Abstract
Few germline genetic variants have been robustly linked with breast cancer outcomes. We conducted trans-ethnic meta genome-wide association study (GWAS) of overall survival (OS) in 3973 breast cancer patients from the Pathways Study, one of the largest prospective breast cancer survivor cohorts. A locus spanning the UACA gene, a key regulator of tumor suppressor Par-4, was associated with OS in patients taking Par-4 dependent chemotherapies, including anthracyclines and anti-HER2 therapy, at a genome-wide significance level (\(P = 1.27 \times 10^{ - 9}\)). This association was confirmed in meta-analysis across four independent prospective breast cancer cohorts (combined hazard ratio = 1.84, \(P = 1.28 \times 10^{ - 11}\)). Transcriptome-wide association study revealed higher UACA gene expression was significantly associated with worse OS (\(P = 4.68 \times 10^{ - 7}\)). Our study identified the UACA locus as a genetic predictor of patient outcome following treatment with anthracyclines and/or anti-HER2 therapy, which may have clinical utility in formulating appropriate treatment strategies for breast cancer patients based on their genetic makeup.
Similar content being viewed by others
Introduction
The past decade has witnessed remarkable advances in our knowledge of the genetic architecture of breast cancer susceptibility from numerous genome-wide association studies (GWAS). To date, over 300 independent breast cancer risk variants that passed genome-wide significance (\(P \, < \, 5 \times 10^{ - 8}\)) have been identified1,2. In contrast, parallel efforts to discover genetic variants associated with breast cancer prognosis lag far behind, with only 11 GWAS published to date and only four loci reaching or approaching the canonical genome-wide significance3,4,5,6,7,8,9,10,11,12,13.
Several factors may have hindered the progress in GWAS of breast cancer prognosis. First, unlike breast cancer risk as a binary phenotype that is available from most cancer epidemiology studies and can be readily aggregated from multiple studies to a large sample size required for GWAS analysis, breast cancer prognosis is a phenotype that is more challenging to capture and requires long term follow-up efforts after diagnosis. Thus, breast cancer survival outcome data are available only from a limited number of studies, but even then, survival bias is a major concern in many of those studies. Second, heterogeneity in tumor subtype, histopathological presentation, treatment received, and comorbidities can have major effect on prognosis, which may have contributed to the lack of success in replicating loci across the prior GWAS of breast cancer prognosis. Detailed clinical annotation data are critical to control for potential confounding or interaction effects in GWAS of cancer prognosis, as well as to the meaningful interpretation of these findings. However, such data are often unavailable from studies where genotype data have been generated.
Despite these challenges, there is evidence supporting a strong genetic influence on breast cancer prognosis14,15. In animal studies, mammary tumor progression varies by genetic strain, and candidate germline polymorphisms are prognostic16. In human studies, familial concordance of breast cancer mortality was observed among mother–daughter and sister–sister pairs17, and the hereditary component in breast cancer prognosis appears to be independent of patient, tumor, and treatment variables18. Indeed, variants identified from GWAS of breast cancer susceptibility are rarely associated with prognosis19,20, indicating distinct genetic architecture underlying susceptibility and prognosis. The agnostic GWAS approaches are expected to discover new genes and biological mechanisms underlying breast cancer progression and drug response, to identify potential targets for treatment, and to provide clinically useful biomarkers for risk stratification and prediction of treatment outcomes.
In the present study, we report findings from GWAS of breast cancer prognosis in the context of the Pathways Study, one of the largest contemporary breast cancer survivor cohorts with rich patient outcome data through long-term active follow-up, and accompanying pathological, clinical, treatment, and epidemiological data. We sought to replicate our promising findings in three independent breast cancer cohorts, followed by meta-analysis to synthesize the results across race/ethnicity and cohorts, and lastly, a transcriptome-wide association study (TWAS) to further confirm the causal gene.
Results
Replication of prior GWAS findings
To date, 24 lead variants and one suggestive causal variant from 11 GWAS of breast cancer prognosis have been reported3,4,5,6,7,8,9,10,11,12,13, only four of which reached or approached genome-wide significance. We attempted to replicate these variants in the Pathway Study, by matching patient sub-population, survival outcomes, and covariates with the original study (Table 1). Only one of the 24 variants (rs421379, \(P = 0.015\)) was nominally significant (\(P \le 0.05\)) and had consistent direction of effect in our replication analysis as the prior GWAS. rs421379 on chr 5 was reported to associate with breast-cancer specific death (BCSD) in young (age ≤40 years) European patients6. The sample size and event number of this patient subpopulation were limited in our study (nine events out of 109 patients). When tested in European-descent patients of all adult ages, no association with BCSD was found (\(P = 0.92\)), in contrast to the previous GWAS9.
GWAS of breast cancer prognosis in the Pathways Study
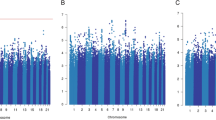
In GWAS on OS within 2801 European-descent patients, the largest racial/ethnic group in the Pathways Study (Supplementary Table 1), no variants reached genome-wide significance. Clinical characteristics, including age at diagnosis, cancer grade and stage, hormonal receptor status, HER2 status, and treatment, were adjusted as covariates in the Cox regression model (see “Methods” section). The strongest association was on chromosomes 6 and 15 (\(P = 3.63 \times 10^{ - 7}\) and \(5.95 \times 10^{ - 7}\), respectively; Supplementary Fig. 1 and Supplementary Table 2). Trans-ethnic meta-GWAS across all populations (European, East Asian, Hispanic, and African) identified the same locus on chromosome 15 with close to genome-wide significance (\(P = 9.42 \times 10^{ - 8}\) for rs11855431, Fig. 1 and Table 2 and Supplementary Figs. 2 and 3).
