Abstract
The drastic distortion of potentiodynamic polarization curves measured at high potential scan rates prevents the extraction of accurate kinetic parameters. In this work, we start by measuring potentiodynamic polarization curves of AA7075 at scan rates ranging from 0.167 mV·s−1 to 100 mV·s−1, in an acidic 0.62 M NaH2PO4 solution and a near-neutral 3.5 wt% NaCl solution. Changes in potentiodynamic polarization curves are observed not only at different scan rates and electrolytes but also between replicated experiments. Contrary to what was reported in previous studies, the disturbance of charging current associated with high scan rates does not satisfactorily explain the potentiodynamic polarization shape. Instead, the high field model that incorporates the kinetics of anodic oxide growth successfully captures the features of experimental potentiodynamic polarization curves. Compared to Tafel’s theory, the high field model explains remarkably the changing kinetics with scan rates, electrolytes, and the variance between measurements performed at different sites.
Similar content being viewed by others
Introduction
Fueled by the need for energy efficiency and the reduction of greenhouse gas emissions, there is an important push of the automotive industry towards lightweighting1,2,3. In this regard, aluminum alloys (AA) have become the materials of choice to compete with traditional steel applications1,4. However, within an AA, the presence of more noble alloying elements, responsible for increasing strength, creates micro-galvanic coupling. As a result, these phases lead to localized corrosion and, in some cases, subsequent mechanical failure due to stress corrosion cracking5,6.
Corrosion is often investigated electrochemically by potentiodynamic polarization (PDP) experiments, both at the macro7,8,9,10 and micro11,12,13,14,15 scales. However, the corrosion metrics extracted from PDP using the Tafel kinetics laws are sensitive to the fitting procedure and experimental parameters16,17,18. Notably, it is recommended to measure the anodic and cathodic branches separately, starting from the corrosion potential (Ecorr), and to use a low scan rate of 0.167 mV·s−1 19. However, under these conditions, the measurements are affected by irreversible surface changes and the accumulation of corrosion products20. As such, there is no consensus for the choice of optimal PDP parameters, which leads to inconsistency in interpreting the corrosion kinetics from PDP experiments. Moreover, the degradation of AA by localized corrosion is misinterpreted by macroscale PDP since the damage is concentrated near microstructural features. Therefore, localized corrosion must be investigated at the scale of these microstructural features to accurately assess the long-term durability of AA, which is a critical need in the industry. Since hundreds to thousands of measurements are needed to map a sample’s electrochemical properties using microscale techniques, microscale PDP is typically performed at a high scan rate (>10 mV·s−1) in a single scan direction11,21,22,23. Under these conditions, the system is away from its steady-state, and the extracted Tafel parameters will not accurately reflect the corrosion kinetics20,24. Hence, at the microscale, PDP is currently relegated as a qualitative corrosion measurement.
Despite the convenience of measuring PDP at a higher scan rate, the effect of scan rates has been seldom studied, and, in these few studies, the scan rates were limited to a narrow range of 0.1 mV·s−1 to 10 mV·s−1. The changes appearing at higher scan rates were mostly attributed to double-layer capacitance20,25,26 and mass transport24,27. However, the metals’ active dissolution and passivation kinetics under high scan rate PDP has received little attention. This is surprising considering the extensive work on the kinetics of oxide growth on metals28,29,30,31,32,33,34,35,36. Notable work includes the study of aluminum passivation using the high field model by H.S. White29 and H.S. Isaac31, and the development of the point defect model by D.D. Mcdonald37. Both models show an inherent dependence with scan rates that could account for the changes appearing in PDP at higher scan rates.
Herein, the PDP obtained from AA7075 (nominal composition in Supplementary Table 1) in 0.62 M NaH2PO4 and 3.5 wt% NaCl solutions at scan rates up to 100 mV·s−1 were analyzed by numerical simulations, taking into account effects of double-layer capacitance as well as the high field model. For both electrolytes, the passivation kinetics described by the high field model played the largest role in predicting the trends observed when varying scan rates.
Results and discussion
Complex trends emerge from PDP at high scan rates
The experimental PDP curves of AA7075 in 0.62 M NaH2PO4 and 3.5 wt% NaCl are presented in Fig. 1. Prior to the PDP measurement, the sample was at OCP until steady-state is reached: ~20 min for OCP to stabilize at −0.82 V vs. SCE in 0.62 M NaH2PO4 and ~5 min for OCP to stabilize at −0.78 V vs. SCE in 3.5 wt% NaCl. Before starting the potential scan in the positive direction, a potential of −0.25 V vs. OCP was then applied for 20 s to reduce the transient effects such as charging current and diffusion of O2.
PDP measured in (a) 0.62 M NaH2PO4 and (b) 3.5 wt% NaCl solution. The data with a 95% confidence interval is presented in the SI (Supplementary Fig. 1).
In 0.62 M NaH2PO4, the Ecorr and corrosion current density (jcorr) measured at 0.167 mV·s−1 (black line in Fig. 1a) are −0.77 V vs. SCE and 9.8 µA·cm−2, respectively, which is in agreement with values from the literature38. Under acidic conditions (pH of 3.6), HER is the dominant cathodic reaction39,40. At the anodic branch, the current steadily increases until it reaches a current plateau, the critical passivation current, where corrosion proceeds uniformly. At scan rates of 5, 25, and 100 mV·s−1 (red, blue, and green lines in Fig. 1a), the Ecorrapp all shift to a similar potential value, ∼ −0.848 V vs. SCE comparatively to −0.77 V vs. SCE at 0.167 mV·s−1. Moreover, the anodic branch current magnitude increases with increasing scan rates. The differences from the high scan rates PDP translate into different values of Ecorr and jcorr for each scan rate (Supplementary Table 2). Thus, the ‘apparent’ superscript is used here to distinguish between the Ecorr and jcorr obtained at 0.167 mV·s−1 from the ones obtained at higher scan rate.
