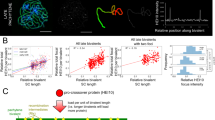
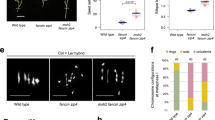
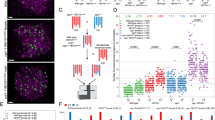
Abstract
Meiosis is a specialized eukaryotic division that produces genetically diverse gametes for sexual reproduction. During meiosis, homologous chromosomes pair and undergo reciprocal exchanges, called crossovers, which recombine genetic variation. Meiotic crossovers are stringently controlled with at least one obligate exchange forming per chromosome pair, while closely spaced crossovers are inhibited by interference. In Arabidopsis, crossover positions can be explained by a diffusion-mediated coarsening model, in which large, approximately evenly spaced foci of the pro-crossover E3 ligase HEI10 grow at the expense of smaller, closely spaced clusters. However, the mechanisms that control HEI10 dynamics during meiosis remain unclear. Here, through a forward genetic screen in Arabidopsis, we identified high crossover rate3 (hcr3), a dominant-negative mutant that reduces crossover interference and increases crossovers genome-wide. HCR3 encodes J3, a co-chaperone related to HSP40, which acts to target protein aggregates and biomolecular condensates to the disassembly chaperone HSP70, thereby promoting proteasomal degradation. Consistently, we show that a network of HCR3 and HSP70 chaperones facilitates proteolysis of HEI10, thereby regulating interference and the recombination landscape. These results reveal a new role for the HSP40/J3-HSP70 chaperones in regulating chromosome-wide dynamics of recombination via control of HEI10 proteolysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Seeds of Arabidopsis transgenic plants used for this study are freely available on request. GBS data in this study are available in the ArrayExpress database at EMBL-EBL (http://www.ebi.ac.uk/arrayexpress) under the accession numbers E-MTAB-10168, E-MTAB-11586, E-MTAB-12663, E-MTAB-12692, E-MTAB-12694, E-MTAB-12695, E-MTAB-12696, E-MTAB-12697, E-MTAB-12726 and E-MTAB-13412–13415. RNA-seq data have been deposited at EMBL-EBI under accession numbers E-MTAB-12699 and E-MTAB-13417. Source data are provided with this paper.
Code availability
All custom codes involved in the paper are available at https://github.com/KyuhaChoi-Lab/HCR3.
References
Villeneuve, A. M. & Hillers, K. J. Whence meiosis? Cell 106, 647–650 (2001).
Hunter, N. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7, 1–36 (2015).
Mercier, R., Mézard, C., Jenczewski, E., Macaisne, N. & Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 66, 297–327 (2015).
Pyatnitskaya, A., Borde, V. & De Muyt, A. Crossing and zipping: molecular duties of the ZMM proteins in meiosis. Chromosoma 128, 181–198 (2019).
Berchowitz, L. E. & Copenhaver, G. P. Genetic interference: don’t stand so close to me. Curr. Genomics 11, 91–102 (2010).
Berchowitz, L. E., Francis, K. E., Bey, A. L. & Copenhaver, G. P. The role of AtMUS81 in interference-insensitive crossovers in A. thaliana. PLoS Genet. 3, e132 (2007).
Crismani, W. et al. FANCM limits meiotic crossovers. Science 336, 1588–1590 (2012).
Séguéla-Arnaud, M. et al. Multiple mechanisms limit meiotic crossovers: TOP3α and two BLM homologs antagonize crossovers in parallel to FANCM. Proc. Natl Acad. Sci. USA 112, 4713–4718 (2015).
Agarwal, S. & Roeder, G. S. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102, 245–255 (2000).
De Muyt, A. et al. E3 ligase Hei10: a multifaceted structure-based signaling molecule with roles within and beyond meiosis. Genes Dev. 28, 1111–1123 (2014).
Ziolkowski, P. A. et al. Natural variation and dosage of the HEI10 meiotic E3 ligase control Arabidopsis crossover recombination. Genes Dev. 31, 306–317 (2017).
