Abstract
Breast tissue enhances on contrast MRI and is called background parenchymal enhancement (BPE). Having high BPE has been associated with an increased risk of breast cancer. We examined the relationship between BPE and the amount of fibroglandular tissue on MRI (MRI-FGT) and breast cancer risk factors. This was a cross-sectional study of 415 women without breast cancer undergoing contrast-enhanced breast MRI at Memorial Sloan Kettering Cancer Center. All women completed a questionnaire assessing exposures at the time of MRI. Prevalence ratios (PR) and 95% confidence intervals (CI) describing the relationship between breast cancer risk factors and BPE and MRI-FGT were generated using modified Poisson regression. In multivariable-adjusted models a positive association between body mass index (BMI) and BPE was observed, with a 5-unit increase in BMI associated with a 14% and 44% increase in prevalence of high BPE in pre- and post-menopausal women, respectively. Conversely, a strong inverse relationship between BMI and MRI-FGT was observed in both pre- (PR = 0.66, 95% CI 0.57, 0.76) and post-menopausal (PR = 0.66, 95% CI 0.56, 0.78) women. Use of preventive medication (e.g., tamoxifen) was associated with having low BPE, while no association was observed for MRI-FGT. BPE is an imaging marker available from standard contrast-enhanced MRI, that is influenced by endogenous and exogenous hormonal exposures in both pre- and post-menopausal women.
Similar content being viewed by others
Introduction
Mammographic percent density (MPD) is a measure of the proportion of the normal breast occupied by fibroglandular tissue (FGT), seen as dense (white) areas on a mammogram. While MPD is considered to be one of the strongest, established risk factors for breast cancer1, there are other features (e.g., texture features) on mammogram that have also been implicated in risk2.
In the United States women at a high risk of breast cancer (i.e., ≥20% lifetime risk) are recommended to undergo annual screening with contrast-enhanced magnetic resonance imaging (MRI) in addition to mammography3. The amount of fibroglandular tissue in the breast can be assessed volumetrically on MRI (MRI-FGT) and is known to be correlated with MPD4,5. Much like MPD, MRI-FGT has been shown to be associated with breast cancer risk6, and sensitive to endogenous (e.g., menopause) and exogenous (e.g., tamoxifen, aromatase inhibitors) hormonal exposures7,8,9. Like mammogram, there are other image features from MRI that may be associated with breast cancer risk.
Contrast-enhanced MRI uses an intravenously injected contrast agent, to help visualize tumors through the identification of distinct patterns of contrast dispersion10,11. The MRI signal from normal FGT also enhances to varying degrees and is called background parenchymal enhancement (BPE). BPE is recorded as the proportion of FGT in the breast that enhances. Having high BPE has been associated with an increased risk of breast cancer in some6,12,13,14,15,16,17, but not all18,19 studies. Notably, this association is thought to be independent of MRI-FGT6,16. BPE has been shown to be highly influenced by both endogenous (e.g., menopausal status7, serum estrogen concentrations20, body mass index [BMI]21,22) and exogenous (e.g., menopausal hormone therapy [MHT]23,24, tamoxifen9,25, aromatase inhibitors8,26) hormonal exposures. However, many of these studies have been small or did not consider relevant confounders.
The objective of this study was to contribute to our understanding of BPE and MRI-FGT as imaging markers of breast cancer risk by examining their relationship with established breast cancer risk factors.
Results
Distribution of Breast Cancer Risk Factors
The median age at MRI was 49 years, 48% of women were postmenopausal, and 90% self-identified as White (Table 1). Most women (82% of premenopausal and 88% of postmenopausal), were having an MRI for high-risk breast cancer screening purposes. As expected, BPE and MRI-FGT were both higher in premenopausal than in postmenopausal women (P < 0.0001 for both) (Table 2). Weighted Cohen’s kappa coefficients for repeat reads of BPE and MRI-FGT were 0.87 (95% CI 0.76, 0.98) and 0.92 (95% CI 0.83, 1.00) (i.e., ‘almost perfect agreement’), respectively. Notably BPE and MRI-FGT were not correlated (Pearson correlation coefficient and p-value: postmenopausal women r = −0.00658, p = 0.93; premenopausal women: r = −0.09307 p = 0.17). The distribution of different breast cancer risk factors in the study population are shown in Tables 1 and 3.