In 3.5 wt% NaCl, at pH 7, the Ecorr and jcorr measured at 0.167 mV·s−1 (black line in Fig. 1b) are −0.78 V vs. SCE and 1.60 µA·cm−2, respectively, which is in agreement with values from the literature41,42,43. Both the value of jcorr and the magnitude of all currents before the onset of pitting are smaller in NaCl compared with those measured in acidic NaH2PO4. This observation can be attributed to a less reactive environment for the cathodic reaction and a slower oxide dissolution rate in the neutral solution. The anodic branch does not present the typical passive region since localized pitting corrosion occurs in the presence of aggressive Cl– ions and pitting initiates at a potential (Epit) very close to Ecorr44. As the scan rate increases (red, blue, and green lines in Fig. 1b), distinct and severe distortions are observed on each PDP curve. Larger shifts of the Ecorrapp are observed for all scan rates in NaCl compared to NaH2PO4. The Ecorrapp shift is accompanied by a change in the cathodic branch shape: at 0.167 mV·s−1 (black line in Fig. 1b), the cathodic branch appears relatively flat, while at higher scan rates (red, blue, and green lines in Fig. 1b), its slope increases. Moreover, the potential at which stable pitting takes place becomes more positive with the increase in scan rate.
At higher scan rates, a qualitative evaluation of the corrosion process can be drawn from the shifts of the corrosion potential and pitting potential, but due to the drastic distortions observed in the Tafel plots, the extracted apparent Tafel parameters cannot be used quantitatively (Supplementary Table 2). For a specific electrolyte solution, the intrinsic corrosion metrics should not change with scan rates, meaning that the Tafel fit is also capturing processes that accompany a change in scan rate.
The Inability of Capacitive Current to Describe High Scan Rates PDP
The PDP current density (jpdp) can be expressed as a convolution of the current originating from faradaic and capacitive processes.
Where jpdp is the PDP current density, jf is the faradaic current density and jcap is the capacitive current density.
Based on Eq. (2), jcap increases with the scan rate and could potentially explain the PDP trends observed at higher scan rates in Fig. 125,45.
Where C(t) is the capacitance and \(\frac{{\partial V}}{{\partial t}}\) is the potential scan rate.
In order to evaluate the effect of jcap on high scan rates PDP, the PDP curves at scan rates of 5, 25, and 100 mV·s−1 were simulated by calculating jpdp using Eq. (1). The measured PDP current at 0.167 mV·s−1 was used as an approximation for jf (condition where jcap → 0), while jcap was calculated from Eq. (2). However, determining C(t) is not an easy task as evidenced by the large discrepancy in reported values for AA707546,47,48,49,50 which can range from 10 to 3500 μF·cm−2. The inconsistency in measured C(t) for an oxide-covered metal can be attributed in part to interference from corrosion occurring at the metal/oxide/electrolyte interface. C(t) is most often measured by EIS where the chosen equivalent circuit has a major impact in evaluating C(t), but the choice of the equivalent circuit and its physical interpretation remain a contentious subject among corrosion specialists48,49,51. Moreover, corrosion of the metal matrix can lead to changes in real surface area due to the etching of the oxide into a porous structure52,53,54, causing the value of C(t) to change during the measurements.
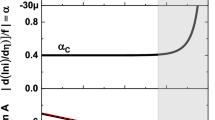
Since we are solely interested in evaluating C(t) for AA7075 and not the corrosion kinetics, the EIS measurements were restricted to the higher frequency region (105 to 1 Hz) where the capacitive process dominates. The oxide/electrolyte interface is modeled using a solution resistance (Rs) for the electrolyte and a constant phase element (CPE) in parallel with a polarization resistance (Rp) to represent the non-ideal capacitive behavior of the oxide film (Fig. 2a). Here, the CPE is defined as a combination of the electrochemical double layer (Cdl) and oxide (Cox) in series, where the total capacitance is often defined by Eq. (3).
According to Gharbi et al.55, Eq. (3) is not accurate for CPE and is only valid at very high frequency or scan rate, where C(t) would be dominated by Cox. At frequency, within the range of typical PDP (1 mV·s−1 to 100 mV·s−1) the measured C(t) would be more reflective of Cdl. This would explain the frequency dependence observed for C(t) on pure aluminum, where they measured a change in C(t) from 35 µF·cm−2 at scan rate of 1 mV·s−1 to 15 µF·cm−2 at scan rate of 2000 mV·s−1. Thus, C(t) was extracted using the Brug equation (Eq. (4)), since it is suited to describe processes with a surface time-constants distribution56,57.
Where α and Q are CPE parameters, Rp is the polarization resistance and Rs is the solution resistance. Also, to account for any change in C(t) during the measurement of PDP, EIS measurements were performed at multiple potentials and after conditioning of the sample by PDP at 5 mV·s−1 and 100 mV·s−1 (Fig. 2b, c). The α for all spectra were above 0.85 (Supplementary Tables 3, 4) and based on Fig. 3, the extracted C(t) is different between both electrolytes but does not vary significantly as a function of the applied potential or the sample conditioning.
The addition of jcap can partially explain some of the changes observed in high scan rates PDPs (Fig. 4) such as the shift of the Ecorrapp, but it does not correctly predict the overall shape of the curves for any scan rates and electrolytes. For example, in 0.62 M NaH2PO4 with a C(t) of 300 μF·cm−2 (blue line in Fig. 4a), the addition of capacitive current predicts correctly the Ecorrapp shift for the PDP performed at a 100 mV·s−1. However, it fails to do so for slower scan rates, even when accounting for increasing C(t) at slower scan rates. It also fails to fit the anodic branch and to explain any other observed trend, like the overlap of both the cathodic branches and Ecorrapp observed for scan rates of 5, 25, and 100 mV·s−1 (Fig. 1a).
A gap, similar to that seen for the anodic branches, would be expected based on the jcap calculated from Eq. (2) with a constant C(t). Similarly, attempts to fit the PDP curves obtained in 3.5 wt% NaCl are unsuccessful (Fig. 4b), even if we disregard the pitting occurring after Ecorr that is not directly related to the capacitive current. Here C(t) would need to vary drastically, up to 1 or 2 orders of magnitude, as a function of the electrolyte, the potential, and the scan rates, to account for all the observed trends in PDPs at high scan rates. However, such an extensive increase in C(t) is not supported by our EIS measurements (Fig. 3). Thus, the capacitive current alone cannot explain the complex trends observed at different scan rates in both electrolytes, which is surprising since it has often been alluded to be the main contributor to PDP distortion20,25.