Qiao, H. et al. Antagonistic roles of ubiquitin ligase HEI10 and SUMO ligase RNF212 regulate meiotic recombination. Nat. Genet. 46, 194–199 (2014).
Reynolds, A. et al. RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat. Genet. 45, 269–278 (2013).
Bhalla, N., Wynne, D. J., Jantsch, V. & Dernburg, A. F. ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet. 4, e1000235 (2008).
Serra, H. et al. Massive crossover elevation via combination of HEI10 and recq4a recq4b during Arabidopsis meiosis. Proc. Natl Acad. Sci. USA 115, 2437–2442 (2018).
Chelysheva, L. et al. The Arabidopsis HEI10 is a new ZMM protein related to Zip3. PLoS Genet. 8, e1002799 (2012).
Kong, A. et al. Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science 319, 1398–1401 (2008).
Morgan, C. et al. Diffusion-mediated HEI10 coarsening can explain meiotic crossover positioning in Arabidopsis. Nat. Commun. 12, 4674 (2021).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
France, M. G. et al. ZYP1 is required for obligate cross-over formation and cross-over interference in Arabidopsis. Proc. Natl Acad. Sci. USA 118, e2021671118 (2021).
Capilla-Pérez, L. et al. The synaptonemal complex imposes crossover interference and heterochiasmy in Arabidopsis. Proc. Natl Acad. Sci. USA 118, e2023613118 (2021).
Lambing, C., Kuo, P. C., Tock, A. J., Topp, S. D. & Henderson, I. R. ASY1 acts as a dosage-dependent antagonist of telomere-led recombination and mediates crossover interference in Arabidopsis. Proc. Natl Acad. Sci. USA 24, 13647–13658 (2020).
Zhang, L. et al. Topoisomerase II mediates meiotic crossover interference. Nature 511, 551–556 (2014).
Nageswaran, D. C. et al. HIGH CROSSOVER RATE1 encodes PROTEIN PHOSPHATASE X1 and restricts meiotic crossovers in Arabidopsis. Nat. Plants 7, 452–467 (2021).
Ahuja, J. S. et al. Control of meiotic pairing and recombination by chromosomally tethered 26S proteasome. Science 355, 408–411 (2017).
Haversat, J. et al. Robust designation of meiotic crossover sites by CDK-2 through phosphorylation of the MutSγ complex. Proc. Natl Acad. Sci. USA 119, e2117865119 (2022).
He, W. et al. Regulated proteolysis of MutSγ controls meiotic crossing over. Mol. Cell 78, 168–183 (2020).
Cheng, C.-H. et al. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 20, 2067–2081 (2006).
Bhagwat, N. R. et al. SUMO is a pervasive regulator of meiosis. eLife 10, e57720 (2021).
He, W. et al. SUMO fosters assembly and functionality of the MutSγ complex to facilitate meiotic crossing over. Dev. Cell 56, 2073–2088 (2021).
Rao, H. B. D. P. et al. A SUMO-ubiquitin relay recruits proteasomes to chromosome axes to regulate meiotic recombination. Science 355, 402–407 (2017).
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P. & Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20, 665–680 (2019).
Jiang, Y., Rossi, P. & Kalodimos, C. G. Structural basis for client recognition and activity of Hsp40 chaperones. Science 365, 1313–1319 (2019).
Lee, D. H., Sherman, M. Y. & Goldberg, A. L. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 4773–4781 (1996).
Mogk, A., Bukau, B. & Kampinga, H. H. Cellular handling of protein aggregates by disaggregation machines. Mol. Cell 69, 214–226 (2018).
Shen, L., Kang, Y. G. G., Liu, L. & Yu, H. The J-domain protein J3 mediates the integration of flowering signals in Arabidopsis. Plant Cell 23, 499–514 (2011).
Barghetti, A. et al. Heat-shock protein 40 is the key farnesylation target in meristem size control, abscisic acid signaling and drought resistance. Genes Dev. 31, 2282–2295 (2017).
