the second stage of the exposure when 18O was significantly enriched. A most intriguing feature of the present dataset is the included alloy GB. The demonstrated Cr concentration maps (Fig. 10a, d) were calculated from different heights, 17.5 nm apart from each other.
The concentration profile in Fig. 10a originates from a region nearer the exposed alloy surface. A stripe of approximately 14 nm width is completely depleted in Cr (< 0.05 at.%), proving that Cr diffusion through the metal interior is negligible at these exposure temperatures. As a consequence of the rapid transport of Cr along the GB, DIGM initiated35. The original location of the GB is approximated by a dashed line in the Cr concentration map. in addition, the Cr distribution map emphasizes a negligible contribution of Cr diffusion in the matrix. Neither the interface of the chromia with grain02 nor the interface of the original GB with grain01 exhibits a considerable gradient in Cr concentration. Closer to the IOF, the Cr depletion in grain02 appears less regular. Even though, the transition between the depleted region and the matrix is equally sharp, only a section of the IG oxide is connected with the Cr-depleted zone.
Discussion
The transport of oxygen through a complex surface oxide of a Ni-base alloy after exposure to pressurized water was previously reported26. The study underlined the potential of SIMS depth profiling in combination with labeled oxygen isotopes to prove inward transport. However, no conclusive results on the diffusion paths could be reported. The ToF-SIMS analysis presented in Fig. 5 aims to sustain model predictions by providing information on the lateral distribution of the respective oxygen isotopes. The limitations due to the insufficient resolution of the maps demonstrate the importance to use APT for the investigations. The high concentration of 18O in oxide portions that formed in the vicinity of the IOF (Fig. 10) added further indirect proof for inward oxygen transport. Nevertheless, only a detailed analysis of interfaces between the alloy and oxide phases that formed during the first stage of exposure reveals the transport path. Considerable 18O enrichment at these interfaces (Figs. 7 and 9).
As outlined above, several acclaimed studies aimed to explain oxygen transport during internal oxidation. Stott et al. reported discrepancies in the relative depth of oxygen penetration from estimated values using bulk diffusivity of oxygen in Ni-Cr system during internal oxidation9,36. Considerable enhancement of diffusion along alloy/oxide interfaces was proposed. The model predictions were also adapted by other groups20 for the unexpectedly fast IG corrosion of Alloy 600 in hydrogenated steam. Nevertheless, direct and unambiguous proof for preferred O diffusion along the interface of internal oxide phases could not be delivered until today. To our knowledge, the demonstrated APT analysis of distinct sample regions after two-stage oxygen tracer exchange is the first visual evidence for the proposed transport mechanism. In more general cases, APT was demonstrated to reveal the enrichment of elements along metal/oxide interfaces that could be connected to elemental transport. For example, the outward transport of Ni in alloy 600 during severe internal oxidation was proven by Langelier and Co-workers37.
Another morphological feature of the formed oxide is the apparent porosity. It cannot be stated with certainty to which extent pore networks developed already during the exposure. In general, interconnected pores could allow the access of molecular species to the oxidation front38,39,40. The phenomena were also observed for IG oxide formed during exposure to hydrogenated water35. The diffusion of O along the alloy/oxide interface was unequivocally proven. However, the additional contribution of gaseous transport cannot be excluded.