Defining Scan Rate Dependent Processes for Modeling High Scan Rates PDP
Since the addition of capacitive current failed to predict the trends observed in PDP at higher scan rates, we now investigate the current originating from the growth of the oxide layer as described by the high field model. For reactive materials like aluminum alloys, classified as ‘valve metals’, the corrosion process is inhibited by the spontaneous formation of an insulating oxide film in ambient conditions32,58. Radiotracer and secondary ion mass spectrometry59,60,61,62 experiments confirmed that the oxide layer grows at the metal/oxide interface by the migration of OH−/O2− species (Fig. 5a), and at the oxide/electrolyte interface by the migration of Al3+ (Fig. 5b). Equations (5) to (7) summarize the passivation process under such conditions. However, direct dissolution of Al can still occur via field-assisted ejection of Al3+ species in solution63,64 (Fig. 5c).
In the high field model, the migration of ionic species across the oxide film is considered the rate-limiting step for the metal oxidation rather than charge transfer. Mott and Cabrera derived the current expression, by defining the potential drop within the oxide film as the main driving force for the migration of ions65.
Where jhf is the anodic current density derived under the high field model, A and βa are kinetic parameters that describe the mobility of ions in the oxide, E is the magnitude of the electric field, d is the oxide film thickness, η is the overpotential, Eapp is the applied potential, and \(E_{{{{\mathrm{Al}}}}_2{{{\mathrm{O}}}}_3}\)is the equilibrium potential for the formation of Al2O3 described by Eq. (7).
The jhf can be converted into an oxide film growth rate from Faraday’s electrolysis law. A passivation efficiency factor (εp) was introduced by Lee et al.64 to differentiate between the jhf fraction that contributes to oxide growth and direct dissolution of Al.
where εp is the passivation efficiency (1 = 100% passivation efficiency), M is the molar mass of Al2O3, ρ is the density of Al2O3, n is the number of electrons transferred, and F is the Faraday’s constant.
The oxide film growth is also balanced by the chemical dissolution of Al2O3 in solution29,33 (Fig. 5d), where the dissolution rate is known to be enhanced in acidic/alkaline condition64,66,67.
Where Rdiss is the dissolution rate of Al2O3.
To determine the electric field magnitude, which depends on the overpotential and the oxide film thickness (Eq. (9)), the change in oxide film thickness during polarization is calculated by integrating the rate of film growth (Eq. (11)), and film dissolution (Eq. (12)).
Where d0 is the thickness of the air-formed oxide film already present at the surface before immersion in the electrolyte. Finally, the anodic current contribution, jhf, can be calculated by solving Eqs. (8) and (13) together.
The cathodic current density (jc) for an AA in an aerated aqueous solution originates from both the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR)62,68.
To make things simpler as we are focusing on the anodic current described by the high field model, we assume that both cathodic reactions can be described by a single Tafel-like expression and that it is not strongly affected by the change in oxide thickness.
Where jc is the cathodic current density, jc0 is the cathodic exchange current density, Ec is the mixed equilibrium potential for the cathodic reactions, βc is the cathodic Tafel slope.
So now, the PDP current density, jpdp, can be expressed as the sum of the anodic high field current density, the cathodic current density, and the capacitive current density.
PDP curves were simulated in COMSOL Multiphysics v6.2 using the DAE and ODE physics in 0D to calculate jpdp from Eq. (17), jhf from Eqs. (8) and (13), jc from Eq. (16) and jcap from Eq. (2) (details are in section 8 of the SI and the COMSOL report is also available). To limit the number of fitting variables, the passivation kinetic parameters A, βa, discussed in more details in the following sections, were taken from reference28, by approximating that they do not change significantly between different Al alloys and from an electrolyte to another. The density of Al2O3 (ρ) was taken from the literature, however, it can change according to the electrolyte composition and anodization conditions since it will affect the morphology and composition of the oxide film59,60,69. The number of electrons, n, involved in the growth of Al2O3 is assumed to be 6, based on Eqs. (5) and (7). Ec was approximated by the respective value of OCP in 0.62 M NaH2PO4 and 3.5 wt% NaCl, while values of \(E_{{{{\mathrm{Al}}}}_2{{{\mathrm{O}}}}_3}\)were taken from the Pourbaix diagram factoring in the electrolyte pH70. Based on our EIS measurements, C(t) of 16 µF·cm−2 was used for 0.62 M NaH2PO4 and 21 µF·cm−2 for 3.5 wt% NaCl. Although, there is an increase in C(t) as the scan rate decreases, the effect on jcap is negligible within the range of scan rates used in this work (Fig. 4), and C(t) was assumed constant. The parameters taken from the literature are estimates that might not strictly apply in the context of an alloy, but nevertheless, the objective is to demonstrate that the high field model used in this work can explain the experimental PDP trends. All parameters used for the simulations are presented in Table 1. The remaining parameters, most of them hard to characterize accurately, were left to be fitted (Rdiss, d0, εp, jc0, βc), and a single set of parameters was used to fit measurements from all the scan rates simultaneously (Table 2).
The Anodic High Field Current as the Dominant Factor in High Scan Rates PDP
The simulated jhf inherently changes with increasing scan rates (Fig. 6a, c). At the beginning of the potential scan, jhf increases exponentially but the progressive thickening of the oxide film (Supplementary Fig. 2) will affect the magnitude of the electric field, E (Eq. (9)). Eventually, E and the oxide growth rate (Eq. (13)), which is controlled by jhf and Rdiss, will settle into a dynamic equilibrium, and jhf will converge to a steady-state (jss). At higher scan rates, a higher potential is reached for the same oxide thickness relative to a slower scan rate (Supplementary Fig. 2a, b), resulting in a larger E and jhf. The perception of jhf is further skewed because it is plotted as a function of the potential; for a potential window of 250 mV, the oxide growth is compressed over a 2.5 s interval for a scan rate of 100 mV·s−1 but it is stretched over 1497 sec at 0.167 mV·s−1. The end result, as particularly observed in Fig. 6a, is jhf convergence to a steady-state at a less negative potential and a higher current density with increasing scan rates. An analytical expression of the steady-state current (jss) as a function of the scan rate can be derived from Eq. (8) and Eq. (13) under the assumption of 100% faradaic efficiency and no chemical dissolution34,35,36.