Kim, J. et al. Arabidopsis HEAT SHOCK FACTOR BINDING PROTEIN is required to limit meiotic crossovers and HEI10 transcription. EMBO J. 41, e109958 (2022).
Rowan, B. A., Patel, V., Weigel, D. & Schneeberger, K. Rapid and inexpensive whole-genome genotyping-by-sequencing for crossover localization and fine-scale genetic mapping. G3 5, 385–398 (2015).
Berchowitz, L. E. & Copenhaver, G. P. Fluorescent Arabidopsis tetrads: a visual assay for quickly developing large crossover and crossover interference data sets. Nat. Protoc. 3, 41–50 (2008).
Modliszewski, J. L. et al. Elevated temperature increases meiotic crossover frequency via the interfering (Type I) pathway in Arabidopsis thaliana. PLoS Genet. 14, e1007384 (2018).
Lloyd, A., Morgan, C., Franklin, F. C. H. & Bomblies, K. Plasticity of meiotic recombination rates in response to temperature in Arabidopsis. Genetics 208, 1409–1420 (2018).
Fang, N. N. et al. Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat. Cell Biol. 16, 1227–1237 (2014).
de Felippes, F. F., Wang, J. & Weigel, D. MIGS: miRNA-induced gene silencing. Plant J. 70, 541–547 (2012).
McLoughlin, F., Kim, M., Marshall, R. S., Vierstra, R. D. & Vierling, E. HSP101 interacts with the proteasome and promotes the clearance of ubiquitylated protein aggregates. Plant Physiol. 180, 1829–1847 (2019).
Yoo, H., Bard, J. A. M., Pilipenko, E. V. & Drummond, D. A. Chaperones directly and efficiently disperse stress-triggered biomolecular condensates. Mol. Cell 82, 741–755 (2022).
Prusicki, M. A. et al. Live cell imaging of meiosis in Arabidopsis thaliana. eLife 8, e42834 (2019).
Fozard, J. A., Morgan, C. & Howard, M. Coarsening dynamics can explain meiotic crossover patterning in both the presence and absence of the synaptonemal complex. eLife 12, e79408 (2023).
Durand, S. et al. Joint control of meiotic crossover patterning by the synaptonemal complex and HEI10 dosage. Nat. Commun. 13, 5999 (2022).
Morgan, C. et al. Evolution of crossover interference enables stable autopolyploidy by ensuring pairwise partner connections in Arabidopsis arenosa. Curr. Biol. 31, 4713–4726 (2021).
Wu, G., Rossidivito, G., Hu, T., Berlyand, Y. & Poethig, R. S. Traffic lines: new tools for genetic analysis in Arabidopsis thaliana. Genetics 200, 35–45 (2015).
Melamed-Bessudo, C., Yehuda, E., Stuitje, A. R. & Levy, A. A. A new seed-based assay for meiotic recombination in Arabidopsis thaliana. Plant J. 43, 458–466 (2005).
Charng, Y.-Y., Liu, H.-C., Liu, N.-Y., Hsu, F.-C. & Ko, S.-S. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol 140, 1297–1305 (2006).
Fernandes, J. B., Seguela-Arnaud, M., Larcheveque, C., Lloyd, A. H. & Mercier, R. Unleashing meiotic crossovers in hybrid plants. Proc. Natl Acad. Sci. USA 115, 2431–2436 (2017).
Schneeberger, K. et al. SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nat. Methods 6, 550–551 (2009).
Schiml, S., Fauser, F. & Puchta, H. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 80, 1139–1150 (2014).
Mair, A., Xu, S.-L., Branon, T. C., Ting, A. Y. & Bergmann, D. C. Proximity labeling of protein complexes and cell type specific organellar proteomes in Arabidopsis enabled by TurboID. eLife 8, e47864 (2019).
van Tol, N., Rolloos, M., van Loon, P. & van der Zaal, B. J. MeioSeed: a CellProfiler-based program to count fluorescent seeds for crossover frequency analysis in Arabidopsis thaliana. Plant Methods 14, 32 (2018).