Cr-depletion in alloy regions adjacent to the oxide interface is scarcely detectable in proximity histograms of Ni5Cr. After 2 h oxidation at 600 °C, samples with 20 at.% exhibit a region of approximately 15 nm length that is depleted in Cr. Closer to the termination of the oxide network, Cr concentration maps (Fig. 10) indicated no apparent consumption of the solute from the matrix. This is interesting since this oxide portion depicts the early stage of IG oxide growth. One possible explanation for the delayed onset of Cr depletion in the alloy could be negligible diffusion of the solute to the surface. In other words, at this stage of oxidation, the Cr flux that arrives at the IOF is immediately consumed by the expansion of IG oxide along the GB. In contrast, the STEM-EDX maps in Fig. 4 of both alloys demonstrate depletion of Cr along the GB. This leads to the conclusion that the majority of Cr originates from the GB far below the IOF. Alloy regions neighboring penetrating oxide in grain interiors of the surface oxides are scarcely depleted in Cr. The formation of Cr2O3 is therefore solely sustained by transport along the GB. In accordance with severe Cr depletion, Ni is enriched along the GB. DIGM was also observed on some of the protected GBs, especially in the Ni-20Cr specimens (Fig. 3). Since the focus of this study was the understanding of O transport, the well-explained mechanism is not considered in detail. Briefly summarized, the short-circuit diffusion of Cr towards the IOF is non-symmetric35,41. During consumption of Cr and simultaneous depletion, the GB migrates towards the IG oxide (Fig. 10). For more details, readers are referred to previous studies on the oxidation behavior of Cr-containing alloys in the intermediate temperature regime41,42,43,44.
The internal oxide scale morphologies in Ni-5Cr and Ni-20Cr samples after oxidation in CO-CO2 mixtures were consistent. Despite the reduction of O availability, both compositions revealed GBs that were protected by Cr2O3 on the surface as well as GB that were decorated by interconnected IG oxide. Owing to the overall lower Cr reservoir, more GBs were attacked in samples with 5 at.% Cr. Especially GBs below protective oxide caps (Fig. 3a) demonstrated migrated interfaces and were more frequently observed in the alloy with 20 at.% Cr. However, APT analysis of IG oxide termination exhibited traces of a migrated GB (Fig. 10). Literature studies reported DIGM in Ni-5Cr model alloys after long-term exposure (100–1000 h) in hydrogenated water at 330 °C35,45. Therefore, it appears plausible to assume that also DIGM will initiate in Ni-5Cr samples at longer oxidation in CO-CO2. A particularity of IG attack in Ni-Cr alloys during exposure to hydrogenated water is the formation of oxide branches that reach into the alloy31,46. Even though not every IG oxide was only confined to the GB line, apparent growth of oxide branches that reach into the alloy was not observed during our analysis. One reason for the absence of these features might be sufficient Cr supply at 600 °C that ensures expansions along the IG region and thereby prevent the accumulation of growth stresses that would be suspected to cause transgranular expansion of oxide lattices.
For both Cr contents, specimens formed Ni extrusions during IG oxidation. These phases appear homogeneously distributed in individual grains. The density of Ni nodules varies depending on the grain characteristics. Additionally, the surfaces of Ni-20Cr samples exhibited more Ni extrusions than the ones of Ni-5Cr after the same experiments. As initially stated, Ni diffusion to the surface was attributed to intense internal oxidation11. Studies that are more recent also demonstrated extensive growth of metallic Ni extrusions in the course of IG oxidation in hydrogenated steam or water47,48,49. Growth of internal Cr2O3 is suggested to block diffusion of Ni to the termination of GBs on the surface. Additionally, the Ni transport is sustained by the evolution of compressive stress fields within the alloy during volume increase due to oxide growth37. These assumptions are also supported by an older study on the internal oxidation of binary Ni alloys under load. At moderate strain rates, Ni-Cr alloys demonstrated an increased Ni flux to the surface and deeper penetration of the (IG) oxidation front50. Internal oxidation can occur in Ni-base systems even with very high Cr concentrations. For example, dislocation networks in Alloy 690 (30 at.% Cr) were demonstrated to facilitate O penetration that resulted in the growth of Cr2O3 in grain regions below the surface51. The present study could not reveal a significant contribution of further short-circuit pathways, especially sub-surface dislocations. It is emphasized once again, that their relevance is well-documented in literature and a minor role also in the analyzed model system cannot be excluded.