Association between BPE and MRI-FGT with breast cancer risk factors
Table 4 shows results from multivariable-adjusted models for BPE and MRI-FGT in premenopausal women. A positive association between BPE and BMI was observed, but this result did not reach statistical significance (PR = 1.14, 95% CI 0.96, 1.35). There was also a positive association between BPE and use of oral contraceptives at the time of MRI, such that women who reported using oral contraceptives tended to have a higher prevalence of high BPE, however, this finding did not reach statistical significance (PR = 1.45, 95% CI 0.98, 2.15). Conversely, premenopausal women with documented BRCA mutations were less likely to have higher BPE than non-carriers (PR = 0.40, 95% CI 0.19, 0.83). It was thought that this relationship could be explained by use of preventive medications (e.g., tamoxifen) in this high-risk group. However, none of the women identified as premenopausal BRCA mutation carriers reported use of preventive medications at the time of MRI. In multivariable-adjusted models of MRI-FGT in premenopausal women, BMI was significantly associated with MRI-FGT, with increasing BMI associated with a lower prevalence of high MRI-FGT (PR = 0.66, 95% CI 0.57, 0.77 per five-unit increase in BMI). We also found a significant positive association between having a personal history of LCIS and the prevalence of high MRI-FGT (PR = 1.22, 95% CI 1.02, 1.45).
In postmenopausal women (Table 5), BPE tended to be lower with increasing age, although this did not reach statistical significance (p = 0.09). A significant positive association between BMI and BPE was observed such that each five-unit increase in BMI was associated with a 44% higher prevalence of high BPE (PR = 1.44, 95% CI 1.08, 1.93). Compared to women who were nulliparous, those who were 30 years or older at the time of first full-term pregnancy had a lower prevalence of high BPE (PR = 0.33, 95% CI 0.13, 0.86). Only BMI was significant associated with MRI-FGT in postmenopausal women, with a 34% decrease in the prevalence of high MRI-FGT seen with every five-unit increase in BMI (PR = 0.66, 95% CI 0.56, 0.78) (Table 5).
Sensitivity analyses
Sensitivity analyses were conducted restricting to women who self-reported White/Caucasian or reported having an MRI for high-risk screening purposes and the results did not differ (results not shown).
BPE and MRI-FGT and use of preventive medications
Finally, the use of preventive medications was associated with low BPE in both pre- and post-menopausal women (Table 6). The impact of these medications on BPE is so strong that all women using these medications had low BPE (p = 0.07 in premenopausal women and p = 0.05 in postmenopausal women). We therefore could not include these variables in the multivariable models for BPE due to small (zero) cell counts. No association between current use of preventive medications and MRI-FGT was observed (p > 0.05) (Table 6).
Discussion
BPE and MRI-FGT are characteristics of normal breast tissue that are routinely assessed by radiologists from standard contrast-enhanced MRI. Prior studies have shown these markers to be independently associated with breast cancer risk, contributing distinct information about a woman’s risk6,16. To better understand these relationships and how they could be used to inform recommendations for screening and prevention, it is necessary to understand the factors that impact these imaging markers. The results of the current study show that BPE is highly dependent on hormonal exposures, with a positive association observed between BMI (postmenopausal women only) and a strong inverse association with use of preventive medications (e.g., tamoxifen, aromatase inhibitors).
BPE was found to be positively associated with BMI in both pre-and post-menopausal women. This relationship was most clear (and statistically significant) for postmenopausal women where for every 5-unit increase in BMI the prevalence of high BPE increases by about 40%. While a positive association was also observed in premenopausal women, this association did not reach statistical significance. These results are consistent with those of two prior studies observing a positive association between BMI and BPE. In a small study of 214 women, Hellgren et al.21 found that women with obesity (BMI > 30 kg/m2) had an almost 5-fold higher odds (95% CI 1.2, 19.4) of having high versus low BPE compared to women with a BMI < 25 kg/m2. A second study found an association between BMI and BPE in unadjusted analyses22. Ours is the largest multivariable-adjusted study to-date, able to also examine this relationship stratified by menopausal status. Consideration of menopausal status is important given the complex relationship between BMI, menopausal status, and breast cancer risk.
