Abstract
Von Hippel-Lindau disease (VHL) is an autosomal dominant, inherited syndrome with variants in the VHL gene causing predisposition to multi-organ benign and malignant neoplasms. A germline VHL variant is identified in 95–100% of individuals with a clinical diagnosis of VHL. Here, we present the case of an individual with a clinical diagnosis of VHL disease where peripheral blood DNA analysis did not detect a VHL variant. Sequencing of four tumor tissues (ccRCC, pheochromocytoma, lung via sputum, liver) revealed a VHL c.593 T > C (p.Leu198Pro) variant at varying allele fractions (range: 10–55%) in all tissues. Re-examination of the peripheral blood sequencing data identified this variant at 6% allele fraction. Tumor analysis revealed characteristic cytomorphological, immunohistochemical reactivity for alpha-inhibin, and CAIX, and reduced pVHL reactivity supported VHL-related pseudohypoxia. This report of a rare case of VHL mosaicism highlights the value of tissue testing in VHL variant negative cases.
Similar content being viewed by others
Introduction
Von Hippel-Lindau disease (VHL) is an autosomal dominant syndrome that predisposes individuals to benign and malignant neoplasms in various organs1. The most common neoplasms affecting individuals with VHL are hemangioblastoma of the retina, hemangioblastoma of the central nervous system (CNS), endolymphatic sac tumor, clear cell renal cell carcinoma (ccRCC), pheochromocytoma (PCC), paraganglioma, and pancreatic neuroendocrine neoplasm or cyst2. VHL is caused by a germline pathogenic variant in the tumor suppressor gene VHL and has a prevalence of ~1 in 36,0003. Tumorigenesis typically follows the two-hit mechanism where the wild-type VHL allele becomes lost or inactivated through multiple mechanisms, including somatic methylation of the VHL promoter region, point mutations, small insertions/deletions, or loss-of-heterozygosity (often through large deletions)4.
Patients with suspected VHL disease undergo confirmation through molecular genetic analysis of the unaffected DNA. A germline VHL variant (sequence or copy number variant) is identified in 95–100% of individuals who fulfill the clinical criteria for VHL5,6,7,8. A study of 945 VHL families found the majority of individuals have a missense variant (52%), followed by frameshift (13%), large deletion (11%), nonsense (11%), splice site (7%), and in-frame deletion/insertion (6%)9. It is typically believed that up to 5% of individuals with a clinical diagnosis of VHL have negative genetic test results from germline DNA analysis6,7,8. However, a national study in Denmark found no disease-causing VHL variant in 21% (15/71) assumed VHL patients that underwent genetic testing, which included patients with a hemangioblastoma of the CNS10. This may be due to postzygotic mosaicism, phenotypic coincidence, methylation, alteration in another gene that results in a phenotype similar to VHL, or a cryptic variant in VHL not detected by conventional methods4,11,12.
Only a few case studies13,14,15 have reported VHL mosaicism; the rate of mosaicism in VHL is largely unknown and may be under-reported. Several studies have attempted to quantify the rate of mosaicism within VHL, the most notable being Sgambati et al (2000)16. This study found mosaic variants in ~5% (2/42) of patients with a clinical diagnosis of VHL, but without a family history. A recent study used Next Generation Sequencing (NGS) in combination with single mutation-specific PCR methods in a cohort of gene-negative patients with either clinical VHL or suspected VHL17. This combination method was able to detect mosaic variants in 8.5% (4/47) of their cohort17. Another paper by the same group performed deep sequencing (>1000×) on eight patients suggestive of VHL, with two patients fulfilling clinical VHL criteria. A pathogenic VHL variant was detected at 1.7% and 5.7% variant allele fractions (VAFs) in the two cases with clinical VHL18. Of note, tumor testing was performed in only one study where half the patients (2/4) with mosaic variants had tumor tissue analyzed17. All studies defined mosaicism in terms of a variant with a low VAF, with no specific cutoff provided.
Advances in NGS allow for high throughput and more sensitive detection of variants at lower VAFs and more cases of mosaic VHL may emerge19. To further ascertain and counsel these cases, tumor analysis of the VHL gene and tumor immunohistochemistry will become increasingly important. Here, we present a case of a suspected mosaic individual who fulfilled clinical criteria for VHL but initially received negative germline VHL results through multigene panel testing on DNA extracted from peripheral blood leukocytes. We describe further evaluation with tumor tissue analysis as a complementary tool in the workup of such variant elusive VHL cases.
