Abstract
Gametogenesis, which is essential to the sexual reproductive system, has drastically changed during plant evolution. Bryophytes, lycophytes and ferns develop reproductive organs called gametangia—antheridia and archegonia for sperm and egg production, respectively. However, the molecular mechanism of early gametangium development remains unclear. Here we identified a ‘non-canonical’ type of BZR/BES transcription factor, MpBZR3, as a regulator of gametangium development in a model bryophyte, Marchantia polymorpha. Interestingly, overexpression of MpBZR3 induced ectopic gametangia. Genetic analysis revealed that MpBZR3 promotes the early phase of antheridium development in male plants. By contrast, MpBZR3 is required for the late phase of archegonium development in female plants. We demonstrate that MpBZR3 is necessary for the successful development of both antheridia and archegonia but functions in a different manner between the two sexes. Together, the functional specialization of this ‘non-canonical’ type of BZR/BES member may have contributed to the evolution of reproductive systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the manuscript or the Supplementary Information. All sequence reads were deposited in the Sequence Read Archive (SRA) under project accession number PRJNA993618 and the DNA Data Bank of Japan (DDBJ) under accession numbers DRA016656 and DRA016671. Sequence and motif data in the manuscript or Supplementary Materials can be found in the databases MarpolBase MpTak v.6.1 (https://marchantia.info) and Pfam in InterPro (https://www.ebi.ac.uk/interpro/). Source data are provided with this paper.
Change history
22 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41477-024-01702-2
References
Berger, F. & Twell, D. Germline specification and function in plants. Annu. Rev. Plant Biol. 62, 461–484 (2011).
Ishizaki, K. Evolution of land plants: insights from molecular studies on basal lineages. Biosci. Biotechnol. Biochem. 81, 73–80 (2017).
Hisanaga, T. et al. Building new insights in plant gametogenesis from an evolutionary perspective. Nat. Plants 5, 663–669 (2019).
Kohchi, T., Yamato, K. T., Ishizaki, K., Yamaoka, S. & Nishihama, R. Development and molecular genetics of Marchantia polymorpha. Annu. Rev. Plant Biol. 72, 677–702 (2021).
Shimamura, M. Marchantia polymorpha: taxonomy, phylogeny and morphology of a model system. Plant Cell Physiol. 57, 230–256 (2016).
Bowman, J. L. et al. The renaissance and enlightenment of Marchantia as a model system. Plant Cell 34, 3512–3542 (2022).
Yamaoka, S., Inoue, K. & Araki, T. Regulation of gametangia and gametangiophore initiation in the liverwort Marchantia polymorpha. Plant Reprod. 34, 297–306 (2021).
Yamaoka, S. et al. Generative cell specification requires transcription factors evolutionarily conserved in land plants. Curr. Biol. 28, 479–486.e5 (2018).
Cui, Y. et al. Three-dimensional morphological analysis revealed the cell patterning bases for the sexual dimorphism development in the liverwort Marchantia polymorpha. Plant Cell Physiol. 64, 866–879 (2023).
Higo, A. et al. Transcription factor DUO1 generated by neo-functionalization is associated with evolution of sperm differentiation in plants. Nat. Commun. 9, 5283 (2018).
Higo, A. et al. Transcriptional framework of male gametogenesis in the liverwort Marchantia polymorpha L. Plant Cell Physiol. 57, 325–338 (2016).
Koi, S. et al. An evolutionarily conserved plant RKD factor controls germ cell differentiation. Curr. Biol. 26, 1775–1781 (2016).
Rövekamp, M., Bowman, J. L. & Grossniklaus, U. Marchantia MpRKD regulates the gametophyte–sporophyte transition by keeping egg cells quiescent in the absence of fertilization. Curr. Biol. 26, 1782–1789 (2016).
Hisanaga, T. et al. A cis-acting bidirectional transcription switch controls sexual dimorphism in the liverwort. EMBO J. 38, e100240 (2019).
Iwasaki, M. et al. Identification of the sex-determining factor in the liverwort Marchantia polymorpha reveals unique evolution of sex chromosomes in a haploid system. Curr. Biol. 31, 5522–5532.e7 (2021).