In analyses stratified by tumor ER status, an imputed variant passed genome-wide significance in GWAS within ER-positive (ER+) patients from Pathways European population (rs113113429 on chr 13, imputation Rsq = 0.91255, MAF = 0.053, \(P = 2.89 \times 10^{ - 8}\); Supplementary Fig. 4). However, no variants in the locus were in high LD with rs113113429 (Supplementary Fig. 5), making it less likely a true hit. No genome-wide significant finding was observed in patients with ER-negative (ER−) cancer (top variant rs11690772 on chr 2, \(P = 1.98 \times 10^{ - 7}\); Supplementary Fig. 6). Trans-ethnic meta-GWAS stratified by tumor ER status did not yield any genome-wide significance finding (lowest \(P = 6.91 \times 10^{ - 7}\) and \(1.67 \times 10^{ - 7}\) for ER+ and ER− patients, respectively; Supplementary Figs. 4 and 6),
UACA locus affects patient outcomes after doxorubicin or anti-HER2 therapy
The top locus on chr 15 identified in our trans-ethnic meta-GWAS of OS contains the UACA gene, a key regulator of tumor suppressor Par-421. Recent studies have suggested that Par-4 inhibition is involved in breast cancer recurrence and resistance to chemotherapy22,23,24. We thus tested the interaction between chemotherapy status and rs11855431, the lead variant of the UACA locus, for its effect on OS, and found the interaction was significant (\(P = 6.1 \times 10^{ - 3}\)). Since anti-HER2 agents and doxorubicin were shown to lead to Par-4-dependent multinucleation and cell death22, we further tested the interaction with these two Par-4 dependent agents vs. with all other chemotherapeutic agents. The results confirmed the interactive effect on OS to be specific to anti-HER2 and doxorubicin therapies (\(P = 2.4 \times 10^{ - 4}\) vs. \(P = 0.80\) for all other chemo treatment). Subsequent GWAS performed separately in these two treatment groups within the European population (Supplementary Figs. 7 and 8 and Supplementary Table 2) revealed that UACA was the only locus associated with OS at genome-wide significance in patients taking Par-4 dependent chemotherapies (Hazard ratio (HR) = 2.79, \(P = 4.19 \times 10^{ - 9}\) for the minor allele (T) of lead variant rs720251; Figs. 2 and 3), with no effect in the remaining patients (HR = 1.20, \(P = 0.19\); Fig. 3 and Supplementary Fig. 7). The association remained significant in trans-ethnic meta-GWAS within patients taking Par-4 dependent chemotherapies (\(P = 1.27 \times 10^{ - 9}\) for the lead variant rs62019060), where the G allele of rs62019060 was consistently associated with poorer patient survival in all four populations (HR = 3.01, 3.19, 1.90, 1.51, and allele frequency = 0.101, 0.099, 0.092, 0.354 in European, East Asian, Hispanic, African population, respectively, Fig. 1 and Table 2, and Supplementary Fig. 9). In contrast, no association was observed for rs62019060 in trans-ethnic meta-GWAS of the remaining patients (\(P = 0.21\)). The three lead variants, rs62019060, rs720251, and rs11855431 are in high linkage disequilibrium (LD) in the 1000 Genomes EUR population (all pairwise r2 ≥ 0.90, see “Methods” section). No other genes involving in the UACA-Par-4 pathway associated with OS in patients taking Par-4 dependent chemotherapies (Supplementary Fig. 10).
KM plot in the Pathways European population (a), and the SBCSS + SBCS cohort (b). Patients were separated by whether they took Par-4 dependent chemotherapies. Patients with imputation dosage <0.5 were considered having genotype CC. Patients with imputation dosage ≥1.5 were considered having genotype TT. Patients with imputation dosage in-between were considered having genotype CT.
Because Par-4 has been reported to play an important role in breast cancer recurrence22,23,24, we then evaluated the impact of UACA locus on recurrence and progression-free survival (PFS), which includes both recurrence and death, among patients treated with anti-HER2 or doxorubicin chemotherapies. Although rs720251, the lead variant in European population, was associated with both recurrence (HR = 2.02, raw \(P = 6.24 \times 10^{ - 5}\), Fig. 4) and PFS (HR = 2.17, raw \(P = 1.12 \times 10^{ - 7}\); Fig. 4), the associations were not as strong as with OS despite using the same patients. As after cancer diagnosis, patients can progress to death through two mutually exclusive courses: death after recurrence versus death without recurrence, we used a competing risk model to investigate how the UACA locus may affect these two courses. rs720251 was observed to associate with both death after recurrence (subdistribution hazard ratio (SHR) = 2.59, \(P = 7.7 \times 10^{ - 5}\)) and death without recurrence (SHR = 2.23, \(P = 1.1 \times 10^{ - 2}\)), suggesting a more general effect beyond recurrence. In addition, we found rs720251 associated with both breast cancer-specific death (SHR = 2.49, \(P = 1.3 \times 10^{ - 6}\)) and other cause of death (SHR = 2.79, \(P = 3.9 \times 10^{ - 2}\)). As cardiotoxicity is a well-recognized adverse effect of both anti-HER2 or doxorubicin chemotherapies25,26,27, we then investigated whether the UCAC locus also affected cardiotoxicity in patients received those treatments using a competing risk model. Indeed, we found rs720251 associated with both death due to cardiovascular disease (SHR = 2.80, \(P = 7.7 \times 10^{ - 3}\)) and other cause of death (SHR = 2.61, \(P = 2.6 \times 10^{ - 6}\)).
The “treatment” and “remaining” group correspond to patients received Par-4 dependent chemotherapies and the remaining patients, respectively. The estimated hazard ratio as well as its 95% confidence interval were plotted. Raw P-values from the corresponding cox models were given for direct comparison across outcomes.