BMI is positively associated with breast cancer risk in postmenopausal women and inversely associated with risk in premenopausal women27. This relationship is likely explained in part by the relationship between BMI and hormones (e.g., estradiol)28, where in postmenopausal women, adipose tissue becomes the primary source of circulating estrogens29. There is a growing body of evidence showing BPE to be associated with both endogenous and exogenous hormonal exposures. BPE has been shown to increase with the use of MHT23,24 and to decrease with menopause7 and oophorectomy30 and in response to treatment with tamoxifen9,25, or aromatase inhibitors8,26. Recently we also showed that BPE is significantly positively associated with serum estradiol levels in postmenopausal women20. Together this suggests a plausible mechanism through which BMI could be impacting BPE in postmenopausal women. Further, there is some indication that the relationship between BPE and breast cancer risk may be modified by BMI, however, this requires further investigation16.
In the current study, we found a strong relationship between the use of hormonal medications at the time of MRI and BPE. Specifically, the use of preventive medications (e.g., tamoxifen) was so strongly associated with low BPE in both pre- and postmenopausal women, that all women using these medications had low BPE. We also found that use of oral contraceptives at the time of MRI was associated with a higher prevalence of high BPE, however, this association did not reach statistical significance. Prior work has shown that current or recent use of oral contraceptives is associated with increased breast cancer risk31,32. These results support the hypothesis that BPE is a reflection of current/recent hormonal exposures (endogenous and exogenous) experienced in the breast, however further investigation of the influence of oral contraceptive use on BPE is warranted.
Prior work has shown a positive association between BPE and MHT23,24. We found that use of MHT at the time of MRI was associated with a non-significant increase in the prevalence of high BPE. This is likely due to the small number of women reporting use of MHT, and the high proportion of those reporting use of local therapy. Specifically, of the 33 women who reported MHT use at the time of MRI, 21 reported use of estrogen-only therapy and 16 of these women reported use of local therapy (e.g., Vagifem, Estring). We did not have the sample size to conduct analyses further stratified by subtype, but since it is thought that BPE may be an indicator of local and systemic hormonal exposures this could have important implications for the use of local estrogen therapy in women undergoing treatment for breast cancer with aromatase inhibitors33. Notably we did not see similar associations with MRI-FGT.
MPD has been studied extensively, and it is known to be highly variable across the population. Overall it has been shown to decrease with increasing age, increasing the number of births34,35,36, and increasing BMI34. MPD has also been shown to be influenced by hormonal exposures, decreasing with menopause, and with tamoxifen use in postmenopausal women37, and increasing with administration of combined estrogen and progestin MHT (but not estrogen alone therapy)38. Similarly, we observed an inverse relationship between MRI-FGT and BMI. This was expected given that MRI-FGT is a volumetric measure of the amount of fibroglandular tissue in the breast that has been shown to correlate with MPD4,5,39. Other factors (e.g., parity, MHT) previously associated with MPD were not associated with MRI-FGT in this study. We hypothesize that this is because the current analysis is focused on exposure status at the time of MRI. Prior work by our group has shown that BPE is highly dynamic and responsive to the hormonal environment of the breast, whereas changes in MRI-FGT take longer to be observed on MRI8,9. This supports the idea that BPE may be an imaging biomarker of current hormonal exposures in the breast and could be used as an indicator of response to hormonal medications40. One finding that deserves further consideration is the relationship between LCIS and the prevalence of high MRI-FGT. To our knowledge, this association has not been shown before, however breast density has been shown to play an important role in breast cancer risk among women with LCIS41.
One unexpected finding was that BRCA mutation carrier status was inversely associated with BPE in premenopausal women such that women carrying a BRCA mutation were 60% less likely to have high BPE, compared to non-carriers. It was thought that this association could be related to use of preventive medications (prescribed as chemoprevention in this high-risk population), however, none of the premenopausal BRCA mutation carriers reported use of these medications. Further, because this association is only observed in premenopausal women, we know that the lower BPE is also not due to prophylactic oophorectomy.
A small study conducted in Chinese women found BRCA mutation carriers to have lower BPE than non-carriers in unadjusted analyses42. However, the results of this study (while consistent with our findings) are hard to interpret given that it is not clear if this association is due to other differences between carriers and non-carriers (e.g., use of preventive medications, differences in age or menopausal status). To date no studies have specifically addressed the relationship between BPE and breast cancer risk in BRCA mutation carriers. However, the few studies that have examined the association between BPE and breast cancer risk, suggest that BPE is also a risk factor for breast cancer in these women12.