Results
Patient characteristics
A 55-year-old male presented in the emergency room with shortness of breath, chest pain, and coughing; a chest X-ray found a perihilar mass on the left lung. A CT scan of the chest confirmed the mass on the left lung (measuring 3.1 × 1.8 cm) and noted a left renal lesion. A CT scan of the abdomen and pelvis revealed a large mass on the left kidney and right adrenal gland. An ultrasound guided biopsy showed that the left kidney mass was a renal cell carcinoma. The patient subsequently underwent a left-sided radical nephrectomy and right-sided adrenalectomy. The pathology results confirmed ccRCC (measuring 12 × 11 × 9.5 cm) and identified a pheochromocytoma (measuring 6.7 × 5.8 × 5 cm). Biochemical evaluation of urine and plasma metanephrines and urine catecholamines were within normal limits. Two pieces of lung tissue orally expelled from the patient further supported metastatic ccRCC. While spinal MRI and chest CT identified bone metastases, there were also two spinal lesions (measuring 2.2 mm and 14 mm) consistent with spinal hemangioblastomas. In addition, histologically confirmed liver metastases from ccRCC were noted. The patient succumbed to his disease 10 months after initial presentation. The patient’s family history was unremarkable for VHL and no other VHL-associated manifestations were found on the patient’s subsequent imaging. A clinical diagnosis of Type 2B VHL was presumed, and blood was sent for germline molecular genetic testing.
Initial genetic testing results
The patient underwent germline testing on DNA isolated from peripheral blood leukocytes with a panel of genes targeted at hereditary renal cell cancer and a hereditary paraganglioma-pheochromocytoma. The patient tested negative for any pathogenic, likely pathogenic, or uncertain DNA sequence or copy number variants in FH, FLCN, MET, MITF, MLH1, MSH2, MSH6, PMS2, PTEN, SDHA, SDHB, SDHC, SDHD, TP53, TSC1, TSC2, MAX, MEN1, NF1, RET, SDHAF2, TMEM127, and VHL. Variant allele threshold for review on the germline panel was 10% or greater VAF.
As a molecular genetic diagnosis of VHL was not identified, the daughters of the proband underwent germline molecular genetic analysis of VHL and results were negative. However, given the family history, both daughters were enrolled in full VHL screening including ophthalmologic assessment, brain, spine, and abdominal MRI. They have no VHL manifestations at the ages of 25 and 30.
Morphological and immunohistochemical characteristics of pheochromocytoma
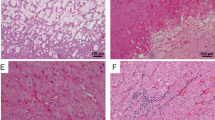
The adrenal tumor tissue underwent detailed morphological assessment by an endocrine pathologist. The adrenal tumor was an encapsulated pheochromocytoma with variable clear cell change and stromal degeneration with increased microvasculature (Fig. 1a, b). For immunohistochemistry analysis, positivity for tyrosine hydroxylase (Fig. 1c) and GATA3 confirmed the diagnosis of pheochromocytoma. The cytomorphological findings (e.g., clear cell change, encapsulation with stromal changes) were highly suggestive of VHL-related pheochromocytoma20. While there was no cyclinD1 overexpression, the tumor was positive for carbonic anhydrase IX (CAIX) and alpha-inhibin (variable and weak) (Fig. 1d, 1e). Alpha-inhibin expression21, along with CAIX expression21,22,23, was consistent with VHL-disease-related pseudohypoxia, especially in the background of morphological findings. Moreover, variable loss of pVHL also supported an altered pseudohypoxia pathway in this tumor (Fig. 1f).
Whole scanned images: The adrenalectomy specimen (hematoxylin and eosin) shows an encapsulated pheochromocytoma with clear cell change, variable fibrohyaline, and myxoid stroma rich in microvasculature (a, b). The tumor cells are diffusely positive for tyrosine hydroxylase (the rate limiting enzyme in catecholamine synthesis) (c). The tumor is positive for carbonic anhydrase IX (d). Alpha-inhibin shows variable weak reactivity in the tumor cells (e). pVHL shows variable loss or significantly reduced staining intensity in the tumor cells (f).
Genetic testing of tumor specimens
Paraffin embedded tissue retrieved from the ccRCC, pheochromocytoma, expelled lung tissue (sputum), and liver biopsy were tested on a targeted hereditary cancer panel24. A likely pathogenic variant in exon 3 of VHL (NM_0000551.3: c.593 T > C; p.Leu198Pro) was detected in all four tissues at varying VAFs (Fig. 2). This missense variant is consistent with Type 2 VHL and has been classified as likely pathogenic in the germline context25,26,27. Although no second hits in VHL were detected, copy number calling revealed evidence of 3p loss-of-heterozygosity in all tumor tissues (kidney, adrenal, lung, and liver).