Where jss is the steady-state current and ν is the scan rate. Thus, the kinetic parameter A and βa can be determined from the slope and intercept of the ln(jss) vs. ν/jss plot.
For the reverse case, where the potential is scanned in the negative direction, jcap becomes negative and the E decreases from the combined effect of the negative potential scan and oxide growth. For example, in 0.62 M NaH2PO4, the large E drives a high current, which is followed by the rapid decay of the anodic current due to the oxide growth and negative potential scan (Supplementary Fig. 3). The simulated PDP curves (Supplementary Fig. 3) show that the high field model can describe the trend for the decay of the anodic current for different scan rates.
In the 0.62 M NaH2PO4 electrolyte, the Ecorr visually corresponds to the potential at which jhf and jc intersect (Fig. 6a) since the contribution from jcap is negligible. At scan rates of 5, 25, and 100 mV·s−1, jhf still increase beyond the steady-state at 0.167 mV·s−1, but they start to diverge as the jhf of higher scan rates continue to increase, while for lower scan rates they settle into a steady-state. However, jc intersect with jhf from scan rates of 5, 25, and 100 mV·s−1 before the jhf are diverging significantly, thus explaining the Ecorrapp overlap and the shift from −0.770 V to −0.848 V vs. SCE (purple dotted line and red, green, blue line in Fig. 6a). Moreover, as can be seen in Fig. 6b, the overall shape and trend of the experimental anodic branches with increasing scan rates match with the simulated jhf. However, the high field model predicts that a steady-state current, jss, will eventually be reached during the potential scan, but in the experimental anodic branch, the current density keeps increasing, a trend that becomes more apparent as the scan rate is lowered. Lee et al.64 attributed the continuous increase in jss to a decrease in passivation efficiency, εp, from a change in the surface pH. In an unbuffered solution, the surface pH would drop, thus hindering the formation of O2− produced by water dissociation (Eq. (4)) at the oxide/electrolyte interface, and reducing the probability for Al3+ to bond with O2− 64,71. In our case, the use of an equimolar buffer solution of 0.564 M NaH2PO4/0.056 M Na2HPO4 (Fig. 7) stabilized jss by preventing a change in surface pH during the potential scan. Moreover, the difference in pH from 3.6 to 5.5, between both electrolytes, contributed to shifting the onset of oxide growth to a more positive potential and decreasing jpdp. This result highlights the impact of surface pH change on the measured PDP, which can be linked to change in properties such as εp, Rdiss, \(E_{{{{\mathrm{Al}}}}_2{{{\mathrm{O}}}}_3}\), A and βa. Nevertheless, even with the use of constant properties, the inclusion of the jhf parameter in this work can still effectively predict the main changes occurring in a PDP curve at a higher scan rate in the acidic NaH2PO4 solution; namely, the shift and the overlap of the cathodic branches and Ecorr, but also the increase of the anodic branch current density at different scan rates.
In the case of PDP in 3.5 wt% NaCl (Fig. 6c), a step function was used to increase Rdiss and decrease εp, in order to simulate the sudden increase in jhf associated with the occurrence of pitting corrosion. At 0.167 mV·s−1, the measured Ecorr corresponds to the intersection of jc with the pitting region of the jhf (purple dotted line and black line in Fig. 6c). While, at higher scan rates 5, 25, and 100 mV·s−1, Ecorrapp is the result of jc being canceled out by the combined contribution of jcap and jhf transient (purple dotted line and red, green, and blue line in Fig. 6c). Before the occurrence of pitting, the inclusion of jhf captures well the movement of Ecorrapp and the PDP shape across all scan rates (Fig. 6d) from a single set of fitting variables. However, the region near the pitting area is not as well described, indicating that the high field model cannot be used to explain pitting corrosion.
Validation of the high field model parameters
Although the simulated PDP was in good agreement with the experimental data, it relied on a large number of fitting variables and validation is necessary to ensure that the fitted parameters in Table 2 have realistic values. The air-formed oxide thickness was measured by X-ray photoelectron spectroscopy (Supplementary Fig. 4 and Table 4) and an average d0 value of 2.38 nm with a 95% confidence interval of 0.51 nm was obtained from three measurements in different sites within a 1 cm2 AA7075 sample. Our values of d0 used in the simulation for both electrolytes are consistent with our oxide thickness measurement and the ones reported in literature49,72,73.
The oxide dissolution rate was estimated by quantification of Al ions in their respective electrolyte by ICP-OES after a 24 h exposure. For 3.5 wt% NaCl, the measured dissolution rate agrees with the fitted Rdiss. For 0.62 M NaH2PO4, there is a larger difference between both values, but the difference is still within 1 order of magnitude. Moreover, the measured dissolution is most likely underestimated since it was performed without any polarization of the sample. Under polarization, the sample oxide dissolution rate would be expected to increase as the surface pH change (Fig. 7).
The kinetic parameters A and βa play a large role in determining the oxide growth rate but it is difficult to characterize them properly. There is a large discrepancy of A and βa reported in the literature relative to the characterization method used. When it is calculated from the slope and intercept of the linear fit of the ln(jss) vs. ν/jss plot using Eq. (18), A is on the order of 10−6 A·cm−2 34,35, comparatively, it is on the order of 10−12 A·cm−2 when it is obtained through fitting30. In our case it follows the same pattern, the A (4.35 x 10−5 A·cm−2) and βa (5.17 x 10−7 cm·V−1) calculated from Fig. 8a, are vastly different from the ones used in our simulation (A = 6.5 x 10−12 A·cm−2 and βa = 3.6 x 10−6 cm·V−1). When they are used to simulate PDP, it predicts accurately the steady-current, jss, but vastly overestimates the initial jpdp and gives a poor fit of the PDP. Moreover, the simulated oxide growth (Fig. 8c) is unrealistic in comparison to our initial simulation (Supplementary Fig. 2a), especially at 0.167 mV·s−1. In 0.62 M NaH2PO4 and most electrolytes, the passivation efficiency is less than 100%, the oxide dissolves chemically and the high field properties are not constant during a measurement, especially in unbuffered electrolytes (Fig. 7). Thus, the assumption from which Eq. (18) were derived are broken and leads to the erroneous evaluation of A and βa.