Validation in patients of European descent in the Genetic Epidemiology Research on Aging (GERA) Cohort
The GERA breast cancer cohort included both a prospective component, which involved 880 incident cases who had breast cancer diagnosis after collection of biospecimens for genotyping, and a retrospective component, which involved 1983 prevalent cases who had cancer diagnosis before biospecimen collection. Of the 880 incident cases, 158 (18%) received anti-HER2 or doxorubicin therapies, in comparison to 34% in the Pathways Study. We observed a non-significant yet consistent trend that UACA locus was associated with OS in patients treated with the Par4-dependent agents (HR = 1.61, \(P = 0.67\) for the minor allele T of rs720251); yet no effect was seen in the remaining patients (HR = 1.13, \(P = 0.71\)).
Based on the hypothesis that the T allele of rs720251 was associated with higher risk of death in patients treated with Par-4 dependent chemotherapies, we expect that the prevalent cases who were treated with these agents represent a survival bias and thus carry T alleles at a lower frequency than that in the incident cases, but no such difference should be expected in cases without such treatment. Indeed, this was what we observed between prevalent and incident cases (Par4-dependent treatment: 7.98% vs. 11.08%, bootstrap \(P = 5.50 \times 10^{ - 3}\); other treatment: 9.75% vs. 11.08%, bootstrap \(P = 0.07\); Fig. 5, see “Methods” section).
The “treatment” and “remaining” group correspond to the GERA prevalent patients with and without Par-4 dependent chemotherapies respectively. T allele frequency at population level was estimated using the GERA incident cases or using all European-descent patients from the Pathways Study (“Pathways”). The estimated T allele frequency as well as its 95% confidence interval from 10,000 bootstrap samples were plotted. The P-values were calculated by comparing the T allele frequency between the two corresponding groups connected by the brackets in the plot from the 10,000 bootstrap replications. Significant P-values (≤0.05) were in bold.
Validation in patients of European descent in the Data Bank and Biorepository (DBBR) cohort
The DBBR cohort included 451 breast cancer patients of European descent treated with anti-HER2 or doxorubicin therapies at Roswell Park Comprehensive Cancer Center. In comparison to the Pathways Study, the DBBR cohort were younger (mean age at diagnosis: 51 vs. 55.7 years) and more likely to receive radiation therapy (82.5% vs. 23.1%) (Supplementary Table 3). None of the four top variants in the UACA locus was significantly associated with OS in this cohort (lowest \(P = 0.56\) for rs11855431, Supplementary Table 4).
Validation in patients of East Asian descent in the Shanghai Breast Cancer Survival Study (SBCSS) and Shanghai Breast Cancer Study (SBCS)
The two Shanghai studies pre-dated the advent of anti-HER2 therapy, and thus very few patients received this treatment. While doxorubicin was the dominant anthracycline agent used in the three US cohorts, >96% of the 1289 SBCSS and SBCS patients treated with anthracycline-containing regimens received epirubicin or pirarubicin. We evaluated 1297 variants within 500 kb around UACA gene for their associations with OS stratified by anthracycline treatment. The most significant variant was rs62016907 (HR = 1.72, \(P = 5.76 \times 10^{ - 5}\), imputation Rsq = 1.00, Fig. 6) in patients with anthracycline treatment, while no association was found for this SNP (HR = 1.06, \(P = 0.48\)) among patients receiving no anthracycline treatment. Notably, rs62016907 was genotyped in the Pathways Study and also associated with OS among patients of East Asian population who received Par-4 dependent chemotherapies (HR = 3.00, \(P = 8.68 \times 10^{ - 3}\)). The lead variant in patients of European population in the Pathways Study, rs720251, was the second most significant variant associated with OS in the SBCSS and SBCS patients treated with anthracyclines (HR = 1.67, \(P = 1.29 \times 10^{ - 4}\), imputation Rsq = 0.92). Again, no association was found between rs720251 and OS among patients without anthracycline treatment (HR = 1.06, \(P = 0.41\)).
Meta-analysis of rs720251 in the UACA locus across all four cohorts
In meta-analysis involving 3359 patients (652 events) treated with Par-4 dependent chemotherapies across the above four prospective cohorts using Han and Eskin’s Random Effects model28, the T allele of rs720251 was significantly associated with OS (combined HR = 1.84, \(P = 1.28 \times 10^{ - 11}\), Fig. 7). Posterior probability calculated from the meta-analysis suggested a genetic effect of this variant in the European and East Asian populations of the Pathways Study, as well as in the SBCSS and SBCS patients (m-value > 0.9).
Pathways-EUR, Pathways-EAS, Pathways-HIS, and Pathways-AFR represents the European, East Asian, Hispanic, and African population of the Pathways Study respectively. GERA corresponds to the incident cases of European descent from the GERA cohort. SBCSS + SBCS represents the SBCSS and SBCS patients. Meta P-value was calculated using METASOFT based on Han and Eskin’s Random Effects model. The forest plot (left) displays P-value, study name, log hazard ratio and its standard error from the cox regression model of the corresponding study. The PM-Plot (right) displays the m-value (the posterior probability that the effect exists) of each study along with its P-value. The size of the symbol represents the study sample size.
Higher UACA gene expression associated with worse breast cancer survival
To confirm that the UACA gene is indeed responsible for the GWAS association and to infer the underlying biological mechanism, we interrogated cis-expression quantitative trait locus (eQTLs) from a meta-analysis of 31,684 blood samples collated by the eQTLGen consortium29. Among genes that are 1 Mb nearby, the lead variant in the trans-ethnic meta-GWAS, rs62019060, was identified as an eQTL for only UACA, with the risk allele G associated with higher expression (Z-score = 20.57, Bonferroni corrected \(P = 6.10 \times 10^{ - 86}\)). Furthermore, of the 49 tissues tested for the GTEx eQTL analysis30, rs62019060 is a significant eQTL for UACA in 17 tissues (multi-tissue meta-analysis \(P = 3.70 \times 10^{ - 124}\), Supplementary Fig. 11). The risk G allele was consistently associated with higher UACA expression across tissues, including breast (Supplementary Fig. 12). In a transcriptome-wide association study (TWAS) of OS among patients of European population treated with Par-4 dependent chemotherapies in the Pathways Study, UACA expression was significantly associated with higher risk of death (HR = 11.38, \(P = 4.68 \times 10^{ - 7}\)). It was also the only gene reaching transcriptome-wide significance (Fig. 8).