A major strength of this study is the collection of data on multiple breast cancer risk factors from both a study questionnaire at the time of MRI, and patients’ medical records. This is particularly important for the assessment of the impact of hormonal medications and lifestyle factors (e.g., smoking and alcohol), where in our study current use (at the time of MRI) was assessed. Inter-reader variability is an important consideration in studies involving the assessment of BPE and MRI-FGT, with many ongoing efforts to develop computer-assisted algorithms for image assessment to reduce potential variability. Here we used a single experienced reader, with repeat BPE and MRI-FGT reads showing very high concordance (i.e., ‘almost perfect agreement’). Although the use of a single reader could potentially limit the generalizability of the study, the high degree of reproducibility of repeat reads by the study radiologist shows strong internal validity. Further, by dichotomizing BPE as low (min/mild) and high (moderate/marked), external validity is increased by reducing the potential for discordance.
Further limitations include the lack of racial diversity in the study population, with 89% of women self-identifying as White, limiting our ability to examine the impact of race on BPE or MRI-FGT. We also lacked information on the timing of MRI with respect to week of the menstrual cycle for pre-menopausal women. The American College of Radiology recommends that MRIs be performed in week 2 of the menstrual cycle when BPE is thought to be at its lowest, thereby maximizing the sensitivity of the MRI for cancer detection43. Information on menstrual cycle week was not consistently available for study participants and so is not part of the current analysis. The impact of this is likely negligible as recent papers have found no association between menstrual cycle week and BPE44,45. Finally, we did not have information on all breast cancer risk factors. Of particular interest could be the relationship between physical activity and BPE. Physical activity is a potentially modifiable risk factor that has been shown to be associated with both circulating hormone levels and body composition46,47.
Prior work has found BPE to be a promising new marker of breast cancer risk providing information beyond that provided by assessment of breast density6,16. The development of abbreviated MRI screening protocols likely means that MRI will increasingly be used to screen women that do not meet the high-risk criteria. This could include women with dense breasts48 or those at higher than average (elevated) risk49. This highlights the need to understand the factors that influence BPE. Here we show that BPE is significantly positively associated with BMI in postmenopausal women. Further, use of preventive medications led to an almost complete reduction in BPE. The hormonally responsive nature of BPE, supported by this and prior studies, suggests that BPE could be an imaging biomarker of hormonal exposures in the breast, potentially used as an indicator of response to hormonal medications, or to further stratify women at high risk of breast cancer undergoing MRI screening.
Methods
This study was reviewed and approved by the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board, and written informed consent was obtained at the time of recruitment from all study participants.
Study population
Patient recruitment and data collection have been published previously20. Briefly, women (N = 504) who had no prior history of any cancer (including ductal carcinoma in situ [DCIS], but excluding nonmelanoma skin cancer) as noted in their medical record were approached in the MRI screening clinic at MSK between August 2012 and March 2014. Of the 449 women (88.9%) who volunteered to participate in the study, 30 were ultimately determined to be ineligible. Reasons for exclusion included insufficient proficiency in English (n = 2), prior personal history of cancer not previously identified during medical record review (n = 14), incomplete study questionnaire (n = 2) and diagnosis of breast cancer within the six months following MRI (n = 12). Individuals missing information on any covariates were also excluded (3 premenopausal women and 1 postmenopausal woman). This left a study population of 415 women for the current analysis.
Data collection
All women completed a self-administered questionnaire at the time of their MRI capturing information related to reproductive history, use of hormonal medications, family history of breast cancer and other risk factors. These included: age at menarche and menopause, parity (number of full-term pregnancies, age at first full-term pregnancy, time since last full-term pregnancy), use of hormonal medications at the time of MRI (e.g., MHT, tamoxifen, raloxifene, aromatase inhibitors), weight and height at the time of MRI, family history of breast cancer, and history of oophorectomy. Data from questionnaires was confirmed, when possible, through review of medical records.
Contrast-enhanced MRI and assessment of BPE and MRI-FGT
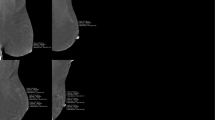
Breast MRIs were conducted using standard imaging protocols as described previously20. BPE and MRI-FGT were assess by a single reader using the proposed American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) criteria50,51. As such, BPE was classified as: minimal, mild, moderate or marked, and MRI-FGT as: a. almost entirely fatty, b. scattered fibroglandular tissue, c. heterogeneous fibroglandular tissue, or d. extreme fibroglandular tissue. The radiologist was blinded to all clinical characteristics of the patients.