Flores-Sandoval, E., Romani, F. & Bowman, J. L. Co-expression and transcriptome analysis of Marchantia polymorpha transcription factors supports class C ARFs as independent actors of an ancient auxin regulatory module. Front. Plant Sci. 9, 1345 (2018).
Nolan, T. M., Vukašinović, N., Liu, D., Russinova, E. & Yin, Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32, 295–318 (2020).
Wang, Z. Y. et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2, 505–513 (2002).
Yin, Y. et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109, 181–191 (2002).
Bowman, J. L. et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304.e15 (2017).
Mecchia, M. A. et al. The BES1/BZR1-family transcription factor MpBES1 regulates cell division and differentiation in Marchantia polymorpha. Curr. Biol. 31, 4860–4869.e8 (2021).
Kawamura, S. et al. MarpolBase Expression: a web-based, comprehensive platform for visualization and analysis of transcriptomes in the liverwort Marchantia polymorpha. Plant Cell Physiol. 63, 1745–1755 (2022).
Tian, Y. et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 9, 1063 (2018).
Dierschke, T. et al. Gamete expression of TALE class HD genes activates the diploid sporophyte program in Marchantia polymorpha. Elife 10, e57088 (2021).
Hisanaga, T. et al. Deep evolutionary origin of gamete-directed zygote activation by KNOX/BELL transcription factors in green plants. Elife 10, e57090 (2021).
Sanchez-Vera, V. et al. The Physcomitrium patens egg cell expresses several distinct epigenetic components and utilizes homologues of BONOBO genes for cell specification. New Phytol. 233, 2614–2628 (2022).
Chen, W. et al. BES1 is activated by EMS1-TPD1-SERK1/2-mediated signaling to control tapetum development in Arabidopsis thaliana. Nat. Commun. 10, 4164 (2019).
Chen, L. G. et al. BZR1 family transcription factors function redundantly and indispensably in BR signaling but exhibit BRI1-independent function in regulating anther development in Arabidopsis. Mol. Plant 12, 1408–1415 (2019).
Cai, H. et al. Brassinosteroid signaling regulates female germline specification in Arabidopsis. Curr. Biol. 32, 1102–1114.e5 (2022).
Su, D. et al. Tomato transcriptional repressor SlBES1.8 influences shoot apical meristem development by inhibiting the DNA binding ability of SlWUS. Plant J. 110, 482–498 (2022).
Ishizaki, K., Chiyoda, S., Yamato, K. T. & Kohchi, T. Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 49, 1084–1091 (2008).
Furuya, T. et al. A glycogen synthase kinase 3-like kinase MpGSK regulates cell differentiation in Marchantia polymorpha. Plant Biotechnol. 39, 65–72 (2022).
Ishizaki, K. et al. Development of gateway binary vector series with four different selection markers for the liverwort Marchantia polymorpha. PLoS ONE 10, e0138876 (2015).
Ishida, S. et al. Diminished auxin signaling triggers cellular reprogramming by inducing a regeneration factor in the liverwort Marchantia polymorpha. Plant Cell Physiol. 63, 384–400 (2022).
Sugano, S. S. et al. Efficient CRISPR/Cas9-based genome editing and its application to conditional genetic analysis in Marchantia polymorpha. PLoS ONE 13, e0205117 (2018).
Ishizaki, K., Johzuka-Hisatomi, Y., Ishida, S., Iida, S. & Kohchi, T. Homologous recombination-mediated gene targeting in the liverwort Marchantia polymorpha L. Sci. Rep. 3, 1532 (2013).
Tsuboyama, S., Nonaka, S., Ezura, H. & Kodama, Y. Improved G-AgarTrap: a highly efficient transformation method for intact gemmalings of the liverwort Marchantia polymorpha. Sci. Rep. 8, 10800 (2018).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T. & Salzberg, S. L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11, 1650–1667 (2016).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Mistry, J. et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 49, D412–D419 (2021).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166 (2019).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Mihara, M., Itoh, T. & Izawa, T. SALAD database: a motif-based database of protein annotations for plant comparative genomics. Nucleic Acids Res. 38, D835–D842 (2010).