Discussion
Breast cancer survival has proven to be a challenging phenotype for GWAS to identify bona fide germline genetic variants that are either prognostic regardless of treatment, or predictive for the efficacy of specific treatment. The goal of the former is rooted in tumor biology, whereas the latter falls into the area of pharmacogenomics and potentially useful for optimizing treatment with specific regimens. To date, most prior GWAS on breast cancer survival were conducted for prognostication, either with all patients combined or stratified by tumor ER status. This is likely due to the fact that many prior analyses were conducted by pooling data from patient populations originally genotyped for analysis of cancer risk, where detailed treatment data were often lacking. Nonetheless, findings from these prior GWAS suffer from low reproducibility. The 11 prior GWAS of breast cancer survival have reported 24 loci with four loci at or close to genome-wide significance (P < 5 × 10−8). However, none of them could be replicated by others. In fact, in three separate analysis of breast cancer-specific death conducted by the same group based on different yet overlapping patient populations, no replicable variants emerged5,11,12. It is thus not surprising that our evaluation of the prior GWAS variants in the Pathways Study could not replicate any with high level of confidence.
Failure in replicating prior GWAS findings for breast cancer prognosis along with no genome-wide significant finding in our initial GWAS within all, ER+, or ER− patients highlights the difficulty in identifying prognostic genetic markers applicable to all patients or to patients of certain subtypes, considering breast cancer is intrinsically heterogeneous. Aside from the heterogeneities in patient populations, tumor subtypes, duration of follow-up, and survival endpoints tested, limited sample size and power is often cited as another major challenge. However, the largest GWAS to date for breast cancer survival included >96,000 patients and close to 8000 events, yet no variants reached genome-wide significance11, whereas prior GWAS for breast cancer risk with a sample size of this magnitude produced many highly replicable hits with modest effect sizes. An important factor to be contemplated that might help explain the conundrum is cancer treatment, which has a major impact on patient outcomes after cancer diagnosis. There is great diversity in treatment modalities, from different surgical procedures to radiation therapy, systemic chemotherapy, or endocrine therapy in the neoadjuvant or adjuvant setting. Each patient’s course of treatment is likely unique and subject to the influence of physician discretion, personal preference, and psychosocial factors. The sheer magnitude of treatment heterogeneities might overwhelm the modest genetic effect, leading to spurious findings difficult to replicate when data structure changes in a separate analysis. It is thus critical to carefully consider patient treatment while conducting GWAS for breast cancer survival.
Based on almost 4000 breast cancer patients in the Pathways Study with detailed treatment data, our analysis began with a GWAS of OS in all patients while controlling for cancer treatment. The top variant located in a locus containing UACA was close to genome-wide significance cutoff. Strong biological evidence supports the role of UACA in helping cancer cells escape Par-4 induced apoptosis by preventing trafficking of Par-4 receptor, GRP78, to the cancer cell surface21, whereas Par-4-dependent cancer cell multinucleation and cell death was shown indispensable for the anti-cancer activities of anti-HER2 agents and anthracyclines22. Subsequent analyses stratified by Par-4-dependent therapies vs. other therapies confirmed the association to be specific to patients who received anti-HER2 and/or anthracycline-containing therapies. Meta-analysis across four independent cohorts confirmed the association with a strong per allele HR of 1.84 for the top variant rs720251. Our eQTL analysis and TWAS further supported the robustness of this finding. We identified a pharmacogenomic marker for anthracyclines and HER2 targeted therapy, two commonly used anti-breast cancer agents. Considering the risk allele being rather common, the observed large effect size, and the consistent effect across diverse populations (MAF > 9% and HR > 1.5), particularly the effect on the East Asian population was independently validated, a genetic test on the UACA locus may bring broad clinical relevance in guiding physicians’ decisions on the use of these agents. In addition, our finding suggests that patients carrying the germline risk alleles likely have higher UACA expression in both normal cells and breast tumor cells. Higher UACA expression in tumor cells results in a reduced sensitivity to therapeutic agents that rely on Par-4 induced apoptosis and hence worse prognosis. In normal cells, it has been shown that activation of p53 in normal cells, e.g., following doxorubicin treatment, promotes normal cells secreting Par-4 to induce tumor cell apoptosis31. However, being a principal binding partner of Par-4, UACA can sequester Par-4 inside normal cells and suppress Par-4 secretion31. Therefore, higher UACA expression in normal cells results in lower extracellular Par-4 level, which again leads to reduced tumor apoptosis and worse prognosis. Such discoveries highlight the potential of targeting the Par-4 pathway as a novel therapeutic approach for improving outcomes of breast cancer24,32,33.
In conclusion, in a large GWAS of breast cancer survival, we identified a genome-wide significant genetic predictor for the efficacy of two widely used breast cancer agents, namely anti-HER2 therapy and anthracyclines. Upon future studies to further validate our findings and to evaluate the clinical utility, we anticipate that the knowledge of the UACA genotypes or expression level would help physicians identify patients who are most likely to benefit from anthracyclines and HER2 targeted therapy, and provide complementary or alternative treatment to those who are not.
Methods
Study population
The Pathways Study is a prospective cohort study of a diverse population of recently diagnosed breast cancer survivors in Kaiser Permanente Northern California (KPNC)34. Recruitment into the cohort was from January 2006 to May 2013. Eligibility criteria were: age ≥ 21 years; current KPNC member; recently diagnosed with invasive breast cancer; no prior history of other invasive cancer other than non-melanoma skin cancer; primary language of English, Spanish, Cantonese, or Mandarin; and lived within a 65-mile radius of a field interviewer. Blood and saliva specimens were collected around the time of enrollment, which was on average less than two months after pathology-confirmed diagnosis. Recurrences were identified through self-report, the KPNC Cancer Registry, or through an algorithm designed to identify possible recurrences through clinical events, such as ICD9 or 10 code, suggesting a recurrence or re-initiation of chemotherapy. All recurrences were confirmed through medical record review.