Oxidation of model Ni-Cr alloys under controlled low \({p}_{{{{{\rm{O}}}}}_{2}}\) conditions at a moderate temperature was beneficial to isolate interfacial atomic transport mechanisms during selective oxidation. Several studies of similar model systems in gaseous atmospheres at reduced oxygen partial pressure are also available in the literature for comparison with the observed oxide scales using oxygen isotopic tracers. Especially during confined oxidation along interfaces, the growth of precipitates can severely alter the oxidation response during exposure to reduced oxygen availability44,52. A well-established explanation suggests the reduction of GB diffusivity by dissolved C as the main reason for the improved resistance against IG corrosion53,54,55. The alloys used in the present study did not form unwanted precipitates such as carbides. The slightly increased N content in Ni-5Cr (Fig. 6) can be mostly retracted to minor accumulation within the oxide. Nevertheless, the TEM diffraction indicates the expected lattice parameters of Cr2O3. It can be assumed that the N impurities do not significantly alter the oxidation mechanism. To differentiate internal and external oxidation, Rhines packs were generally adapted in the literature9,11,50. The oxide networks along GBs were furthermore reported to be mostly continuous. This aligns well with our results after exposure at significantly lower temperatures (600 °C).
Additionally, the investigation of model compositions with 20 at.% Cr increases the comparability of the results with commercial alloy systems (e.g., Alloy 600). The exact mechanisms of IG attack in Alloy 600 samples during moderate temperature exposure in hydrogenated water or steam are still under debate21,44,49. This is unsatisfying since oxidation is considered a precursor event to component failures via more deleterious effects, including stress corrosion cracking21,45. A consensus developed that transport fluxes of O and Cr decide if a fast penetrating Cr2O3 network develops along GBs or if the regions remain protected by a protective cap on the surface44. The present study proves the immense value that is reached by the combination of APT with oxygen tracer exchange studies. The reduction of complexity comes with the reward of direct access to transport mechanisms on the near-atomic scale. The demonstrated results indicate the potential to address research questions that could not be solved over decades. Admittedly, the insight into oxygen transport of this well-defined model scenario provided a number of additional tasks that can be elegantly addressed with the developed research strategy. For example, its elucidation of the correlation between individual grain boundary characters on the susceptibility to IG attack is of high relevance. For modeling, access to individual diffusivities of GBs is valuable. This is also supported by the recent study in which Poplawsky et al. concluded that O diffusion along alloy GBs must be considered to foresee oxidation resistance during the development of high-temperature alloys14.
Methods
High-purity alloys
High-purity Ni-Cr binary alloys were fabricated by SAES Smart Materials. Both elements, Ni and Cr were high-purity precursors leading to an as-cast purity of 0.99x%. The nominal alloy compositions were measured by ICP-MS and APT, and supplemented by glow discharge mass spectroscopy (GDMS) for the low level of impurities (320 appm Fe and 13 appm S being the most significant). Small coupons (≈ 8 x 8 x 2 mm) were prepared for oxidation experiments. The faces of the coupons were ground with SiC paper and polished to a mirror-finish with colloidal silica.
Exposure to environments below Ni/NiO equilibrium
Oxidation exposures at controlled \({p}_{{{{{\rm{O}}}}}_{2}}\) were carried out in a vertical furnace equipped with a turbomolecular pump for efficient change of atmosphere. A mixture of CO/CO2 with a ratio 1:10 leads to an oxygen partial pressure below the dissociation pressure of NiO at 600 °C. The flow rates were controlled by mass flow controllers at high accuracy. At the target temperature, the total pressure in the system remained constant at 800 mbar. Heating and cooling rates were 10 K/min. Baseline exposure to document the expected IG attack had a duration of 1 h. Oxygen isotopic exchange experiments followed a classical two-stage approach. In the first step, the model alloy samples were exposed for 40 min to a mixture of CO/CO2 mixture with natural isotopic abundances. Subsequently, the atmosphere was changed to a mixture of CO and C18O2 (c(18O) = 97%) with the same ratio (1:10). The sample was oxidized for an additional 80 min, for a total oxidation time of 2 h.