Ziolkowski, P. A. et al. Juxtaposition of heterozygous and homozygous regions causes reciprocal crossover remodelling via interference during Arabidopsis meiosis. eLife 4, e03708 (2015).
Lim, E.-C. et al. DeepTetrad: high-throughput image analysis of meiotic tetrads by deep learning in Arabidopsis thaliana. Plant J. 101, 473–483 (2020).
Choi, K. et al. Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res. 28, 532–546 (2018).
Liao, Y., Smyth, G. K. & Shi, W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Walker, J. et al. Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat. Genet. 50, 130–137 (2018).
Chelysheva, L. et al. An easy protocol for studying chromatin and recombination protein dynamics during Arabidopsis thaliana meiosis: immunodetection of cohesins, histones and MLH1. Cytogenet. Genome Res. 129, 143–153 (2010).
Higgins, J. D., Sanchez-Moran, E., Armstrong, S. J., Jones, G. H. & Franklin, F. C. H. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev. 19, 2488–2500 (2005).
Hurel, A. et al. A cytological approach to studying meiotic recombination and chromosome dynamics in Arabidopsis thaliana male meiocytes in three dimensions. Plant J. 95, 385–396 (2018).
Vrielynck, N. et al. Conservation and divergence of meiotic DNA double strand break forming mechanisms in Arabidopsis thaliana. Nucleic Acids Res. 49, 9821–9835 (2021).
Acknowledgements
We thank G. Copenhaver (University of North Carolina at Chapel Hill), A. Levy (The Weizmann Institute of Science) and S. Poethig (University of Pennsylvania) for providing CTLs/FTLs. We thank C. Franklin (University of Birmingham) for providing ASY1 and RAD51 antibodies. We appreciate the support of IJPB plant observatory technological platforms. This research was supported by Suh Kyungbae Foundation SUHF-17020079, Samsung Science and Technology Foundation SSTF-BA2202-09, Next-Generation BioGreen 21 Program PJ01337001 and National Research Foundation of Korea NRF-2020R1A2C2007763 to K.C.; National Research Foundation of Korea NRF-2021R1A6A3A01087206 to J.K.; Agence Nationale de la Recherche CO-PATT-ANR-20-CE12-0006 and Saclay Plant Sciences-SPS ANR-17-EUR-0007 to M.G.; BBSRC grants BB/S006842/1, BB/S020012/1 and BB/V003984/1 to I.R.H.; European Research Council Consolidator Award ERC-2015-CoG-681987 to C.L. and I.R.H.; BBSRC grant BB/X011003/1 to C.L.; BBSRC Discovery Fellowship BB/V005774/1 to C.M.; BBSRC Institute Strategic Programme GEN BB/P013511/1 to M.H.
Author information
Authors and Affiliations
Contributions
H.K., J.K., C.M., I.R.H. and K.C. designed the study. H.K., J.K., N.S., P.K., C.M., A.C., D.B., J.P., Y.L., Y.M.P., J.G., A.H., C.L. and K.C. performed experiments. H.K., J.K., D.B, J.P., Y.L., Y.M.P., I.H., R.M., I.R.H. and K.C. contributed to genetics, genomics and biochemical results. H.K., P.K., C.M., A.C., J.G., A.H., C.L. and M.G. contributed to cytological data. C.M. generated the data of HEI10 foci. P.K., A.C., J.G., A.H., C.L. and M.G. contributed to quantification of MLH1 foci. H.K., J.K., N.S., P.K., C.M., A.C., D.B., J.P., Y.L., Y.M.P., J.A.F, C.L., M.H., M.G., I.R.H. and K.C. analysed the results. N.S. conducted analyses of GBS and RNA-seq data. J.A.F analysed the HEI10 coarsening model. C.L., M.H., M.G., I.H., I.R.H. and K.C. contributed to the supervision. H.K., J.K. and K.C. wrote and revised the paper. All authors commented, discussed and provided input on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Zhukuan Cheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of j3 and j2 T-DNA insertion mutants.