Kurihara, D., Mizuta, Y., Sato, Y. & Higashiyama, T. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168–4179 (2015).
Kurihara, D., Mizuta, Y., Nagahara, S. & Higashiyama, T. ClearSeeAlpha: advanced optical clearing for whole-plant imaging. Plant Cell Physiol. 62, 1302–1310 (2021).
Minamino, N., Norizuki, T., Mano, S., Ebine, K. & Ueda, T. Remodeling of organelles and microtubules during spermiogenesis in the liverwort Marchantia polymorpha. Development 149, dev200951 (2022).
Acknowledgements
We thank Y. Ozawa, Y. Fukaya, A. Suizu, A. Hata, R. Wakasugi, M. Tabara, A. Takeda, H. Kato, Y. Sakai, A. Mamiya and C. Goto for assistance and kind support. This work was funded by the Ministry of Education, Culture, Sports, Science and Technology, Japan (Scientific Research on Priority Areas and Scientific Research on Innovative Areas (grants 19H04860 and 20H05780 to S.Y., 20H04884 and 22H04733 to R.N., 19H05675 to T.K., 25113005 to T.A., and 17H06476, 20H05407 and 22H04720 to Y.K.), Japan Society for the Promotion of Science (grants 19K21189, 20K15813 and 23K05811 to T.F., 20K15824 and 22K15149 to N.M., 23H04751 to K.M., 22H00417 to T.K., 21K06236 to M.K., 19H03244 to T.A. and 17H05008, 20K15815 and 22H02647 to Y.K.), Sasakawa Scientific Research Grant from the Japan Science Society (grant 4363 to T.F.), Ohsumi Frontier Science Foundation (to S.Y.), Takeda Science Foundation (research grant to K.M.), JST PRESTO (grant JPMJPR20D9 to K.M.) and by JST FOREST Program (grant JPMJFR224Q to Y.K.)).
Author information
Authors and Affiliations
Contributions
T.F. and Y.K. conceived and designed the research, coordinated the project and wrote the manuscript. T.F. performed most experiments and data analyses. Y.T. constructed the knock-in lines. N.S., C.Y. and K.M. performed microscopic observations. N.M. performed immunostaining. M.N. and K. Inoue carried out the RNA-seq analysis of archegonia. S.S. contributed to the experiments and data analyses. T.F., S.Y., R.N., K. Ishizaki, T.U., H. Fukaki, T.K., H. Fukuda, M.K., T.A. and Y.K. supervised the project. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Bowen Zheng, Frédéric Berger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Expression profiles and overexpression phenotypes of MpBZRs.
a, Expression profiles of MpBZR3 (Mp2g23000) in the RNA-seq data sets were visualised. b, Chromatic expression images of MpBZR3 in the expression database for M. polymorpha, MBEX: Marpolbase Expression. c, Tissue-specific gene expression pattern of MpBZR1 and MpBZR2 in the expression database for M. polymorpha, MBEX: Marpolbase Expression. Dots indicate each replicate. d, Relative transcript levels of MpBZRs in the overexpression lines of MpBZRs. Relative expression levels were calculated relative to the expression of MpEF1α. Detail information of all WT and transgenic plants were listed in Supplementary Table 2. Total RNAs were extracted from thalli grown from gemmae of WT male (Tak-1) and WT male (proMpCYCD;1:MpCYCD;1Nter-tdTomato-NLS, CYCD reporter) for 11 days and explants of the overexpression lines of MpBZRs grown for 20 days. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 3; biological replicates). Significant differences were examined using two-sided Welch’s t test (*P < 0.05, **P < 0.01). e,f, Morphology of thalli grown from gemmae of WT male (Tak-1) and WT male (CYCD reporter) for 10 days. g, h, Morphology of the proMpEF1α:MpBZR3-Citrine-2. i, Morphology of the proMpEF1α:MpBZR2-Citrine-4. j, Morphology of the proMpEF1α:MpBZR1-Citrine-4. Scale bars: 1 mm in e,f,h–j, and 3 mm in h.