Material characterization
Scanning electron microscopy (SEM) and site-specific preparation of specimens for transmission electron microscopy (TEM) and APT were accomplished using two dual beam SEM/focused ion-beam (FIB) systems: an FEI Quanta 3D and FEI Helios 3D. SEM imaging was performed at 5 kV in both secondary electron (SE) and backscattered electron (BSE) imaging modes. For sample preparation, oxidized GBs were first protected by a Pt cap deposited by the FIB’s gas injection system. FIB lift-outs were then prepared for TEM and APT using well-established protocols56. During preparation, the specimens were shaped and/or thinned using a 30 kV Ga+ ion-beam, followed by a 2 kV Ga+ ion cleaning procedure to minimize ion-beam damage. For APT samples, the clean-up step lasted until the region of interest (metal/oxide interface) appeared within 100 nm below the specimen apex.
Scanning transmission electron microscopy (STEM) was performed using an aberration (CS) corrected, JEOL ARM200CF operated at 200 keV equipped with a JEOL Centurio energy dispersive spectrometer (EDS) with a 0.9 sR collection angle. Annular dark-field (ADF) data were collected with a convergence angle of 20 mrad and a collection angle ranging from 79–294 mrad. Digital imaging data collection was performed using Gatan Microscopy Suite (GMS) version 3.
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) measurement was performed at Environmental Molecular Sciences Laboratory (EMSL), which is located at Pacific Northwest National Laboratory. A TOF.SIMS5 instrument (IONTOF GmbH, Münster, Germany) was used. Dual-beam interlaced mode depth profiling was used. A 1.0 keV Cs+ beam was used as the sputtering beam and a 50 keV Bi\({}_{3}^{\,++}\) beam was used as the analysis beam for signal collection. The Cs+ sputtering beam (≈ 77 nA) was scanned over a 300*300 μm2 area. The Bi\({}_{3}^{\,++}\) beam was focused to be about 5 μm diameter with a beam current was about 0.10 pA with a 10 kHz frequency. The Bi\({}_{3}^{\,++}\) beam was scanned over an area of 100*100 μm2 at the Cs+ sputter crater center.
The APT analysis of oxidized Ni-Cr alloys was limited to sample volumes that included significant oxide below the original sample surface. Material transport along oxide/metal interfaces was suspected to contribute extensively to the overall oxidation mechanism and was the primary target for APT analyses. APT measurements used a LEAP 4000X HR in laser pulsing mode (λ = 355 nm, pulsing frequency 125–250 Hz). The pulse energies were adjusted to 80 pJ to maintain a Cr++/Cr+ ratio of ≈ 2. 3D reconstructions of APT data were performed with the commercial Integrated Visualization and Analysis Software (IVAS) package from Cameca, v 3.8.12. Further analysis and visualization of the reconstructed data were conducted with the open-source software GNU Octave. A custom dedicated toolbox, adapting many routines initially developed by Peter Felfer in MatLab.
APT data handling
The analysis of APT datasets was conducted in multiple steps. 3D reconstruction of ion positions was performed with the commercial Integrated Visualization and Analysis Software (IVAS) package from Cameca. The visualization of data and all additional analysis were conducted with the open-source software GNU Octave. A dedicated toolbox adapting the general routines suggested by Peter Felfer was implemented for the advanced handling of isotopes within the analyzed data.
Data availability
All data that support the results of the present study are available from the corresponding author upon reasonable request.
References
Airey, G. P. Microstructural aspects of the thermal treatment of inconel alloy 600. Metallography 13, 21–41 (1980).
Andresen, P. L. Effects of temperature on crack growth rate in sensitized type 304 stainless steel and alloy 600. Corrosion 49, 714–725 (1993).
Bruemmer, S. M. & Was, G. S. Microstructural and microchemical mechanisms controlling intergranular stress corrosion cracking in light-water-reactor systems. J. Nucl. Mater. 216, 348–363 (1994).
Evans, H. E., Donaldson, A. T. & Gilmour, T. C. Mechanisms of breakaway oxidation and application to a chromia-forming steel. Oxid. Met. 52, 379–402 (1999).