BPE and MRI-FGT are usually similar between breasts11. To confirm this, readings were conducted in both breasts and were only found to be discordant in one individual. In this instance, the higher value of the two breasts was assigned. Finally, to assess agreement between repeat reads, a set of MRIs (n = 19) were randomly selected to be re-read for both BPE and MRI-FGT.
Statistical Analysis
This was a cross-sectional study examining the relationship between BPE, MRI-FGT and breast cancer risk factors. BI-RADS categories of BPE and MRI-FGT were collapsed to create dichotomous variables categorizing BPE as minimal/mild and moderate/marked, and MRI-FGT as predominantly fatty/scattered and heterogeneous/extreme.
Breast cancer risk factors considered included: age at MRI (continuous), menopausal status (see below), current BMI (continuous), first-degree family history of breast cancer (yes, no), personal history of breast biopsy (yes, no), history of high-risk benign lesions (none, atypical hyperplasia (atypical ductal hyperplasia [ADH] or atypical lobular hyperplasia [ALH]), lobular carcinoma in situ (LCIS), age at menarche (<13 years of age, ≥13 years of age, based on the median age at menarche in the study population), parous (yes/no), number of full-term pregnancies (nulliparous, 1, 2, ≥3), and age at first full-term pregnancy (nulliparous, <25, 25–<29, ≥30 years). BRCA1/2 mutation status categories were collapsed (negative, positive [BRCA1-positive, BRCA2-positive, BRCA1- and BRCA2-positive, positive-unknown type, variant of unknown significance (VUS)], untested [Table 3]) because of insufficient numbers in some subgroups. Use of hormonal medications at the time of MRI was also captured and included use of oral contraceptives (premenopausal women only; yes/no), MHT (postmenopausal women only; yes (any type)/no), and preventive medications (current use of tamoxifen, raloxifene, aromatase inhibitors; yes/no). Other exposures including smoking status (yes/no), alcohol consumption (yes/no), and the number of drinks per week (none, <7, ≥7) were also examined.
If a woman reported that she had not had a menstrual period in the previous 12 months or had a personal history of a bilateral oophorectomy she was considered postmenopausal at the time of MRI. Five women were either missing information on age at the last menstrual period (n = 2) or had a period between 6 and 12 months of enrollment (n = 3). Eleven women, ranging in age from 44 to 64 years reported having had a simple hysterectomy, making it challenging to determine their menopausal status. For these groups of women, a prior analysis in this study population that included serum measurements of estradiol and estrone found that all had hormone levels were within the postmenopausal range. This indicated that they were indeed postmenopausal at the time of MRI20.
Mutually adjusted prevalence ratios (PR) and 95% confidence intervals (CI) were estimated using modified Poisson regression (i.e., Poisson regression using a log link and with robust error variance)52. Models did not include an adjustment for MRI-FGT because there was no association between BPE and MRI-FGT in our data (thus it did not meet the requirement of a confounder). Further, for analyses related to BMI, there was concern about over-adjustment given the strong relationship between BMI and MRI-FGT. All analyses were conducted stratified by menopausal status. Analyses were also conducted restricting to women who were having an MRI for high-risk screening purposes (i.e., excluding women with an abnormal mammogram or lump, N = 348) and then again in those that self-reported White/Caucasian race/ethnicity (N = 369).
Concordance between repeat BPE and MRI-FGT reads was assessed using Cohen’s kappa coefficients. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary NC) and all p values are 2-sided.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
McCormack, V. A. & dos Santos Silva, I. Breast Density and Parenchymal Patterns as Markers of Breast Cancer Risk: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 15, 1159–1169 (2006).
Hopper, J. L. et al. Going Beyond Conventional Mammographic Density to Discover Novel Mammogram-Based Predictors of Breast Cancer Risk. J. Clin. Med 9, 627 (2020).
Saslow, D. et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA: a cancer J. clinicians 57, 75–89 (2007).
Thompson, D. J. et al. Assessing the usefulness of a novel MRI-based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: the UK MARIBS study. Breast Cancer Res 11, R80–R80 (2009).