Extended Data Fig. 2 Classification of each subgroup of BZR/BES transcription factors.
a,BZR/BES transcription factors in various species were classified based on the comparison of their amino acid sequences. Details of all proteins are listed in Supplementary Data 2. b, Comparison analysis of sequence motif in BZR/BES transcription factors in Arabidopsis thaliana (At), Solanum lycopersicum (Sl), Oryza sativa (Os) Ceratopteris richardii (Cr), Selaginella moellendorffii (Sm), Physcomitrium patens (Pp), Marchanita polymorpha (Mp), Anthoceros agrestis (Aa), and Penium margaritaceum (Pm) was performed in SALADA DB.
Extended Data Fig. 3 Alignments for a domain and motif of BZR/BES transcription factors.
a, Multiple sequence alignment of the DNA-binding domain of BZR/BES transcription factors in Arabidopsis thaliana (At), Solanum lycopersicum (Sl), Oryza sativa (Os) Ceratopteris richardii (Cr), Selaginella moellendorffii (Sm), Physcomitrium patens (Pp), Marchanita polymorpha (Mp), Anthoceros agrestis (Aa), and Penium margaritaceum (Pm) was constructed using the MAFFT software. Motif 1 and motif 2 detected in analysis using SALADA DB are involved in the DNA-binding domain. Feature of secondary structures in AtBZR1 is also indicated. b, Multiple sequence alignment of the type-B specific N-terminal motif (motif 16) detected in analysis using SALADA DB is indicated. The motif 16 is conserved in the type-B subgroup of BZR/BES transcription factors in land plants.
Extended Data Fig. 4 Phenotypes of the β-oestradiol-inducible MpBZR3 overexpression lines.
a–c Transgenic plants, proMpE2F:XVE»MpBZR3-2 male, -3 female, -10 male, -12 male, -19/Mpbnbko male, -22/Mpbnbko male, -13 female, -14 female, -23/Mpbnbko female, and -25/Mpbnbko female were grown from gemmae for 4 days. And then these plants were treated with or without 10 µM β-oestradiol for 1 day. Relative expression levels were normalized by the expression of MpEF1α and calculated relative to comparison to proMpE2F:XVE»MpBZR3-2 male without β-oestradiol treatment for a or proMpE2F:XVE»MpBZR3-10 male without β-oestradiol treatment for b,c, respectively. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 3; biological replicates). Significant differences were examined using two-sided Welch’s t test (*P < 0.05, **P < 0.01). d, Morphology of the β-oestradiol-inducible MpBZR3 overexpression lines, proMpE2F:XVE»MpBZR3-2 female and -3 male. Transgenic plants grown from gemmae for 4 days were treated with or without 10 µM β-oestradiol for 0, 8, 12, and 16 days. e, Visualisation of induced gametangium-like structures in the proMpE2F:XVE»MpBZR3-2 female and -3 male treated with 10 µM β-oestradiol for 16 days. Gametangia and induced gametangium-like structures were treated with ClearSee solution and stained by SCRI Renaissance 2200 (Blue) and SYBR Green I (Green). f, Visualisation of developing sperm-like structures in the proMpE2F:XVE»MpBZR3-2 treated with 10 µM β-oestradiol for 14 days. Sperm-like structures were immunostained by α-AC-tubulin (Green) and stained by Hoechst 33342 (Blue). Left and right panels show a blight field image and a fluorescent image, respectively. Scale bars: 3 mm in d, 100 µm in e, 10 μm in f.
Extended Data Fig. 5 Genetic relationship between MpBZR3 and MpBNB.