Although Eq. (18) is inaccurate regarding A and βa, it can still be used to confirm that the oxide growth follows a high field kinetic. Moreover, jss is accurately calculated by Eq. (18) and can be used to extract jss at lower scan rates from PDP measurements obtained at high scan rates. This is of particular interest for microscale PDP, where they are typically performed at scan rates upward of 50 mV·s−1. Especially, if the objective is to evaluate the corrosion rate occurring at OCP, it is not necessary to obtain a perfect fit but rather to extrapolate jss for low scan rate (≤0.167 mV·s−1), where the system is close to steady-state.
Before the occurrence of pitting, Rdiss and more importantly jc, are higher in NaH2PO4 compared to NaCl (Table 2). This is consistent with results from the literature, where corrosion is found to be more active under acidic or alkaline conditions in a process known as cathodic dissolution74,75. The greater dissolution rate of Al2O3 in acidic conditions leads to higher cathodic activity and higher repassivation rate, which increases jc and jhf, respectively28. Similarly, in 0.62 M NaH2PO4, εp is smaller than 1 because in acidic conditions, the production of O2− from the water dissociation reaction, essential to produce Al2O3 (see the mechanism in Fig. 5), is unfavorable. But the value of εp in 3.5 wt% NaCl is exceptionally high, knowing that it should be bound between 0 and 1. Physically, this high passivation efficiency rendered by the model means that the electric field, E, drops faster than what would normally be predicted by the high field model from an increase in oxide layer thickness (Eqs. (9) and (13)). It is not clear if it is a sign that the high field model does not apply in this case, especially since a ln(jss) vs. ν/jss plot could not be obtained to confirm the high field growth of the oxide due to the occurrence of pitting before the appearance of a steady-state current. Alternatively, this could be the result of unaccounted processes that are not presently considered in the high field model, especially considering the good prediction obtained using the high field model (Fig. 6d). For example, all properties were assumed to be constant, but this assumption might be incorrect for certain electrolytes or AA. The oxide layer properties are known to change as it grows from the incorporation of ions present in the electrolyte59, especially for chloride ions69. For unbuffered electrolytes, there can be a significant change in pH at the surface of the metal which in turn can affect the dissolution rate and potential for oxide growth, \(E_{{{{\mathrm{Al}}}}_2{{{\mathrm{O}}}}_3}\) (Fig. 7). However, from these results, it is still clear that oxide growth plays a large part in the anodic current density observed.
Correlation Between the Increasing Variance from PDP at Higher Scan Rates and the Oxide Layer Thickness
Another characteristic of PDP obtained at high scan rates is the larger variance between replicates. For example, jpdp in 3.5 wt% at 0.167 mV·s−1 varies slightly, but overall, the shape of each replicate is virtually the same and they mostly overlap (Fig. 9a). However, at 100 mV·s−1, the difference between each replicate (Fig. 9b) becomes much more apparent and it translates into a much larger distribution of Ecorrapp and Epit, which was determined by the onset of the pitting current from the PDP (Fig. 9c). Consequently, PDP curves obtained at higher scan rates are less reproducible compared to those obtained at slower scan rates. Furthermore, for this work, the increased variance from high scan rates PDP contributes to a larger error in the fitting procedure.
a 5 PDP replicates at 0.167 mV·s−1. b 5 PDP replicates at 100 mV·s−1, the inset shows an enlargement of the Ecorr region. c Distribution of the Ecorr and Epit at scan rates of 0.167, 5, 25, and 100 mV·s−1. Error bars represent the standard deviation of data of 5 replicates at each scan rate. d Simulated effect of air-formed oxide thickness, d0, and comparison with PDP replicates measured at 100 mV·s−1.
Under the high field model, for the same difference in the value of an AA property, such as the air-formed oxide layer thickness (d0), the disparity in jhf will be larger for higher scan rates, making it more sensitive to a slight difference in properties. This could explain the increase in variance observed between each replicate PDP performed at higher scan rates since a different region of the sample is probed per replicate. To verify this, PDP curves were simulated with d0, ranging from 2.60 to 2.75 nm and they were able to emulate the Ecorrapp trend seen in PDP replicates (Fig. 9d). However, it is unclear what the variance in jhf at the macroscale entails since the properties are averaged over a large area. Investigation at the microscale will be required for a better understanding of the observed variance at high scan rates.
In conclusion, the jhf was the dominant factor over capacitive current in explaining the characteristics of PDP emerging at higher scan rates for AA7075. Compared to the Tafel equations, the high field model can describe, using a single set of parameters, the PDP trends in 2 different electrolytes and for multiple scan rates. Moreover, the kinetics is defined in terms of the oxide layer where some of the parameters (d0, Rdiss, εp) have a physical meaning that is complementary to the information conventionally obtained from PDP measurements (e.g., jcorr, Ecorr, Epit).
This work is also crucial in bringing microscale PDP measurements, which require these high scan rates, to the rank of quantitative techniques, but important issues need to be addressed beforehand:
-
Improve the quality of characterization of the high field model key parameters.
-
A better understanding of the effect of localized corrosion events such as micro-galvanic coupling and local oxide breakdown by chloride ions in relation to the high field model.
Methods
Materials and Sample Preparation
AA7075 samples were provided by the NRC (National Research Council Canada, Saguenay), which were then cut into 8 x 5 cm pieces of 2 mm thickness. Samples were lightly abraded using a general-purpose hand pad (Scotch BriteTM). All samples were successively rinsed with anhydrous ethanol (Greenfield Global, Canada) and Milli-Q water (Millipore, 18.2 MΩ·cm resistivity at 25 °C), and then dried under a stream of air. The electrolyte solutions were prepared with NaCl (99.0% purity, Sigma-Aldrich) and NaH2PO4 (≥99.0%, Sigma-Aldrich) in Milli-Q water. The solution’s pH was measured using a pH meter (XL200, FisherbrandTM) which was calibrated in buffer solutions of pH 4, 7, and 10.