Integrative Genomics Viewer display of the c.593 T > C (p.Leu198Pro) variant in tissue from four tumor specimens (a) and peripheral blood leukocytes (b). The percentage of sequencing reads (gray bars) depicting the variant are beside the biospecimen type. The blue bars depict a cytosine nucleotide instead of a thymine nucleotide at the variant loci. The overall proportion of cytosine:thymine reads is represented by the blue:red bars at the top of each tissue panel. Tumor cellularity was estimated by a pathologist to be 65% in the kidney tissue and 85% in the adrenal gland, lung, and liver tissue.
The VHL p.Leu198Pro variant has been previously reported in the germline of two families25,26,27 and tumor of a patient with sporadic pheochromocytoma28. In the first family, three members carried the variant and all three had bilateral pheochromocytomas and one had an additional sympathetic paraganglioma25,27. A protein prediction research software (symphony) predicted that this missense variant affects a VHL surface protein residue25,29. In the second family, one member presented with an early onset pancreatic neuroendocrine tumor, bilateral pheochromocytomas, and an optic nerve hemangioblastoma26.
Reanalysis of germline testing results
Re-examination of the NGS data from the proband’s initial hereditary cancer testing identified the T allele at c.593 in the VHL gene in ~6% of the sequencing reads (77 reads of the total 1298 at this base pair position). This was below the typical reportable threshold for NGS multigene panel testing of 10%, and therefore was not initially reported in clinical genetic testing.
Discussion
Approximately 95–100% of individuals with clinical VHL receive a positive result when they undergo standard genetic testing on DNA extracted from blood or saliva. This case report explores the rare event of an individual with typical VHL manifestations testing negative for a VHL variant on germline DNA extracted from peripheral blood leukocytes, with subsequent sequencing of four postmortem tumor tissue specimens revealing a VHL pathogenic variant in all four tissues. Tumor analysis revealed protein markers consistent with VHL-driven tumorigenesis and pVHL. Reanalysis of germline genetic testing results on the blood sample detected the variant at 6% VAF, confirming the strong likelihood of somatic mosaicism in this case.
This case illustrates several factors to consider for conditions where somatic mosaicism is suspected based on the proband and family history. Clinical genetic testing results are typically anticipated to be categorical (wild-type, heterozygous, and homozygous), as this most accurately represents the inheritance of Mendelian disease-causing variants. The targeted panel performed on DNA isolated from the patient’s peripheral blood leukocytes was validated to detect variants above a 10% VAF, as this was the expected lower VAF range for a germline heterozygous variant on this panel. Variants present at reduced VAFs may not be reported in all clinical labs. In addition, the lower limit threshold for reporting variants is not a standard value for NGS panels whose intended use is to detect inherited germline variants expected to be heterozygous or homozygous. Communication with the testing laboratory, including clinical suspicions for a specific genetic diagnosis, can be helpful in triggering re-examination of low-level calls or raw data for potentially mosaic results. In addition, negative genetic testing should not be considered as a definitive conclusion and further investigations, including of other tissue types, may be required to arrive at a diagnostic molecular finding. Our VAF of the various affected tissues ranged from as low as 10% to as high as 55% despite adequate tumor cellularity. This variability may be due to tumor biology such as inflammatory cells causing normal tissue contamination and other genomic events and is a reported limitation of VAF from tumor sequencing30.
Mosaicism results from the acquisition of a somatic mutation during embryogenesis, leading to variable mutant cell frequency throughout the body. While previously reported that individuals with mosaic VHL are asymptomatic or present with a mild phenotype13,14,15, Coppin et al. (2014) have reported a severe phenotype in a mosaic VHL patient18. Here we presented an individual with three VHL-associated manifestations, which provides further evidence that mosaicism in VHL can present with a severe phenotype. Cases of mosaicism in similar Mendelian conditions have been described previously with phenotypes less severe than might be expected from these autosomal dominant conditions; however, given the incomplete penetrance and variable expressivity of these conditions themselves, it is difficult to make specific phenotypic predictions31,32. A systematic review of 111 articles reporting on 320 mosaic NF1 individuals found that mosaic NF1 patients present with a milder phenotype and have fewer complications and manifestations31. A study on 39 Tuberous Sclerosis Complex patients with mosaicism (<10% VAF) also found that mosaic patients presented with a milder phenotype32. Further examination of suspected VHL mosaicism is needed to discern the relationship between phenotype severity and mosaicism in this hereditary cancer syndrome.