The Kaiser Permanente Genetic Epidemiology Research on Aging (GERA) Cohort consists of a diverse cohort of more than 100,000 adults who are members of KPNC, and participants in its Research Program on Genes, Environment and Health (RPGEH), which started in 2005. The GERA cohort only included RPGEH participants who had answered a detailed survey in 2007, provided saliva samples for extraction of DNA since July 2008, and given broad consent for the use of their data in studies of health and disease. Our study selected breast cancer patients from the GERA cohort who were successfully genotyped and were not participants of the Pathways Study. The GERA cohort included incident breast cancer cases, who were diagnosed after their saliva samples were collected, as well as prevalent breast cancer cases, who were diagnosed before saliva sample collection.
For both the Pathways Study and GERA cohort, detailed information on cancer diagnosis and treatment were obtained from KPNC electronic health records, including the KPNC Cancer Registry. Cohort members were followed for mortality through the KPNC mortality linkage file, which incorporates information from various sources, including medical system records, Social Security Administration databases, and the National Death Index. In the Pathways Study, deaths reported to us by family members were also included.
The Data Bank and Biorepository (DBBR) cohort included 451 female breast cancer patients selected from Roswell Park Comprehensive Cancer Center Data Bank and Biorepository35. All patients were self-reported white and received either Doxorubicin or HER2 targeted therapy.
The Shanghai Breast Cancer Survival Study (SBCSS) was conducted in urban Shanghai and recruited 5042 patients with breast cancer between March 2002 and April 2006 (participation rate: 80.1%); 98% of patients provided an exfoliated buccal cell sample36,37. The Shanghai Breast Cancer Study (SBCS) is a population-based case–control study that recruited incident patients with breast cancer from urban Shanghai between August 1996 and March 1998 and between April 2002 and February 200536. A total of 3448 patients were recruited (participation rate: 86.7%); 90.6% of participants provided a blood or exfoliated buccal cell sample. Medical charts were reviewed to verify cancer diagnosis and obtain clinical information such as cancer stage, tumor ER and progesterone receptor (PR) status, and primary treatments (surgery/mastectomy, radiation therapy, chemotherapy). The SBCSS also collected detailed information on treatment regimens. Patients with cancer have been followed for survival status and breast cancer recurrence through regular record linkages with the Shanghai Vital Statistics Registry. In the SBCSS, in-person surveys at 18-month, 3, 5, and 10 years after diagnosis were also carried out to update exposure information and collect information on cancer recurrence. Because of a time overlap during recruitment, 1469 women participated in both the SBCSS and SBCS. For these overlapping patients, information from the SBCSS patients were used in the current study. We excluded patients with stage 0 disease, having no genetic information or no information on survival status from the study. A total of 5495 patients were included this study.
All study participants provided written informed consent before participating in the study and the Institutional Review Boards of all institutes involved approved the study protocols. Our study is compliant with the “Guidance of the Ministry of Science and Technology (MOST) for the Review and Approval of Human Genetic Resources”.
Genotyping, quality control, and imputation
Genotyping of the Pathways Study was performed by Johns Hopkins Center for Inherited Disease Research (CIDR) using the Illumina Multi-Ethnic Global Array with inclusion of custom content from the BioVU breast cancer SNP subset. A total of 4480 samples from 4376 patients were successfully genotyped and passed CIDR quality control (QC), where samples were evaluated by sample missing rate, gender mismatch, sex chromosome abnormalities, sample relatedness, and population structure. CIDR’s QC also resulted in variant removal if the variant missing rate was >2%, if there was more than one Mendelian error in the HapMap trios included in genotyping, if variants violated Hardy–Weinberg equilibrium (P < 1 × 10−4) or were discordant between duplicate samples (>1 discordant call) in 97 study duplicates. Positional duplicated variants were also removed. CIDR then took the remaining samples and variants after QC for imputation using the University of Michigan Imputation Server38 and the Haplotype Reference Consortium (HRC) reference panel39. Eagle2 was used for pre-phasing while miminac3 and minimac4 was used for imputation on autosomes and X chromosome respectively. Variant with imputation quality Rsq < 0.3 were excluded from analysis.
As the Pathways Study is a multi-ethnic cohort, we further separated the 4480 samples that passed CIDR QC into four populations (European, Asian, Hispanic, and African) based on self-reported race and ethnicity, and performed sample-level QC within each population. Samples were removed if the missing rate was >5%, the typed and reported sex did not match, there were abnormal inbreeding coefficients, or cryptic relatedness. Duplicate samples with higher sample missing rate were filtered out and population outliers were removed by using EIGENSTRAT40. EIGENSTRAT separated the Asian population into two clusters: East Asian and South Asian. The 72 samples of South Asian descent were excluded from further analysis due to limited sample size. At the end 2801, 450, 392, and 330 samples within European, East Asian, Hispanic, and African population were kept for further analysis.