Instrumentation
Electrochemical measurements were performed with a multi-channel VSP-300 potentiostat (Biologic Science Instruments, France) in a Faradaic cage. A three-electrode corrosion cell (K0235 Flat Cell, Princeton Applied Research, AMETEK Scientific Instrument) with an exposed working area of 1 cm2 was used for all electrochemical measurements, with a saturated calomel electrode (SCE) (CHI 150, CH Instruments) as the reference electrode and a platinum mesh (2.54 cm × 2.54 cm, Goodfellow) as the counter electrode.
Potentiodynamic Polarization Measurements
The experimental procedure was based on standards ASTM G5-9419 and ASTM G61-8676. Naturally aerated 3.5 wt% NaCl and 0.62 M NaH2PO4 solutions were used as the electrolyte solutions for all the electrochemical tests to simulate severe corrosion conditions. In both electrolytes, prior to PDP measurements, the sample was left under open circuit potential (OCP) until the variation in OCP fell below 10 mV per minute. Then the sample potential was held at −0.25 V vs. OCP for 20 s before acquiring complete PDP curves at scan rates of 0.167, 5, 25, and 100 mV·s−1, from −0.25 V vs. OCP to 1 V vs. SCE (with a current density cutoff of 1 mA·cm−2). PDP curves were acquired in 5 replicates per scan rate and the average value is considered for analysis. For each measurement, a new uncorroded 1 cm2 area of the sample was exposed. All the presented current densities (j) are normalized to the geometric area of the working electrode.
Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy (EIS) measurements were performed in potentiostatic mode with a voltage perturbation amplitude of 10 mV (Vrms = 7.07 mV) in the frequency range from 105 to 1 Hz, in both 0.62 M NaH2PO4 and 3.5 wt% NaCl. The measurements were also performed at multiple potentials between −1 V vs. SCE to OCP, as well as before and after PDP measurements. Equivalent circuit fitting was performed using EC-lab (Biologic Science Instruments, France) version 11.33.
X-ray Photoelectron Spectroscopy
XPS analyses were carried out using an Al Kα X-ray photoelectron spectrometer (Thermo Scientific, K-Alpha). The beam size was 400 μm in diameter, the take-off angle of the beam was 90° and ten scans were performed per spectrum. The peak fitting was performed using the Avantage software (Thermo Scientific) using a ‘smart’ background which is based on the ‘Shirley’ background with the additional constraint that it should not be of a greater intensity than the actual data at any point in the region. For asymmetric Al 2p (metal) peaks, tail mix and tail exponents were allowed. A spin-orbit splitting ratio of 1:2 was assigned to aluminum metal peaks 2p1/2 and 2p3/2.
Inductively Coupled Plasma Optical Emission Spectroscopy
To measure the dissolution rate of Al2O3 in 3.5 wt% and 0.62 M NaH2PO4, the AA7075 samples were immersed in both electrolytes for 24 h. Then the sample solutions were collected, by rinsing the AA7075 surface with 5% v/v with nitric acid to dissolve any precipitated Al ions for measurements by ICP-OES (Agilent 5100, USA). The wavelengths of Al at 308.215 nm, 394.401 nm, 396.152 nm with minimal elemental interference were chosen as the analytical line. The Al3+ standard solutions were prepared in concentrations of 1 ppm, 10 ppm, 20 ppm, and 50 ppm by addition of AlCl3 powder to the electrolyte of interest (NaCl or NaH2PO4) which were acidified to a concentration of 5% v/v with nitric acid.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The COMSOL file is available from the corresponding author upon reasonable request.
References
Cole, G. & Sherman, A. Light weight materials for automotive applications. Mater. Charact. 35, 3–9 (1995).
Fridlyander, I. et al. Aluminum alloys: promising materials in the automotive industry. Met. Sci. Heat. Treat. 44, 365–370 (2002).
Abro, S. H., Chandio, A., Channa, I. A. & Alaboodi, A. S. Role of automotive industry in global warming. Pak. J. Sci. Ind. Res. A: Phys. Sci. 62, 197–201 (2019).
Dursun, T. & Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 56, 862–871 (2014).
Speidel, M. O. Stress corrosion cracking of aluminum alloys. Metall. Trans. A 6, 631 (1975).
Sieradzki, K. & Newman, R. Stress-corrosion cracking. J. Phys. Chem. Solids 48, 1101–1113 (1987).
Al Saadi, S., Yi, Y., Cho, P., Jang, C. & Beeley, P. Passivity breakdown of 316L stainless steel during potentiodynamic polarization in NaCl solution. Corros. Sci. 111, 720–727 (2016).
Yi, Y., Cho, P., Al Zaabi, A., Addad, Y. & Jang, C. Potentiodynamic polarization behaviour of AISI type 316 stainless steel in NaCl solution. Corros. Sci. 74, 92–97 (2013).
Liu, X., MacDonald, D. D., Wang, M. & Xu, Y. Effect of dissolved oxygen, temperature, and pH on polarization behavior of carbon steel in simulated concrete pore solution. Electrochim. Acta 366, 137437 (2021).
Morshed-Behbahani, K., Zakerin, N., Najafisayar, P. & Pakshir, M. A survey on the passivity of tempered AISI 420 martensitic stainless steel. Corros. Sci. 183, 109340 (2021).
Li, Y., Morel, A., Gallant, D. & Mauzeroll, J. Oil-Immersed Scanning Micropipette Contact Method Enabling Long-term Corrosion Mapping. Anal. Chem. 92, 12415–12422 (2020).
Shkirskiy, V. et al. Nanoscale Scanning Electrochemical Cell Microscopy and Correlative Surface Structural Analysis to Map Anodic and Cathodic Reactions on Polycrystalline Zn in Acid Media. J. Electrochem. Soc. 167, 041507 (2020).
Yule, L. et al. Nanoscale electrochemical visualization of grain-dependent anodic iron dissolution from low carbon steel. Electrochim. Acta 332, 135267 (2020).
Yule, L. C., Bentley, C. L., West, G., Shollock, B. A. & Unwin, P. R. Scanning electrochemical cell microscopy: A versatile method for highly localised corrosion related measurements on metal surfaces. Electrochim. Acta 298, 80–88 (2019).