a, Gene structures for J3 and J2. Positions of T-DNA insertion (black triangles), hcr3 mutation (red triangle) and RT–PCR primers (arrows) are shown. b, Agarose gels showing RT–PCR products of J3 and J2 in Col, hcr3, j3-1, j3-3 and j2-2. c, Plot showing normalized transcript reads for J3 and J2 in RNA-seq of Col seedlings (J3, n = 4, J2, n = 4) and buds (J3, n = 4, J2, n = 4). Data are presented as mean values ± s.d. values of normalized transcript reads. n = the number of biologically independent samples. d, Principle component analysis of three replicates of RNA-seq libraries in Col, hcr3, j3-1, j3-3 and j2-2 seedlings. e, As for (d) but showing integrative genomic viewer windows showing the transcript levels for J3 and J2. f, Immunoblot analysis of transiently expressed epitope Myc-tagged and endogenous J3 and J2 in Col protoplasts. IB, immunoblot. CB, Coomassie blue. Anti-J3 antibody detects both J3 and J2 proteins (J3/J2). g,h, Immunoblot (g) and quantification (h) analyses of J3/J2 using anti-J3 antibody in seedlings and buds of Col, hcr3, j3-1, j3-3 and j2-2. J3/J2, seedling Col (n = 5), hcr3 (n = 5), j3-1 (n = 5), j3-3 (n = 5), j2-2 (n = 5); bud Col (n = 3), hcr3 (n = 3), j3-1 (n = 3), j3-3 (n = 3), j2-2 (n = 3). Immunoblots of tubulin and Coomassie blue staining were used for loading control and quantification. Black dots represent the normalized intensities of immunoblot replicates. n = the number of independent experiments. Red dots and horizontal lines indicate mean ± s.d. of intensities of immunoblot replicates (two-sided Welch’s t-test). i,j, Representative images (i) and quantification (j) of flowering time in Col, hcr3, j3-1, j3-3 and j2-2. Black dots represent leaf numbers of individual plants and red dots and horizontal lines indicate mean ± s.d. values of leaf numbers from individual plants (two-sided Welch’s t-test).
Extended Data Fig. 2 Functional redundancy between J3 and J2 in meiotic crossover frequency.
a, Seed (triangles) and pollen (circles) FTLs across the Arabidopsis genome. Lines represent the interval positions. b,c, Crossover frequency (cM) of pollen (b) and seed (c) FTLs in Col, j3-1, j3-3 and j2-2. d, 420 crossover frequency (cM) in Col, j3-1, j3-1, j2-2/+ j2-2 and j3-1/+ j2-2. e, As for (d) but showing Col, hcr3, j3-1, j3-1 J3::HA-J2 (T1), j3-1 meiMIGS-J2 (T1) and transgenic plants (T1) expressing HA-J2G156R under the J3, RPS5A and SPO11-1 promoters. Coloured (b,c) or black (d,e) dots represent cM values of individual plants. n = the number of individual plants. Black (b,c) or red (d,e) dots and horizontal lines indicate mean ± s.d. of cM values from individual plants (two-sided Welch’s t-test). f, Yeast two-hybrid analysis showing protein interactions of J3 and J3G155R with themselves and J2. g,h, Co-immunoprecipitation analysis showing dimerization of J3 and J3G155R with themselves (g) and J2 (h) in Arabidopsis protoplasts.
Extended Data Fig. 3 Functional redundancy between J3 and J2 in pollen development, embryogenesis and meiotic chromosome segregation.