Halvarsson, M., Tang, J. E., Asteman, H., Svensson, J. E. & Johansson, L. G. Microstructural investigation of the breakdown of the protective oxide scale on a 304 steel in the presence of oxygen and water vapour at 600 °C. Corros. Sci. 48, 2014–2035 (2006).
Wagner, C. Reaktionstypen bei der oxydation von Legierungen. Z. Elektrochemie 63, 772–782 (1959).
Wagner, C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 99, 369–380 (1952).
Rhines, F. A metallographic study of internal oxidation in the alpha solid solutions of copper. Trans. Met. Soc. AIME 137, 246–290 (1940).
Stott, F. H. et al. The transport of oxygen to the advancing internal oxide front during internal oxidation of nickel-base alloys at high temperature. Solid State Ionics 12, 365–374 (1984).
Martínez-Villafañe, A., Stott, F. H., Chacon-Nava, J. G. & Wood, G. C. Enhanced oxygen diffusion along internal oxide-metal matrix interfaces in ni-al alloys during internal oxidation. Oxid. Met. 57, 267–279 (2002).
Wood, G. C., Stott, F. H., Whittle, D. P., Shida, Y. & Bastow, B. D. The high-temperature internal oxidation and intergranular oxidation of nickel-chromium alloys. Corros. Sci. 23, 9–25 (1983).
McIntyre, N. S., Chan, T. C. & Chen, C. Characterization of oxide structures formed on nickel-chromium alloy during low pressure oxidation at 500-600 °C. Oxid. Met. 33, 457–479 (1990).
Kruska, K., Schreiber, D. K., Olszta, M. J., Riley, B. J. & Bruemmer, S. M. Temperature-dependent selective oxidation processes for Ni-5Cr and Ni-4Al. Corros. Sci. 139, 309–318 (2018).
Poplawsky, J. D. Measuring oxygen solubility in Ni grains and boundaries after oxidation using atom probe tomography. Scripta Mater. 210, 114411 (2022).
Hart, E. On the role of dislocations in bulk diffusion. Acta Metall. Mater. 5, 597 (1957).
Frischat, G. H. Mass transport in solids. Angew. Chem. Int. Edit. 13, 384–398 (1974).
Basu, S. N. & Halloran, J. W. Tracer isotope distribution in growing oxide scales. Oxid. Met. 27, 143–155 (1987).
Harrison, L. G. Influence of dislocations on diffusion kinetics in solids with particular reference to the alkali halides. Trans. Faraday Soc. 57, 1191–1199 (1961).
Capell, B. M. & Was, G. S. Selective internal oxidation as a mechanism for intergranular stress corrosion cracking of Ni-Cr-Fe alloys. Metall. Mater. Trans. A 38, 1244–1259 (2007).
Persaud, S. Y., Korinek, A., Huang, J., Botton, G. A. & Newman, R. C. Internal oxidation of alloy 600 exposed to hydrogenated steam and the beneficial effects of thermal treatment. Corros. Sci. 86, 108–122 (2014).
Volpe, L., Burke, M. G. & Scenini, F. Oxidation behaviour of solution-annealed and thermally-treated Alloy 690 in low pressure H2-Steam. Corros. Sci. 167, 108,514 (2020).
Pande, C. S., Suenaga, M., Vyas, B., Isaacs, H. S. & Harling, D. F. Direct evidence of chromium depletion near the grain boundaries in sensitized stainless steels. Scripta Metall. Mater. 11, 681–684 (1977).
Becker, P., Panasko, M. & Young, D. J. Cyclic oxidation of heat resisting steels. Oxid. Met. 64, 281–301 (2005).
Kilner, J. A., Steele, B. C. H. & Ilkov, L. Oxygen self-diffusion studies using negative-ion secondary ion mass spectrometry (SIMS). Solid State Ionics 12, 89–97 (1984).