Pao, L., Muto, I. & Sugawara, Y. Pitting at inclusions of the equiatomic CoCrFeMnNi alloy and improving corrosion resistance by potentiodynamic polarization in H2SO4. Corros. Sci. 191, 109748 (2021).
Revie, R. W. Corrosion and corrosion control: an introduction to corrosion science and engineering, 4th edition (John Wiley & Sons, 2008).
Poorqasemi, E., Abootalebi, O., Peikari, M. & Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 51, 1043–1054 (2009).
Stephens, L. et al. Development of a model for experimental data treatment of diffusion and activation limited polarization curves for magnesium and steel alloys. J. Electrochem. Soc. 164, E3576 (2017).
ASTM Standards. G5-94, Standard reference test method for making potentiostatic and potentiodynamic anodic polarization measurements. Annual book of ASTM Standards, ASTM International, 3 (2004).
Zhang, X., Jiang, Z. H., Yao, Z. P., Song, Y. & Wu, Z. D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 51, 581–587 (2009).
Williams, C. G., Edwards, M. A., Colley, A. L., Macpherson, J. V. & Unwin, P. R. Scanning micropipet contact method for high-resolution imaging of electrode surface redox activity. Anal. Chem. 81, 2486–2495 (2009).
Ebejer, N., Schnippering, M., Colburn, A. W., Edwards, M. A. & Unwin, P. R. Localized high resolution electrochemistry and multifunctional imaging: Scanning electrochemical cell microscopy. Anal. Chem. 82, 9141–9145 (2010).
Takahashi, Y. et al. Nanoscale visualization of redox activity at lithium-ion battery cathodes. Nat. Commun. 5, 5450 (2014).
Fischer, D. A., Vargas, I. T., Pizarro, G. E., Armijo, F. & Walczak, M. The effect of scan rate on the precision of determining corrosion current by Tafel extrapolation: A numerical study on the example of pure Cu in chloride containing medium. Electrochim. Acta 313, 457–467 (2019).
ASTM Standard. G102-89, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. Annual Book of ASTM Standards, ASTM International, 3 (2006).
Birbilis, N., Padgett, B. N. & Buchheit, R. G. Limitations in microelectrochemical capillary cell testing and transformation of electrochemical transients for acquisition of microcell impedance data. Electrochim. Acta 50, 3536–3544 (2005).
Otieno-Alego, V., Hope, G., Flitt, H. & Schweinsberg, D. The corrosion of a low alloy steel in a steam turbine environment: the effect of oxygen concentration and potential scan rate on input parameters used to computer match the experimental polarization curves. Corros. Sci. 37, 509–525 (1995).
Mi, C., Lakhera, N., Kouris, D. A. & Buttry, D. A. Repassivation behaviour of stressed aluminium electrodes in aqueous chloride solutions. Corros. Sci. 54, 10–16 (2012).
Boxley, C. J., Watkins, J. J. & White, H. S. Al2O3 Film Dissolution in Aqueous Chloride Solutions. Electrochem. Solid-State Lett. 6, B38 (2003).
Lee, S. & White, H. S. Dissolution of the native oxide film on polycrystalline and single-crystal aluminum in NaCl solutions. J. Electrochem. Soc. 151, B479 (2004).
Lee, H., Xu, F., Jeffcoate, C. S. & Isaacs, H. S. Cyclic Polarization behavior of aluminum oxide films in near neutral solutions. Electrochem. Solid-State Lett. 4, B31 (2001).
Linarez Pérez, O. E., Fuertes, V. C. & Pérez, M. A. & López Teijelo, M. Characterization of the anodic growth and dissolution of oxide films on valve metals. Electrochem. Commun. 10, 433–437 (2008).
Boxley, C. J. & White, H. S. Relationship Between Al2O3 Film Dissolution Rate and the Pitting Potential of Aluminum in NaCl Solution. J. Electrochem. Soc. 151, B265 (2004).
Gudić, S., Radošević, J., Krpan-Lisica, D. & Kliškić, M. Anodic film growth on aluminium and Al–Sn alloys in borate buffer solutions. Electrochim. Acta 46, 2515–2526 (2001).
Hasenay, D. & Šeruga, M. The growth kinetics and properties of potentiodynamically formed thin oxide films on aluminium in citric acid solutions. J. Appl. Electrochem. 37, 1001–1008 (2007).
Williams, D. & Wright, G. Nucleation and growth of anodic oxide films on bismuth—I. Cyclic voltammetry. Electrochim. Acta 21, 1009–1019 (1976).
Macdonald, D. D. The point defect model for the passive state. J. Electrochem. Soc. 139, 3434 (1992).
Burleigh, T., Rennick, R. & Bovard, F. Corrosion potential for aluminum alloys measured by ASTM G 69. Corrosion 49 (1993).
Abdel-Gaber, A., Abd-El-Nabey, B., Sidahmed, I., El-Zayady, A. & Saadawy, M. Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros. Sci. 48, 2765–2779 (2006).
Khaled, K. & Al-Qahtani, M. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 113, 150–158 (2009).
Osório, W. R., Freitas, E. S. & Garcia, A. EIS and potentiodynamic polarization studies on immiscible monotectic Al–In alloys. Electrochim. Acta 102, 436–445 (2013).
Arnott, D., Hinton, B. W. & Ryan, N. Cationic film-forming inhibitors for the corrosion protection of AA 7075 aluminum alloy in chloride solutions. Mater. Perform. 26, 42–47 (1987).
Andreatta, F., Terryn, H. & De Wit, J. Corrosion behaviour of different tempers of AA7075 aluminium alloy. Electrochim. Acta 49, 2851–2862 (2004).
Nisancioglu, K. & Holtan, H. Measurement of the critical pitting potential of aluminium. Corros. Sci. 18, 835–849 (1978).
Newman, J. & Thomas-Alyea, K. E. Electrochemical systems, 3rd edition (John Wiley & Sons, 2012).
Martin, F., Cheek, G., O’grady, W. & Natishan, P. Impedance studies of the passive film on aluminium. Corros. Sci. 47, 3187–3201 (2005).
Orlikowski, J., Ryl, J., Jarzynka, M., Krakowiak, S. & Darowicki, K. Instantaneous impedance monitoring of aluminum alloy 7075 corrosion in borate buffer with admixed chloride ions. Corrosion 71, 828–838 (2015).