Notably, the two offspring of this proband had negative germline testing, which included analysis of VHL prior to the identification of this mosaic finding. The eventual identification of a molecular cause for their father’s phenotype may have clinical implications for these two daughters. However, given the findings from this report, the risk of VHL disease to the daughters may be significantly decreased and may play a role in counseling them to continue to undergo intensive VHL surveillance. The current gold-standard for mosaic testing involves analysis of tissue from all three germ layers and may not be possible in many clinical scenarios, including in rapidly deteriorating patients. This case report shows the reality of mosaic testing and proves the value in completing tissue testing on available tissue as the result provides valuable information that can be used to guide the surveillance and treatment of family members.
Methods
Ethics approval and clinical trial registration
Written informed consent for this study was obtained from the family of the proband following a clinical research protocol approved by the Research Ethics Board at University Health Network (#16-5831). This research study is registered at clinicaltrials.gov (clinical trials registration number: NCT03857594).
Genetic analysis
Genomic DNA extracted from peripheral blood leukocytes, and quantified using a spectrophotometer, underwent two targeted NGS panels at a commercial laboratory (Ambry Genetics Corporation, Aliso Viejo, California) for clinical testing. Ambry Genetics is a CLIA-certified, CAP accredited laboratory, which performs clinical genetic testing. The requested assays included NGS of all coding base pairs and 5 base pairs of intronic region flanking the exons of 12 genes associated with hereditary renal cell cancer and hereditary paraganglioma-pheochromocytoma predisposition. The genes and reference sequences used for analysis are: FH- NM_000143, MAX- NM_002382, MEN1-NM_130799.2, NF1- NM_001042492, RET- NM_020975, SDHA- NM_004168, SDHAF2-NM_017841, SDHB- NM_003000, SDHC- NM_003001, SDHD- NM_003002, TMEM127- NM_017849, and VHL- NM_000551. Sequence enrichment of the targeted coding exons and adjacent intronic nucleotides was carried out by a bait-capture methodology using long biotinylated oligonucleotide probes followed by polymerase chain reaction and NGS. Sequence reads were aligned to the reference human genome (GRCh37) using NovoAlign (version 3.02.07; Novocraft Technologies, Selangor, Malaysia) and variant calls generated using the Genome Analysis Toolkit (version 3.2.2; Broad Institute, Cambridge, MA). Suspect variant calls, other than those classified as “likely benign” or “benign”, were verified by Sanger sequencing. A minimum coverage of 25×, Q score of 30 and VAF of >10% were required for candidate variants to pass quality control metrics for reporting. Any regions with an insufficient depth of coverage for heterozygous variant calling were analyzed by Sanger sequencing. The sequence of copy number variants which did not meet internal quality control metrics for confidence were confirmed by a secondary methodology, such as chromosomal microarray or Sanger sequencing, prior to reporting.
Paraffin embedded clinical blocks were retrieved postmortem and DNA from four separate tissues (ccRCC, pheochromocytoma, expelled lung tissue, and liver biopsy). The tissue underwent pathology review by experienced clinical pathologists who determined histology and tumor cellularity. DNA was extracted from the four tissues and used in 59-gene NGS panel (Clinical Genome Diagnostics Laboratory, University Health Network, Toronto, Ontario) for detection of pathogenic variants associated with hereditary cancer syndromes, using methods previously described for analysis of tumor DNA from FFPE material24. For variant analysis, a custom bioinformatic pipeline was used with targeted sequence reads aligned to the reference human genome (hg19) using Burrows-Wheeler Aligner (version 0.7.12)33. Duplicate reads were marked (Picard Mark Duplicates; version 1.130) and the Genome Analysis Toolkit (version 3.3.0; Broad Institute, Cambridge, MA) best practices followed Base Quality Score Recalibration. Somatic single nucleotide variants and small insertion/deletions were detected by Varscan2 (version 2.3.8) and copy number status was determined by CNVkit (version 0.7.11)34,35. Loss-of-heterozygosity was detected using pureCN (version 1.12.0) in R (version 3.5.0), with input data from CNVkit and MuTect (version 1.1.5)36,37. All variants were filtered using Alissa software (Agilent, Santa Clara, CA) to detect variants with a VAF > 10% and to remove benign variants. All variants were manually reviewed in IGV (version 2.3)38. All genomic regions achieved at least 25× coverage.