Klifa, C. et al. Magnetic resonance imaging for secondary assessment of breast density in a high-risk cohort. Magn. Reson Imaging 28, 8–15 (2010).
King, V. et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260, 50–60 (2011).
King, V. et al. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur. Radiol. 22, 2641–2647 (2012).
King, V. et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 264, 670–678 (2012).
King, V. et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. breast J. 18, 527–534 (2012).
Turnbull, L. W. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR biomedicine 22, 28–39 (2009).
Morris, E. A. Diagnostic breast MR imaging: current status and future directions. Magn. Reson. imaging Clin. North Am. 18, 57–74 (2010).
Dontchos, B. N. et al. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated with Breast Cancer Risk. Radiology 276, 371–380 (2015).
Arasu, V. A. et al. Population-Based Assessment of the Association Between Magnetic Resonance Imaging Background Parenchymal Enhancement and Future Primary Breast Cancer Risk. J. Clin. Oncol. 37, 954–963 (2019).
Grimm, L. J. et al. Relationship between Background Parenchymal Enhancement on High-risk Screening MRI and Future Breast Cancer Risk. Academic Radiol. 26, 69–75 (2019).
Telegrafo, M., Rella, L., Stabile Ianora, A. A., Angelelli, G. & Moschetta, M. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn. Reson Imaging 34, 173–176 (2016).
Watt, G. P. et al. Association of breast cancer with MRI background parenchymal enhancement: the IMAGINE case-control study. Breast Cancer Res 22, 138–138 (2020).
Niell, B. L. et al. Quantitative Measures of Background Parenchymal Enhancement Predict Breast Cancer Risk. Ajr. Am. J. Roentgenol. 217, 64–75 (2021).
Vreemann, S. et al. Amount of fibroglandular tissue FGT and background parenchymal enhancement BPE in relation to breast cancer risk and false positives in a breast MRI screening program: A retrospective cohort study. Eur. Radio. 29, 4678–4690 (2019).
Bennani-Baiti, B., Dietzel, M. & Baltzer, P. A. MRI Background Parenchymal Enhancement Is Not Associated with Breast Cancer. PloS one 11, e0158573 (2016).
Brooks, J. D. et al. MRI background parenchymal enhancement, breast density and serum hormones in postmenopausal women. Int. J. cancer 143, 823–830 (2018).
Hellgren, R. et al. The association between breast cancer risk factors and background parenchymal enhancement at dynamic contrast-enhanced breast MRI. Acta Radiol. (Stockh., Swed.: 1987) 61, 1600–1607 (2020).
Gillman, J., Chun, J., Schwartz, S., Schnabel, F. & Moy, L. The relationship of obesity, mammographic breast density, and magnetic resonance imaging in patients with breast cancer. Clin. imaging 40, 1167–1172 (2016).
Delille, J.-P. et al. Hormone Replacement Therapy in Postmenopausal Women: Breast Tissue Perfusion Determined with MR Imaging—Initial Observations. Radiology 235, 36–41 (2005).
Pfleiderer, S. O. R. et al. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res 6, R232–R238 (2004).
Schrading, S., Schild, H., Kühr, M. & Kuhl, C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology 271, 45–55 (2014).
Mousa, N. A., Eiada, R., Crystal, P., Nayot, D. & Casper, R. F. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause (N. Y., N. Y.) 19, 420–425 (2012).
Bhaskaran, K. et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet (Lond., Engl.) 384, 755–765 (2014).
Key, T. J. & Pike, M. C. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur. J. cancer Clin. Oncol. 24, 29–43 (1988).
Gérard, C. & Brown, K. A. Obesity and breast cancer – Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 466, 15–30 (2018).
Price, E. R. et al. The impact of bilateral salpingo-oophorectomy on breast MRI background parenchymal enhancement and fibroglandular tissue. Eur. Radio. 24, 162–168 (2014).
Hunter, D. J. et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol., Biomark. Prev.: a Publ. Am. Assoc. Cancer Res., cosponsored Am. Soc. Preventive Oncol. 19, 2496–2502 (2010).
Mørch, L. S. et al. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N. Engl. J. Med. 377, 2228–2239 (2017).
Sulaica, E. et al. Vaginal estrogen products in hormone receptor-positive breast cancer patients on aromatase inhibitor therapy. Breast cancer Res. Treat. 157, 203–210 (2016).