The GERA cohort was genotyped using four Affymetrix Axiom arrays custom-designed for individuals of Non-Hispanic White (EUR), East Asian (EAS), African-American (AFR), and Latino (LAT) race/ethnicity41,42. Samples were assigned to different arrays based on their self-reported race/ethnicity. As only limited number of minority breast cancer patients were included in the GERA cohort, our analyses focused on European-descent patients genotyped using EUR array. As some EUR arrays were run at a time when Affymetrix had completely upgraded its reagent protocol from Kit A to Kit O, we included reagent kit as a covariate in our Cox regression models of the GERA cohort. We performed both sample-level and variant-level QC on these samples. In sample-level QC, samples were removed if the missing rate was >5%, the typed and reported sex did not match, there were abnormal inbreeding coefficients, or cryptic relatedness. Population outliers were removed using EIGENSTRAT. In variant-level QC, variants were removed if the missing rate was >2%, if there was more than one Mendelian error in HapMap trios included in genotyping, if variants violated Hardy–Weinberg equilibrium (P ≤ 1 × 10−6). Monomorphic variants were also excluded before imputation. Similar to the Pathways Study, we performed imputation using the University of Michigan Imputation Server and the HRC reference panel. Eagle2 and miminac3 were used for pre-phasing and imputation respectively on autosomes, while ShapeIT and minimac4 were used for pre-phasing and imputation respectively on X chromosome. Variant with imputation quality Rsq < 0.3 were excluded from analysis. Ultimately, 2909 samples within the European ancestry population were kept for analysis, including 880 incident cases and 1983 prevalent cases.
For replication in the DBBR cohort, we selected five variants (rs11855431, rs720251, rs62019060, rs6494889, and rs28607477) for genotyping using Applied Biosystems TaqMan SNP Genotyping assays. rs6494889 and rs28607477 were highly correlated with the three lead variants (rs11855431, rs62019060, and rs720251) in the Pathways EUR cohort (R2 of imputation dosage >0.94). rs62019060 failed genotyping and only the four remaining variants were used for association analysis.
The SBCSS and SBCS samples were genotyped primarily using one of the Affymetrix SNP 6.0 array, Illumina OncoArray, Illumina MEGA, or Illumina iCOGS platforms. Stringent criteria were used for QC for each dataset43. QC procedure include: samples were excluded if they (i) had genotyping call rate <95%; (ii) were male based on genotype data; (ii) had a close relationship with a Pi-HAT estimate >0.25; (iii) were heterozygosity outliers; (iv) were ancestry outliers. SNPs were excluded if they had (i) a call rate <95%; (ii) no clear genotyping clusters; (iii) a minor allele frequency <0.001; (iv) a Hardy–Weinberg equilibrium test of P < 1 × 10−6; (v) genotyping concordance <95% among the duplicated QC samples. The cleaned data were imputed using 1000 Genomes as reference. Details on the methodology of the parent studies have been described previously36,37. Only variants with MAF > 5% and Rsq \(\ge\)0.3 were included in this study. A total of 1297 variants were analyzed for the UACA locus by pooling patients from SBCSS and SBCS (15:70446893-71555932 in genome build 37).
Statistical analysis
In the Pathways Study, we performed Cox proportional hazards regression for overall survival (OS) on each variant’s imputation dosage while controlling for age at diagnosis, body mass index, tumor grade, stage of disease, ER, progesterone receptor, and HER2 status, hormonal therapy, chemotherapy, radiation therapy, surgery type, and all population stratification principal components deemed significant by the Tracy-Widom statistic derived from EIGENSTRAT (Supplementary Table 1). The same covariates were included in the cox regression model when testing the association between recurrence/progression-free survival (PFS) and rs720251. Time to event was calculated from the date of diagnosis. The last date of follow-up for OS and breast cancer recurrence in the Pathways Study was 12/31/2017. The “survival” and “GWASTools” packages in R were used to perform single variant association analysis within each population for variants with MAF > 5% in the corresponding population. MR-MEGA was adopted for trans-ethnic meta-analysis across populations. To correct for genomic inflation, the standard errors of beta coefficients from the Cox models were multiplied by the square root of the genomic-inflation factor (\(\lambda\)) before running MR-MEGA when \(\lambda \ge 1.06\). GWAS were also run in two separate patient groups: patients receiving Par-4 dependent chemotherapies versus the remaining patients. Specifically, the former group included patients who received doxorubicin as well as patients who were HER2+ and received HER2 targeted agents lapatinib, pertuzumab, or trastuzumab. In association analyses for death after recurrence and death without recurrence (all cause of death), as well as for analyses of breast-cancer specific death (BCSD) or death due to cardiovascular disease versus other cause of death, we used cumulative incidence functions to handle the two competing risks.
In the analysis of GERA cohort, reagent kit was added to the covariate list described above to control for possible effects of using two different Affymetrix reagent kits during genotyping. The last date of follow-up for OS in the GERA cohort was 9/30/2018.
Cox proportional hazards regression of OS was performed on variants of the UACA locus in the DBBR cohort and the SBCSS + SBCS cohort separately using the same model and covariates as described above in the analysis of the Pathways Study, including the principal components to control for population stratification. Principal components were not available for the association analysis in the DBBR cohort as only four variants were genotyped. The end of follow-up for OS in the DBBR cohort was 12/31/2019.
METASOFT was used in meta-analysis of the effect on OS of rs720251 across all four cohorts with Han and Eskin’s Random Effects model, which was optimized to detect associations under heterogeneity28. Beta coefficients from the Cox models and genomic inflation-corrected standard errors (described above in running MR-MEGA) were used as inputs for METASOFT. This model also calculates the posterior probability that an effect exists in each cohort as an m-value44. An m-value > 0.9 suggests existence of a genetic effect in that cohort. METASOFT was also used for meta-GWAS within ER− patients across Pathways European and African populations. ForestPMPlot was used to create the forest plot and the PM plot.
Risk allele identification for GWAS study PMID:31904872
We download the accompanying genotyping and clinical data from https://figshare.com/articles/dataset/IJC_Clinical_features_and_SNPs_genotype_xlsx/11474403/1, and test association between the four GWAS variants and disease-free survival in ER+ patients while controlling for available covariates (tumor stage, hormone treatment, and chemotherapy status).
Linkage disequilibrium calculation
Population-specific measures of linkage disequilibrium between SNP pairs were calculated using LDpair45 based on reference haplotypes from Phase 3 (Version 5) of the 1000 Genomes Project.