Immunohistochemistry
Immunohistochemical staining for GATA3 (Biocare Medical, Pacjeco, CA; catalog number: BC-CM405bl dilution: 1:100), tyrosine hydroxylase (Abcam, Cambridge, UK; catalog number: ab75875-2; dilution: 1:500), cyclinD1 (Cell Marque, Rocklin, CA; catalog number: CMQ-241R16; dilution: 1:250), alpha-inhibin (Cedarlane, Burlington, ON; catalog number: MCA951S; dilution: 1:100) and CAIX (Leica, Wetzlar, Germany; catalog number: CAIX-L-CE; dilution: 1:100) was performed in our clinical diagnostic immunohistochemistry laboratory using appropriate positive and negative controls. pVHL immunohistochemistry was performed in an institutional research laboratory according to the manufacturer’s instructions (OriGene, Rockville, MD; catalog number: TA506222; dilution 1:500), along with appropriate positive and negative controls.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Tumor bam files from the four tissues that underwent targeted sequencing have been deposited in the European Genome-phenome Archive repository under accession code EGAS00001005895. The germline data derived for this study are not publicly available as they contain information that may compromise the research participant’s privacy but may be accessed by qualified researchers through R.H.K. (raymond.kim@uhn.ca).
Code availability
No custom code was used to analyze the data and all tools used within the customized bioinformatics pipeline are included in the Methods section, with the version used.
References
Kim, J. J., Rini, B. I. & Hansel, D. E. in Diseases of DNA Repair (ed. Ahmad, S. I.) 228–249 (Springer New York, 2010).
Binderup, M. L. M. et al. Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan. Med. J. 60, B4763 (2013).
Maher, E. R. et al. Von Hippel-Lindau disease: a genetic study. J. Med. Genet. 28, 443–447 (1991).
Prowse, A. H. et al. Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am. J. Hum. Genet. 60, 765–771 (1997).
Maher, E. R., Neumann, H. P. & Richard, S. von Hippel-Lindau disease: a clinical and scientific review. Eur. J. Hum. Genet. 19, 617–623 (2011).
Cho, H.-J., Ki, C.-S. & Kim, J.-W. Improved detection of germline mutations in Korean VHL patients by multiple ligation-dependent probe amplification analysis. J. Korean Med. Sci. 24, 77–83 (2009).
Stolle, C. et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum. Mutat. 12, 417–423 (1998).
Hes, F. J. et al. Frequency of Von Hippel-Lindau germline mutations in classic and non-classic Von Hippel-Lindau disease identified by DNA sequencing, Southern blot analysis and multiplex ligation-dependent probe amplification. Clin. Genet. 72, 122–129 (2007).
Nordstrom-O’Brien, M. et al. Genetic analysis of von Hippel-Lindau disease. Hum. Mutat. 31, 521–537 (2010).
Binderup, M. L. M., Galanakis, M., Budtz-Jørgensen, E., Kosteljanetz, M. & Luise Bisgaard, M. Prevalence, birth incidence, and penetrance of von Hippel-Lindau disease (vHL) in Denmark. Eur. J. Hum. Genet. 25, 301–307 (2017).
Ricketts, C. J. et al. A germline 1;3 translocation disrupting the VHL gene: a novel genetic cause for von Hippel-Lindau. J. Med. Genet. https://doi.org/10.1136/jmedgenet-2020-107308 (2020).
Lenglet, M. et al. Identification of a new VHL exon and complex splicing alterations in familial erythrocytosis or von Hippel-Lindau disease. Blood 132, 469–483 (2018).
Wu, P. et al. Mosaicism in von Hippel-Lindau disease with severe renal manifestations. Clin. Genet. 84, 581–584 (2013).
Murgia, A. et al. Somatic mosaicism in von Hippel-Lindau Disease. Hum. Mutat. 15, 114 (2000).
Santarpia, L. et al. Mosaicism in von Hippel-Lindau disease: an event important to recognize. J. Cell. Mol. Med. 11, 1408–1415 (2007).
Sgambati, M. T. et al. Mosaicism in von Hippel-Lindau disease: lessons from kindreds with germline mutations identified in offspring with mosaic parents. Am. J. Hum. Genet. 66, 84–91 (2000).
Coppin, L. et al. Optimization of next-generation sequencing technologies for von Hippel Lindau (VHL) mosaic mutation detection and development of confirmation methods. J. Mol. Diagn. 21, 462–470 (2019).