Boyd, N. F. et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol. Biol. (Clifton, N. J.) 472, 343–360 (2009).
Ginsburg, O. M., Martin, L. J. & Boyd, N. F. Mammographic density, lobular involution, and risk of breast cancer. Br. J. Cancer 99, 1369–1374 (2008).
Loehberg, C. R. et al. Assessment of mammographic density before and after first full-term pregnancy. Eur. J. Cancer Prev.: Off. J. Eur. Cancer Prev. Organ. (ECP) 19, 405–412 (2010).
Cuzick, J., Warwick, J., Pinney, E., Warren, R. M. & Duffy, S. W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl. Cancer Inst. 96, 621–628 (2004).
Greendale, G. A. et al. Postmenopausal hormone therapy and change in mammographic density. J. Natl. Cancer Inst. 95, 30–37 (2003).
Lee, N. A. et al. Fatty and fibroglandular tissue volumes in the breasts of women 20-83 years old: comparison of X-ray mammography and computer-assisted MR imaging. Ajr. Am. J. Roentgenol. 168, 501–506 (1997).
Heller, S. L., Lin, L. L. Y., Melsaether, A. N., Moy, L. & Gao, Y. Hormonal Effects on Breast Density, Fibroglandular Tissue, and Background Parenchymal Enhancement. RadioGraphics 38, 983–996 (2018).
Minami, C. A. et al. Do Body Mass Index and Breast Density Impact Cancer Risk Among Women with Lobular Carcinoma In Situ. Ann. Surgical Oncol. 27, 1844–1851 (2020).
You, C. et al. The clinicopathological and MRI features of patients with BRCA1/2 mutations in familial breast cancer. Gland Surg. 10, 262–272 (2021).
American College of Radiology. ACR practice parameter for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast.
Dontchos, B. N., Rahbar, H., Partridge, S. C., Lehman, C. D. & DeMartini, W. B. Influence of Menstrual Cycle Timing on Screening Breast MRI Background Parenchymal Enhancement and Diagnostic Performance in Premenopausal Women. J. Breast Imaging 1, 205–211 (2019).
Lee, C. H. et al. Outcome of Screening MRI in Premenopausal Women as a Function of the Week of the Menstrual Cycle. Ajr. Am. J. Roentgenol. 214, 1175–1181 (2020).
Brown, J. C., Kontos, D., Schnall, M. D., Wu, S. & Schmitz, K. H. The Dose-Response Effects of Aerobic Exercise on Body Composition and Breast Tissue among Women at High Risk for Breast Cancer: A Randomized Trial. Cancer Prev. Res. (Phila., Pa.) 9, 581–588 (2016).
McTiernan, A. et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obes. (Silver Spring, Md.) 14, 1662–1677 (2006).
Comstock, C. E. et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 323, 746–756 (2020).
Grimm, L. J., Mango, V. L., Harvey, J. A., Plecha, D. M. & Conant, E. F. Implementation of Abbreviated Breast MRI for Screening: AJR Expert Panel Narrative Review. Ajr. Am. J. Roentgenol. 218, 202–212 (2022).
Morris, E. ACR BI-RADS® magnetic resonance imaging. (2013).
Sickles, E. A., D'Orsi, C. J. & Bassett, L. W. ACR BI-RADS Mammography, (2013).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Acknowledgements
This study was funded by the National Institutes of Health (grant no: R03CA162207 and R01CA183086) and the Canadian Institutes of Health Research (grant no: RN312225-376411).
Author information
Authors and Affiliations
Contributions
J.D.B. conceived of the design of the study, oversaw analysis, and drafted the manuscript. R.A.G.C. conducted the data analysis. R.A.G.C., J.S.S., M.C.P., I.O., J.L.B. and E.A.M. contributed to study design, data analysis and drafting of manuscript. J.D.B., J.S.S., and I.O. contributed to data acquisition. All authors revised the manuscript for important intellectual content and have read and approved the final manuscript. All authors take accountability for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brooks, J.D., Christensen, R.A.G., Sung, J.S. et al. MRI background parenchymal enhancement, breast density and breast cancer risk factors: A cross-sectional study in pre- and post-menopausal women. npj Breast Cancer 8, 97 (2022). https://doi.org/10.1038/s41523-022-00458-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-022-00458-2