Risk allele frequency comparison
Using non-parametric bootstrapping, we compared the risk allele (T) frequency of rs720251 in the GERA prevalent breast cancer patients taking Par-4 dependent chemotherapies (\(F_R\)) with its population frequency estimated from the GERA incident breast cancer patients (\(F_P\)). The null hypothesis is\(H_0:F_R \ge F_P\), and the alternative hypothesis is \(H_a:F_R < F_P\). We re-sampled the imputation dosage of rs720251 with replacement 10,000 times in the prevalent patients taking the particular treatment as well as in all incident patients and then calculated T allele frequency each time (\(F_{R,i}\) and \(F_{P,i}\), where \(i = 1, \ldots ,10000\)). The bootstrap P-value was calculated as \(P = \mathop {\sum }\nolimits_{i = 1}^{10000} I(F_{R,i} \ge F_{P,i})/10000\). The same procedure was used to compare T allele frequency of rs720251 between the GERA prevalent patients without Par-4 dependent chemotherapies and all GERA incident patients. The analysis was repeated to use all European-descent patients from the Pathways Study to estimate T allele frequency at population level.
eQTL analysis
cis-eQTL summary statistics from the eQTLGen consortium were download from the eQTLGen website (https://www.eqtlgen.org/cis-eqtls.html). cis-eQTLs with FDR < 0.05 were considered significant. The cis-eQTL analysis across tissues in Genotype-Tissue Expression (GTEx) Project were obtained from the GTEx Portal on 4/21/2020.
Transcriptome-wide association study
Imputation dosages of variants with MAF > 5% and Rsq \(\ge 0.8\) in the Pathways European population were used as input to PrediXcan46 with whole-blood prediction model trained in 922 whole-blood samples from Depression Genes and Networks (DGN)47. Predicted gene expression was tested for association with OS using Cox proportional hazards regression with same covariates as in the model of GWAS (see “Statistical analysis” section above). A total 11,520 genes were tested, yielding a transcriptome-wide significance cutoff \(4.34 \times 10^{ - 6}\).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Individual-level genotype and imputation data of the Pathways Study is available through dbGaP (accession number: phs001534.v1.p1).
Code availability
We used R (version 3.4.1) extensively for data analysis and creating plots. Additional software used in the study included PLINK 1.9 https://www.cog-genomics.org/plink/, EIGENSOFT v6.1.4 https://www.hsph.harvard.edu/alkes-price/software/, MR-MEGA v0.1.5 https://genomics.ut.ee/en/mr-mega, METASOFT v2.0.1 http://genetics.cs.ucla.edu/meta_jemdoc/index.html, ForestPMPlot v1.0.2 http://genetics.cs.ucla.edu/meta_jemdoc/index.html, PrediXcan https://github.com/hakyimlab/PrediXcan.
References
Zhang, H. et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 52, 572–581 (2020).
Ferreira, M. A. et al. Genome-wide association and transcriptome studies identify target genes and risk loci for breast cancer. Nat. Commun. 10, 1741 (2019).
Song, N. et al. Prediction of breast cancer survival using clinical and genetic markers by tumor subtypes. PLoS ONE 10, e0122413 (2015).
Khan, S. et al. Polymorphism at 19q13.41 predicts breast cancer survival specifically after endocrine therapy. Clin. Cancer Res. 21, 4086–4096 (2015).
Guo, Q. et al. Identification of novel genetic markers of breast cancer survival. J. Natl Cancer Inst. 107, djv081 (2015).
Rafiq, S. et al. Identification of inherited genetic variations influencing prognosis in early-onset breast cancer. Cancer Res. 73, 1883–1891 (2013).
Shu, X. O. et al. Novel genetic markers of breast cancer survival identified by a genome-wide association study. Cancer Res. 72, 1182–1189 (2012).
Azzato, E. M. et al. A genome-wide association study of prognosis in breast cancer. Cancer Epidemiol. Biomark. Prev. 19, 1140–1143 (2010).
Rafiq, S. et al. A genome wide meta-analysis study for identification of common variation associated with breast cancer prognosis. PLoS ONE 9, e101488 (2014).
Chou, W. C. et al. A functional variant near XCL1 gene improves breast cancer survival via promoting cancer immunity. Int. J. Cancer 146, 2182–2193 (2020).
Escala-Garcia, M. et al. Genome-wide association study of germline variants and breast cancer-specific mortality. Br. J. Cancer 120, 647–657 (2019).
Escala-Garcia, M. et al. A network analysis to identify mediators of germline-driven differences in breast cancer prognosis. Nat. Commun. 11, 312 (2020).
Kadalayil, L. et al. Germline variation in ADAMTSL1 is associated with prognosis following breast cancer treatment in young women. Nat. Commun. 8, 1632 (2017).
Ribelles, N., Santonja, A., Pajares, B., Llácer, C. & Alba, E. The seed and soil hypothesis revisited: current state of knowledge of inherited genes on prognosis in breast cancer. Cancer Treat. Rev. 40, 293–299 (2014).
Hunter, K. Host genetics influence tumour metastasis. Nat. Rev. Cancer 6, 141–146 (2006).
Lifsted, T. et al. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int. J. Cancer 77, 640–644 (1998).
Hartman, M. et al. Is breast cancer prognosis inherited? Breast Cancer Res. 9, R39 (2007).
Verkooijen, H. M. et al. Breast cancer prognosis is inherited independently of patient, tumor and treatment characteristics. Int. J. Cancer 130, 2103–2110 (2012).
Bayraktar, S. et al. The relationship between eight GWAS-identified single-nucleotide polymorphisms and primary breast cancer outcomes. Oncologist 18, 493–500 (2013).
Fasching, P. A. et al. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum. Mol. Genet. 21, 3926–3939 (2012).
Burikhanov, R. et al. Novel mechanism of apoptosis resistance in cancer mediated by extracellular PAR-4. Cancer Res. 73, 1011–1019 (2013).