a, Relative expression levels of MpBNB and MpBZR3 in WT and Mpbnbko. Plants were grown from gemmae under the white light condition for 10 days, then these were cultured under the condition for reproductive induction, white light supplemented with far-red light, for 14 and 21 days. Each sample contains all apical notches without gametangiophores or with gametangiophore up to stage 1 taken from a single plant. Notches with gametangiophore at stage 2 or later were removed. Relative expression levels were calculated relative to the expression of MpEF1α. n = 3 plants from independent gemma from a single genotype. b, The β-oestradiol-inducible MpBZR3 overexpression lines, proMpE2F:XVE»MpBZR3-12 male, -22/Mpbnbko male, -13 female and -25/Mpbnbko female, grown from gemmae for 4 days were treated with or without 10 µM β-oestradiol for 12 days. c,d, Visualisation of induced gametangium-like structures in proMpE2F:XVE»MpBZR3-10 male, -19/Mpbnbko male, -13 female, and -23/Mpbnbko female treated with 10 µM β-oestradiol for 16 days. Gametangia and induced gametangium-like structures were treated with ClearSee solution and stained by SCRI Renaissance 2200 (white). Dotted lines and blue areas in c indicate the outlines of ectopic antheridium-like structures and spermatogenous-like cells in ectopic antheridium-like structures, respectively. Red area in d show a large cavity in ectopic archegonium-like structures. Scale bars: 3 mm in b, 100 µm in c,d.
Extended Data Fig. 6 The construction and observation of MpBZR3-Citrine knock-in lines.
a, Structures of MpBZR3 loci in WT and MpBZR3-Citrine knock-in lines and a part of the knock-in vector sequences. Black arrowheads indicate primer positions for genotyping using in b. b, Genotyping of WT and MpBZR3-Citrine knock-in lines. Black and white arrowheads indicate the predicted sizes of the PCR products from WT and MpBZR3-Citrine knock-in lines, respectively. Asterisk indicates non-specific amplification. The V-chromosome-linked DNA marker rbm27 and the U-chromosome-linked DNA marker rhf73 were used for sexual genotyping. c, Expression of MpBZR3 or MpBZR3-Citrine transcripts in WT and MpBZR3-Citrine knock-in lines. Total RNAs were extracted from stage 3 of archegoniophores or antheridiophores. Expression levels of MpEF1α were also indicated as a control. d, Relative transcript levels of MpBZR3 and MpBNB in WT and MpBZR3-Citrine knock-in lines. Relative expression levels were calculated relative to the expression of MpEF1α. Total RNAs were extracted from stage 3 of archegoniophores or antheridiophores. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 3; biological replicates). P values by two-sided Welch’s t test are indicated. e, Citrine signals in stage 1 antheridiophore or archegoniophore of MpBZR3-Citrineki male and female, respectively. Citrine and Calcofluor White signals are shown in yellow and blue, respectively. f, g, Citrine signals in developing antheridia and archegonia of MpBZR3-Citrineki male and female, respectively. Citrine and Calcofluor White signals are shown in yellow and blue, respectively. Red and orange arrowheads in f indicate predicted antheridium initial cells and developing antheridia at the early phase, respectively. Red, orange, and white arrowheads in g indicate archegonium initial cells, developing archegonia at the early phase, and archegonia at the late phase, respectively. Parts of pictures are using in Figs. 4 and 5. Scale bars: 1 mm in e, 20 µm in f, g.
Extended Data Fig. 7 Construction and observation of the Mpbzr3 genome-edited lines.
a, Genomic structure of the encoding region for the MpBZR3 gene. The coding regions are indicated as black boxes. Orange box indicates the location of the coding region of DNA-binding domain. Black arrowhead indicates the location of target sites of gRNAs for genome editing. b, A part of genomic sequences of MpBZR3 in the genome-edited lines. The wild type (Tak-1) sequence is shown together with the PAM sequence (orange) and the target sequence (underline). The genome-edited line Mpbzr3-1ge have a 1 bp insertion, while Mpbzr3-2ge has a 4 bp deletion respectively (blue). c, Partial amino acid sequences of MpBZR3 in the WT (Tak-1) and two genome-edited lines. The genome-edited lines have frame shift mutation (blue). d, Morphology of thalli grown for 10 days from gemmae of the wild-type lines, WT female (Tak-2) and WT male (Tak-1) and Mpbzr3 genome-edited lines, Mpbzr3-1ge female and Mpbzr3-2ge male. e, Each stage of archegoniophore in WT female (Tak-2) and Mpbzr3 genome-edited lines, Mpbzr3-1ge female and that of antheridiophore in WT male (Tak-1) and Mpbzr3 genome-edited lines, Mpbzr3-2ge male. Stages are defined according to Higo et al. 201611. Scale bars: 1 mm in d, 3 mm in e.