a,b, Anthers containing Alexander-stained pollen grains (a) and plot (b) showing viable pollen grains per anther in Col, hcr3, j3-1, j3-3, j2-2, j3-1 j2-2/+ and j3-1/+ j2-2. Scale bars, 100 μm. Black dot indicates viable pollens of individual anther. Red dot and horizontal lines indicate mean values ± s.d. values of viable pollens for genotype. n = the number of individual anthers. c,d, As for (a,b) but showing representative images (c) and plot (d) for number of seeds per silique. White asterisks indicate aborted seeds. Scale bars, 1 mm. Blue and red dots indicate numbers of plump and aborted seeds per silique, respectively. Black dot and horizontal lines indicate mean values ± s.d. values of seed numbers for genotype. n = the number of individual siliques. e, Table of genotyping results and representative images showing seedling lethality of j3-1 j2-2 from the progeny of self-fertilized j3-1 j2-2/+ and j3-1/+ j2-2. Scale bars, 1 mm (top), 1 cm (bottom). f, As for (e) but showing plot and representative DAPI-stained images of chromosomal abnormalities at metaphase I or anaphase I. Red arrows indicate chromosome fragmentation or interlock. Scale bars, 5 μm. (b,d) Data are presented as mean values ± s.d. (two-sided Welch’s t-test).
Extended Data Fig. 4 Genomic analyses for crossover number and distribution in Col × Ler and J3::J3G155R Col × Ler.
a, Schematic of generation of J3::J3G155R Col × Ler hybrid plants and crossover map populations. b, 420 crossover frequencies in Col × Ler (n = 7) and J3::J3G155R Col × Ler (#4, n = 8, #6, n = 10, #8, n = 10,) F1 hybrid plants. Black dots indicate cM values of individual plants. Red dots and horizontal lines represent mean ± s.d. of cM values from individual plants (two-sided Welch’s t-test). n = the number of individual plants. c, Average crossover number per chromosome in Col × Ler and J3::J3G155R Col × Ler F2 individuals. d, As for (c) but showing normalized crossover frequencies (cM/Mb) along chromosome arms from the telomere (TEL) to the centromere (CEN). e,f, As for (c,d), but showing male and female meiosis. g,h, As for (c,d), but showing recq4a recq4b background.
Extended Data Fig. 5 Genomic crossover maps for F2 individuals from independent J3::J3G155R and SPO11-1::J3G155R Col × Ler plants.
a, CTL5.14 crossover frequencies in Col × Ler (n = 11) and two independent SPO11-1::J3G155R Col × Ler (#1, n = 11, #3, n = 12,) F1 hybrid plants. Black dots indicate cM values of individual plants. Red dots and horizontal lines represent mean ± s.d. of cM values from individual plants (two-sided Welch’s t-test). n = the number of individual plants. b, Histograms showing the ratio of F2 individuals containing different crossover numbers in each population. Vertical dotted red lines indicate the mean value. c, Average number of crossovers per chromosome in F2 individuals in each population. d,e, As for (c) but showing normalized crossover frequencies (cM/Mb) along chromosome arms from the telomere (TEL) to the centromere (CEN) (d) and across the genome (e).
Extended Data Fig. 6 Genome-wide crossover mapping of j3 knockout Col × Ler hybrids and quantification of MLH1 foci in j3 mutants.
a, Gene structure and nucleotide sequences of J3 with hcr3 mutation (red triangle), positions of CRISPR/Cas9 sgRNAs and Cas9-mediated mutations for the j3 null mutants in Ler. Nucleotide deletions (green), insertions (blue). b, Anthers containing Alexander-stained pollens of Ler, j3-5, j3-6 and j3-7. Scale bars, 100 μm. c, As for (b), but showing pollen viability in Col (n = 12), hcr3 (n = 13), j3-1 (n = 6), j3-3 (n = 6), j2-2 (n = 6), Ler (n = 6), j3-5 (n = 7), j3-6 (n = 8) and j3-7 (n = 9). Black dots indicate pollen viability per sample. n = the number of independent samples. d, Seed number per silique in Ler (n = 23) and j3-5 (n = 21). Coloured dots indicate the numbers of plump (blue) and aborted (red) seeds. n = the number of individual siliques. e, 420 crossover frequency in Col × Ler (n = 7), j3-1 × Ler (n = 6), Col × j3-5 (n = 6) and j3-1 × j3-5 (n = 7) F1 hybrids. n = the number of individual plants. f, Histogram of sex-averaged crossover number in Col/Ler (n = 240) and j3-1/j3-5 (n = 96) F2 individuals. g, As for (f), but showing average crossover number per chromosome. h,i, As for (f), but showing normalized crossover frequencies (cM/Mb) along chromosome arms from the telomere (TEL) to the centromere (CEN) (h) and across the genome (i). j, Quantification of immunostained MLH1 foci of meiotic cells at diplotene stage in Col (n = 63), j3-1 (n = 30) and j3-3 (n = 26). Coloured dots indicate numbers of MLH1 foci per cell. Black dots and horizontal lines indicate mean ± s.d. of values of MLH1 foci (two-sided Wilcoxon test, j3-1 P = 1.15 × 10−2, j3-3 P = 5.88 × 10−3). n = the number of independent cells. (c,d,e) Data were presented as mean ± s.d. of values and significance was tested by a two-sided Welch’s t-test.