Alvarez James, V. et al. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 24, 30–44 (2013).
Mabe, N. W. et al. Epigenetic silencing of tumor suppressor Par-4 promotes chemoresistance in recurrent breast cancer. J. Clin. Investig. 128, 4413–4428 (2018).
Guo, H., Treude, F., Krämer, O. H., Lüscher, B. & Hartkamp, J. PAR-4 overcomes chemo-resistance in breast cancer cells by antagonizing cIAP1. Sci. Rep. 9, 8755–8755 (2019).
Copeland-Halperin, R. S., Liu, J. E. & Yu, A. F. Cardiotoxicity of HER2-targeted therapies. Curr. Opin. Cardiol. 34, 451–458 (2019).
Zagar, T. M., Cardinale, D. M. & Marks, L. B. Breast cancer therapy-associated cardiovascular disease. Nat. Rev. Clin. Oncol. 13, 172–184 (2016).
Christidi, E. & Brunham, L. R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 12, 339 (2021).
Han, B. & Eskin, E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am. J. Hum. Genet. 88, 586–598 (2011).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
Aguet, F. et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Burikhanov, R. et al. Paracrine apoptotic effect of p53 mediated by tumor suppressor Par-4. Cell Rep. 6, 271–277 (2014).
Burikhanov, R. et al. Chloroquine-inducible Par-4 secretion is essential for tumor cell apoptosis and inhibition of metastasis. Cell Rep. 18, 508–519 (2017).
Burikhanov, R. et al. Arylquins target vimentin to trigger Par-4 secretion for tumor cell apoptosis. Nat. Chem. Biol. 10, 924–926 (2014).
Kwan, M. L. et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control 19, 1065–1076 (2008).
Ambrosone, C. B., Nesline, M. K. & Davis, W. Establishing a Cancer Center Data Bank and biorepository for multidisciplinary research. Cancer Epidemiol. Biomark. Prev. 15, 1575–1577 (2006).
Zheng, W. et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat. Genet. 41, 324–328 (2009).
Shu, X. O. et al. Soy food intake and breast cancer survival. JAMA 302, 2437–2443 (2009).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284 (2016).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Hoffmann, T. J. et al. Next generation genome-wide association tool: design and coverage of a high-throughput European-optimized SNP array. Genomics 98, 79–89 (2011).
Hoffmann, T. J. et al. Design and coverage of high throughput genotyping arrays optimized for individuals of East Asian, African American, and Latino race/ethnicity using imputation and a novel hybrid SNP selection algorithm. Genomics 98, 422–430 (2011).
Shu, X. et al. Identification of novel breast cancer susceptibility loci in meta-analyses conducted among Asian and European descendants. Nat. Commun. 11, 1217 (2020).
Han, B. & Eskin, E. Interpreting meta-analyses of Genome-Wide Association Studies. PLoS Genet. 8, e1002555 (2012).
Machiela, M. J. & Chanock, S. J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557 (2015).
Gamazon, E. R. et al. A gene-based association method for mapping traits using reference transcriptome data. Nat. Genet. 47, 1091 (2015).
Battle, A. et al. Characterizing the genetic basis of transcriptome diversity through RNA-sequencing of 922 individuals. Genome Res 24, 14–24 (2013).
Acknowledgements
This study was supported by the National Cancer Institute (NCI) at the National Institutes of Health (NIH) (U01 CA195565, PIs: Kushi LH, Ambrosone CB). Genotyping of the Pathways Study was provided by CIDR. CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268201700006I. This work was also supported by NCI grant P30CA016056 involving the use of Bioinformatics Shared Resource, Biostatistics and Statistical Genomics Shared Resource (BSGSR), Data Bank and BioRepository (DBBR), Genomic Shared Resource (GSR), and Biomedical Research Informatics Shared Resources (BRISR) at Roswell Park Comprehensive Cancer Center. Zhu Q was supported, in part, by the NIH grant U24 CA232979. Gandhi S was supported, in part, by the National Center for Advancing Translational Sciences of the NIH under award Number KL2 TR001413 and UL1 TR001412. The SBCSS (DAMD 17-02-1-0607 and R01 CA118229; PI, Shu XO), and SBCS (R01 CA064277, PIs. Zheng W, Shu XO) were supported by grants from Department of Defense Breast Cancer Research Program and NCI. The genotyping of the SBCSS and SBCS samples was supported by NCI grant (R01 CA124558, PI, Zheng W; RO1 CA148677, PIs. Zheng W, Long J) and Ingram Cancer Research Professorships (Zheng W and Shu XO). Sample preparation of the SBCSS and SBCS was conducted at the Survey and Biospecimen Shared Resources, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485). We acknowledge Dr. Andrew Morris for suggestions on applying MR-MEGA.
Author information
Authors and Affiliations
Contributions
Q.Z., S.Y., and L.H.K. conceived the study and wrote the manuscript. Q.Z., E.S., J.L., E.V., K.H.R., and Y.L. analyzed the data. J.M.R, E.V., C.A.L., I.J.E., M.L.K., C.A., S.Y., and L.H.K. coordinated samples and clinical data of the Pathways Study. W.D., C.A., and S.Y. coordinated samples and clinical data of the DBBR cohort. J.L., P.B., W.Z., and X.S. coordinated data from the SBCSS + SBCS cohort. D.R. coordinated genetic and clinical data of the GERA cohort. Q.Z., J.L., S.G., M.L.K, W.Z., X.S., C.A., S.Y., and L.H.K. interpreted data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, Q., Schultz, E., Long, J. et al. UACA locus is associated with breast cancer chemoresistance and survival. npj Breast Cancer 8, 39 (2022). https://doi.org/10.1038/s41523-022-00401-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-022-00401-5
This article is cited by
-
Development and testing of a polygenic risk score for breast cancer aggressiveness
npj Precision Oncology (2023)