Visser, P., Terryn, H. & Mol, J. M. Active corrosion protection of various aluminium alloys by lithium‐leaching coatings. Surf. Interface Anal. 51, 1276–1287 (2019).
Evertsson, J. et al. The thickness of native oxides on aluminum alloys and single crystals. Appl. Surf. Sci. 349, 826–832 (2015).
Frers, S., Stefenel, M., Mayer, C. & Chierchie, T. AC-Impedance measurements on aluminium in chloride containing solutions and below the pitting potential. J. Appl. Electrochem. 20, 996–999 (1990).
Wang, F., Li, Y., Zhang, Y. & Chen, G. A method to select the optimal equivalent electrical circuit applied to study corrosion system of composite coating on magnesium alloy. Phys. Lett. A 384, 126452 (2020).
Kwolek, P. Corrosion behaviour of 7075 aluminium alloy in acidic solution. RSC Adv. 10, 26078–26089 (2020).
Torbati-Sarraf, H., Stannard, T. J., La Plante, E. C., Sant, G. N. & Chawla, N. Direct observations of microstructure-resolved corrosion initiation in AA7075-T651 at the nanoscale using vertical scanning interferometry (VSI). Mater. Charact. 161, 110166 (2020).
Wu, Z., Richter, C. & Menon, L. A Study of Anodization Process during Pore Formation in Nanoporous Alumina Templates. J. Electrochem. Soc. 154, E8 (2007).
Gharbi, O., Tran, M. T., Tribollet, B., Turmine, M. & Vivier, V. Revisiting cyclic voltammetry and electrochemical impedance spectroscopy analysis for capacitance measurements. Electrochim. Acta 343, 136109 (2020).
Orazem, M. E. et al. Dielectric properties of materials showing constant-phase-element (CPE) impedance response. J. Electrochem. Soc. 160, C215 (2013).
Hirschorn, B. et al. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 55, 6218–6227 (2010).
Lohrengel, M. Thin anodic oxide layers on aluminium and other valve metals: high field regime. Mater. Sci. Eng., R. 11, 243–294 (1993).
Wood, G., Skeldon, P., Thompson, G. & Shimizu, K. A model for the incorporation of electrolyte species into anodic alumina. J. Electrochem. Soc. 143, 74 (1996).
Thompson, G. et al. Anodic oxidation of aluminium. Philos. Mag. B 55, 651–667 (1987).
Bunker, B. C. et al. Hydration of Passive Oxide Films on Aluminum. J. Phys. Chem. B 106, 4705–4713 (2002).
Despić, A. & Parkhutik, V. P. Electrochemistry of Aluminum in Aqueous Solutions and Physics of Its Anodic Oxide, in Modern aspects of electrochemistry (Vol. 20, Springer, 1989).
Snizhko, L. O. et al. A model for galvanostatic anodising of Al in alkaline solutions. Electrochim. Acta 50, 5458–5464 (2005).
Lee, H. & Isaacs, H. S. Growth of Anodic Aluminum Oxide Films Without pH-Buffer. ECS Trans. 11, 121 (2008).
Cabrera, N. & Mott, N. F. Theory of the oxidation of metals. Rep. Prog. Phys. 12, 163–184 (1949).
Adhikari, S. & Hebert, K. R. Participation of aluminum hydride in the anodic dissolution of aluminum in alkaline solutions. J. Electrochem. Soc. 155, C189 (2008).
Nguyen, T. H. & Foley, R. T. The Chemical Nature of Aluminum Corrosion: III . The Dissolution Mechanism of Aluminum Oxide and Aluminum Powder in Various Electrolytes. J. Electrochem. Soc. 127, 2563 (1980).
Hughes, A. E., Mol, J. M., Zheludkevich, M. L. & Buchheit, R. G. Active Protective Coatings: New-Generation Coatings for Metals, Vol. 233 (Springer, 2016).
Natishan, P. & O’grady, W. Chloride ion interactions with oxide-covered aluminum leading to pitting corrosion: a review. J. Electrochem. Soc. 161, C421 (2014).
Gimenez, P., Rameau, J. & Reboul, M. Experimental pH potential diagram of aluminum for sea water. Corrosion 37, 673–682 (1981).
McCafferty, E. Surface Chemistry of Aqueous Corrosion Processes. (Springer, 2015).
Strohmeier, B. R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 15, 51–56 (1990).
Alexander, M., Thompson, G., Zhou, X., Beamson, G. & Fairley, N. Quantification of oxide film thickness at the surface of aluminium using XPS. Surf. Interface Anal. 34, 485–489 (2002).
Thomas, S., Birbilis, N., Venkatraman, M. & Cole, I. Corrosion of zinc as a function of pH. Corrosion 68, 015009-1–015009-9 (2012).
Zaid, B., Saidi, D., Benzaid, A. & Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 50, 1841–1847 (2008).
ASTM Standard. G61-86, Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron, Nickel-, or Cobalt-based Alloys. Annual Book of ASTM Standards, ASTM International, 3 (2014).
Acknowledgements
We acknowledge the financial support from G234176 NSERC RGPIN-2020-04609 and G248536 CQRDA/NRC. This work was conducted as part of a project funded by the NRC’s METALTec industrial research group, the Centre québécois de recherche et de développement de l’aluminium (CQRDA), as well as the Canadian Office for Energy Research and Development (OERD). The authors would like to acknowledge Dr. Lihong Shang from McGill Institute for Advanced Materials for her help with the XPS measurements as well as the METALTec industrial research group members and sponsors that supported this investigation and publication.
Author information
Authors and Affiliations
Contributions
H.Z. and D.C. are co-first authors. H.Z. performed all experiments. D.C. performed the COMSOL simulation. H.Z and D.C. analyzed the data, discussed the results, and co-wrote the paper. J.M. supervised the project. A.M. and D.G. discussed the results and advised the work. All authors contributed to revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, H., Chhin, D., Morel, A. et al. Potentiodynamic polarization curves of AA7075 at high scan rates interpreted using the high field model. npj Mater Degrad 6, 20 (2022). https://doi.org/10.1038/s41529-022-00227-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-022-00227-3