Coppin, L. et al. VHL mosaicism can be detected by clinical next-generation sequencing and is not restricted to patients with a mild phenotype. Eur. J. Hum. Genet. 22, 1149–1152 (2014).
Gajecka, M. Unrevealed mosaicism in the next-generation sequencing era. Mol. Genet. Genomics 291, 513–530 (2016).
Mete O., Hannah-Shmouni F., Kim R., Stratakis C. A. in The Spectrum of Neuroendocrine Neoplasia (eds. Asa, S. L., Rosa, S. L., Mete, O.) 409–459 (Springer, 2020).
Mete, O., Pakbaz, S., Lerario, A. M., Giordano, T. J. & Asa, S. L. Significance of alpha-inhibin expression in pheochromocytomas and paragangliomas. Am. J. Surg. Pathol. https://doi.org/10.1097/PAS.0000000000001715 (2021).
Papathomas, T. G. et al. What have we learned from molecular biology of paragangliomas and pheochromocytomas? Endocr. Pathol. 32, 134–153 (2021).
Juhlin, C. C. Challenges in paragangliomas and pheochromocytomas: from histology to molecular immunohistochemistry. Endocr. Pathol. https://doi.org/10.1007/s12022-021-09675-0 (2021).
Care, M. et al. Tumor and germline next generation sequencing in high grade serous cancer: experience from a large population-based testing program. Mol. Oncol. https://doi.org/10.1002/1878-0261.12817 (2020).
Lomte, N. et al. Genotype phenotype correlation in Asian Indian von Hippel-Lindau (VHL) syndrome patients with pheochromocytoma/paraganglioma. Fam. Cancer 17, 441–449 (2018).
Wittström, E., Nordling, M. & Andréasson, S. Genotype-phenotype correlations, and retinal function and structure in von Hippel-Lindau disease. Ophthalmic Genet. 35, 91–106 (2014).
Pandit, R. et al. Germline mutations and genotype-phenotype correlation in Asian Indian patients with pheochromocytoma and paraganglioma. Eur. J. Endocrinol. 175, 311–323 (2016).
Dannenberg, H. et al. Von Hippel-Lindau gene alterations in sporadic benign and malignant pheochromocytomas. Int. J. Cancer 105, 190–195 (2003).
Gossage, L. et al. An integrated computational approach can classify VHL missense mutations according to risk of clear cell renal carcinoma. Hum. Mol. Genet. 23, 5976–5988 (2014).
Strom, S. P. Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol. Med. 13, 3–11 (2016).
García-Romero, M. T., Parkin, P. & Lara-Corrales, I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr. Dermatol. 33, 9–17 (2016).
Giannikou, K. et al. Low-level mosaicism in tuberous sclerosis complex: prevalence, clinical features, and risk of disease transmission. Genet. Med. 21, 2639–2643 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012).
Talevich, E., Hunter Shain, A., Botton, T. & Bastian, B. C. CNVkit: Genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Riester, M. et al. PureCN: copy number calling and SNV classification using targeted short read sequencing. Source Code Biol. Med. 11, 13 (2016).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213 (2013).
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013).
Acknowledgements
We would like to thank the patient and their family for allowing us to complete this study. In addition, we would like to thank the Princess Margaret Applied Molecular Profiling—Drug Development Biomarker lab, Ming Tsao and Jing Xu for completing the pVHL immunohistochemical staining. Thank you to Charlotte Fung and Karen Ott for reviewing the manuscript. This study was funded by the University of Toronto Division of Medical Oncology Strategic Innovation Fund and the Soper Kidney Cancer Foundation. R.H.K. is supported by the Bhalwani Family Charitable Foundation.
Author information
Authors and Affiliations
Contributions
R.H.K. devised the study, secured funding, and supervised the study. L.E.O. completed the analysis with input from J.G., S.G., T.S., and R.H.K. O.M. completed the immunohistochemical staining and interpretation. L.E.O. and R.H.K. wrote the manuscript with support from all authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.G. and E.C. are employed by Ambry Genetics. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oldfield, L.E., Grzybowski, J., Grenier, S. et al. VHL mosaicism: the added value of multi-tissue analysis. npj Genom. Med. 7, 21 (2022). https://doi.org/10.1038/s41525-022-00291-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41525-022-00291-3
This article is cited by
-
Hemangioblastoma and mosaic von Hippel Lindau disease: rare presentation and review of the literature
Child's Nervous System (2023)