Chater, R. J., Weiser, M. & Virtanen, S. Visualizing ion transport mechanisms through oxide scales grown on mixed nickel- and cobalt-base model alloys at 900 °C using FIB-SIMS techniques. J. Vac. Sci. Technol. 36, 03F116 (2018).
Voyshnis, S. et al. Oxide layer growth on nickel-base alloy surfaces in high temperature water and in O2 studied by ToF-SIMS with isotopic tracers. Corros. Sci. 145, 212–219 (2018).
Quadakkers, W. J., Elschner, A., Speier, W. & Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 52, 271–287 (1991).
Naumenko, D., Gleeson, B., Wessel, E., Singheiser, L. & Quadakkers, W. J. Correlation between the microstructure, growth mechanism, and growth kinetics of alumina scales on a FeCrAlY alloy. Metall. Mater. Trans. A 38 A, 2974–2983 (2007).
Viskari, L., Hörnqvist, M., Moore, K. L., Cao, Y. & Stiller, K. Intergranular crack tip oxidation in a Ni-basesuperalloy. Acta Mater. 61, 3630–3639 (2013).
Rapp, R. The transition from internal to external oxidation and the formation of interruption bands in silver-indium alloys. Acta Metall. Mater. 9, 730–741 (1961).
Weiser, M., Schreiber, D. K., Kruska, K., Engelhard, M. H., & Olszta, M. J. Intergranulal oxidation and oxygen transport in Ni-20Cr during exposure to model dry and wet oxidation environments. 20th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, EnvDeg 2021 (Snowmass Village, CO, USA, 2022).
Bachhav, M., Danoix, F., Hannoyer, B., Bassat, J. M. & Danoix, R. Investigation of O-18 enriched hematite (alpha-Fe2O3) by laser assisted atom probe tomography. Int. J. Mass Spectrom. 335, 57–60 (2013).
Felfer, P., Scherrer, B., Demeulemeester, J., Vandervorst, W. & Cairney, J. Mapping interfacial excess in atom probe data. Ultramicroscopy 159, 438–444 (2015).
Felfer, P. & Cairney, J. Advanced concentration analysis of atom probe tomography data: local proximity histograms and pseudo-2D concentration maps. Ultramicroscopy 189, 61–64 (2018).
Schreiber, D. K. et al. Examinations of oxidation and sulfidation of grain boundaries in alloy 600 exposed to simulated pressurized water reactor primary water. Microsc. Microanal. 19, 676–687 (2013).
Stott, F. H. & Wood, G. C. Internal oxidation. Mater. Sci. Tech-Lond 4, 1072–1078 (1988).
Langelier, B., Persaud, S. Y., Newman, R. C. & Botton, G. A. An atom probe tomography study of internal oxidation processes in Alloy 600. Acta Mater. 109, 55–68 (2016).
Brueckman, A., Emmerich, R. & Mrowec, S. Investigation of the high-temperature oxidation of Fe-Cr alloys by means of the isotope 18O. Oxid. Met. 5, 137–147 (1972).
Shida, Y., Wood, G. C., Stott, F. H., Whittle, D. P. & Bastow, B. D. Intergranular oxidation and internal void formation in Ni-40% Cr alloys. Corros. Sci. 21, 581–597 (1981).
Weiser, M., Chater, R. J., Shollock, B. A. & Virtanen, S. Transport mechanisms during the high-temperature oxidation of ternary Equation 11 Co-base model alloys. npj Mater. Degrad. 3, 33 (2019).
Volpe, L., Burke, M. G. & Scenini, F. Understanding the role of diffusion induced grain boundary migration on the preferential intergranular oxidation behaviour of alloy 600 via advanced microstructural characterization. Acta Mater. 175, 238–249 (2019).
Balluffi, R. W. & Cahn, J. W. Mechanism for diffusion induced grain boundary migration. Acta Metall. Mater 29, 493–500 (1981).
Shen, Z., Karamched, P., Arioka, K. & Lozano-Perez, S. Observation and quantification of the diffusion-induced grain boundary migration ahead of SCC crack tips. Corros. Sci. 147, 163–168 (2019).