Extended Data Fig. 8 Phenotypes of the complementation lines.
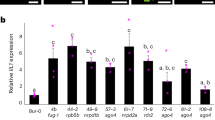
a, Visualisation of antheridia in the antheridial receptacles in Mpbzr3ge mutants. Antheridial receptacles (stage 4) of the WT male, Mpbzr3-2ge, and the complementation lines, proMpBZR3:MpBZR3resist-3xCitrine/Mpbzr3-2ge (Comp-2 and Comp-3) were treated with ClearSee solution and stained by SYBR Green I. Antheridia were highlighted as strong SYBR Green I signal (Green). b, Relative expression levels of antheridium-expressed genes in antheridial receptacles (stage 4) of the WT male, Mpbzr3-2ge, and the complementation lines, Comp-2 and Comp-3. Relative expression levels were normalised by the expression of MpEF1α and calculated relative to comparison to the expression in WT. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 3; biological replicates). Different lowercase letters indicate significant difference (P < 0.05; one-way ANOVA followed by two-sided Tukey-Kramer test). Scale bars: 1 mm in a.
Extended Data Fig. 9 Phenotypes of Mpbzr3ge mutants.
a, b, Cross-sections of stage 2 of antheridial receptacles of the WT male and Mpbzr3-2ge male. Magnified images in a are indicated in b. c, number of discharged sperms from the antheridiophere of WT male and Mpbzr3-2ge male. An antheridia receptacle was placed in 100 µl H2O. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 4; biological replicates). Significant differences were examined by two-sided Student’s t test (*P < 0.05). d, Cross-sections of stage 4 of archegonial receptacles of the WT female and Mpbzr3-1ge female. e, Morphologies of archegonia from the stage 4 of archegonial receptacles of the WT female and Mpbzr3-1ge female. Scale bars: 1 mm in a, 100 µm in b,d, and 500 µm in e. f, Expression levels of gametangium-related genes in Mpbzr3ge mutants. TPM values of gametangium-related genes in the archegonial receptacles (stage 1 and 4) and antheridial receptacles (stage 1 and 4) in the Mpbzr3ge mutants. Data represent the mean ± standard deviation (SD). Dots indicate each replicate (n = 3; biological replicates). FDR values (q values) calculated using EdgeR are indicated. g, Fertility of sperm from Mpbzr3ge male mutants. Spores from sporophyte by crossing between WT female and Mpbzr3-2ge male were cultured on half-strength B5 agar medium with hygromycin B. Some sporelings had hygromycin resistance. In 14 resistant sporelings, 8 were females and 6 were males. The sequence data of MpBZR3 locus in these female plants is indicated. Four-sevenths of female plants had mutations in a similar sequence as that of Mpbzr3-2ge male plants.
Extended Data Fig. 10 Gene expression pattern of BZR/BES transcription factors in Physcomitrium patents.
a, Tissue-specific gene expression pattern of PpBZRs in the expression database, Physcomitrella eFP Browser. Detail information for PpBZRs is indicated in Supplementary Data 2. b, Chromatic expression images of two type-B BZRs, PpBZR3A and PpBZR3B, in Physcomitrella eFP Brwser.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–4.
Supplementary Data
1. List of the differentially expressed genes in the reproductive organs. 2. List of BZR/BES transcription factors.
Supplementary Video 1
Video of discharged sperm cell masses from antheridiophores of WT.
Supplementary Video 2
Video of discharged sperm cell masses from antheridiophores of Mpbzr3-2ge males.
Source data
Source Data Extended Data Fig. 6
Unmodified gels for Extended Data Fig. 6b,c.
Source Data Extended Data Fig. 8
Statistical source data for Extended Data Fig. 8b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furuya, T., Saegusa, N., Yamaoka, S. et al. A non-canonical BZR/BES transcription factor regulates the development of haploid reproductive organs in Marchantia polymorpha. Nat. Plants (2024). https://doi.org/10.1038/s41477-024-01669-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41477-024-01669-0