Volpe, L., Bertali, G., Scenini, F. & Burke, M. G. Effect of temperature on the preferential intergranular oxidation susceptibility of thermally-treated Alloy 600. Corros. Sci. 207, 110565 (2022).
Bruemmer, S. M., Olszta, M. J., Toloczko, M. B. & Schreiber, D. K. Grain boundary selective oxidation and intergranular stress corrosion crack growth of high-purity nickel binary alloys in high-temperature hydrogenated water. Corros. Sci. 131, 310–323 (2018).
Langelier, B. et al. Effects of boundary migration and pinning particles on intergranular oxidation revealed by 2D and 3D analytical electron microscopy. Acta Mater. 131, 280–295 (2017).
Thomas, L. E. & Bruemmer, S. M. High-resolution characterization of intergranular attack and stress corrosion cracking of alloy 600 in high-temperature primary water. Corrosion 56, 572–587 (2000).
Bertali, G., Scenini, F. & Burke, M. G. The intergranular oxidation susceptibility of thermally-treated alloy 600. Corros. Sci. 114, 112–122 (2017).
Persaud, S. Y. et al. Characterization of initial intergranular oxidation processes in alloy 600 at a sub-nanometer scale. Corros. Sci. 133, 36–47 (2018).
Bradley, L., Wood, G. C. & Stott, F. H. The effects of stress on the internal oxidation of Ni-Cr and Ni-Al alloys. Mater. Sci. Forum. 251-254, 341–348 (1997).
Persaud, S.Y., Ramamurthy, S. & Newman, R. C. Internal oxidation of alloy 690 in hydrogenated steam. Corrosion Science 90, 606–613 (2015).
Bertali, G., Burke, M. G., Scenini, F. & Huin, N. The effect of temperature on the preferential intergranular oxidation susceptibility of alloy 600. Metall. Mater. Trans. A 49, 1879–1894 (2018).
Moulin, P., Huntz, A. M. & Lacombe, P. Influence du carbone sur la diffusion du chrome et du nickel en volume et dans les joints de grains de l’alliage Ni-Cr 80/20. Acta Metall. Mater. 27, 1431–1443 (1979).
Hertzberg, J. L. & Was, G. S. The effect of carbon on grain boundary diffusivity in Ni-16Cr-9Fe alloys. Scripta Metall. Mater 33, 1193–1199 (1995).
Giggins, C. S. & Pettit, F. S. Corrosion of metals and alloys in mixed gas environments at elevated temperatures. Oxid. Met. 14, 363–413 (1980).
Thompson, K. et al. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 107, 131–139 (2007).
Acknowledgements
This was supported by the U.S. Department of Energy (DOE) Office of Science, Basic Energy Sciences Materials Science and Engineering Division (FWP 56909). MW acknowledges scientific and financial support from the Deutsche Forschungsgemeinschaft (DFG) through a 1-year research fellowship. Additional support was provided by the EAM, an FAU Competence Center, through Starting Grant EAM-SG21-3. A portion of this research was performed on a project award (10.46936/cpcy.proj.2019.51178/60006716) from the Environmental and Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Biological and Environmental Research program and located at Pacific Northwest National Laboratory (PNNL). PNNL is a multiprogram national laboratory operated by Battelle for the U.S. DOE under Contract DE-AC05-79RL01830.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.W. and D.K.S. designed the experiments. M.W. and M.H.E. conducted the two-stage exposures. M.J.O. elucidate the oxidized cross-sections with TEM. M.W. and D.K.S. conducted sample preparation and APT. The initial outline of the manuscript was done by M.W. and D.K.S. All authors discussed and contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weiser, M., Olszta, M.J., Engelhard, M.H. et al. Visualizing oxygen transport pathways during intergranular oxidation in Ni-Cr. npj Mater Degrad 7, 70 (2023). https://doi.org/10.1038/s41529-023-00387-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-023-00387-w