Extended Data Fig. 7 J3 and J3G155R co-localize with HEI10 in vivo.
a, Representative images of co-immunostained J3/J2 (white), ZYP1 (green) and HEI10-Myc (red) at late-pachytene stage in Col HEI10::HEI10-Myc and hcr3 HEI10::HEI10-Myc. b, As for (a) but showing at diplotene and diakinesis stage in Col HEI10::HEI10-Myc. c, As for (a) but showing immunostained HA-J3, HA-J3G155R and HEI10-Myc at late-pachytene stage in J3::HA-J3 HEI10::HEI10-Myc and at mid-pachytene stage in J3::HA-J3G155R HEI10::HEI10-Myc plants. Three yellow dotted line boxes (1, 2, 3) in the merged images of co-immunostained J3/J2 and HEI10 are enlarged and displayed. Scale bars, 5 μm. Experiments (a–c) were performed at least three times.
Extended Data Fig. 8 J3-HSP70 chaperone machinery restricts crossover frequency.
a, Co-immunoprecipitation analysis of J3 and HSP70 in Arabidopsis protoplasts. b, In vitro pull-down assay of J3 and HSP70-1. c, Co-immunoprecipitation analysis of J2 with HSP70 and HEI10 in Arabidopsis protoplasts. d, Co-localization of J3/J2 and HSP70 with HEI10, PTD and MSH5 in Arabidopsis protoplasts. YN indicates N-terminal 1-158 amino acid resides of yellow fluorescent protein (YFP) and YC, C-terminal 159-238 amino acid residues of YFP for BiFC assay. Scale bars, 20 μm. e, RT–qPCR analysis of HSP70-1-5 genes in unopen flower buds of Col and meiMIGS-HSP70-1 plants. Red and blue dots indicate two technical duplicates of three biological replicates (n = 6 for each genotype) for RT–qPCRs. Mean ± s.d. of values are shown by black dots and horizontal lines. f, Seed FTLs across genome. g, 420 crossover frequencies in meiMIGS-HSP70-1 and meiMIGS-HSP70-3 transgenic lines. 420 Col (n = 12), meiMIGS-HSP70-1 T1 (n = 8), meiMIGS-HSP70-1 T2#5 (n = 6), meiMIGS-HSP70-1 T2#7 (n = 4), meiMIGS-HSP70-2 T1 (n = 8), meiMIGS-HSP70-2 T2#1 (n = 8), meiMIGS-HSP70-2 T2#4 (n = 8), h, As for (g) but showing CTLs in meiMIGS-HSP70-1. CTL1.17 Col (n = 6), meiMIGS-HSP70-1 (n = 7); CTL1.22 Col (n = 5), meiMIGS-HSP70-1 (n = 5); CTL3.15 Col (n = 6), meiMIGS-HSP70-1 (n = 5); CTL4.1 Col (n = 6), meiMIGS-HSP70-1 (n = 5); CTL4.7 Col (n = 6), meiMIGS-HSP70-1 (n = 6). i, As for (g) but showing meiMIGS-HSP70/Ler hybrid plants. Col/Ler (n = 8), meiMIGS-HSP70-1/Ler (n = 8), meiMIGS-HSP70-2/Ler (n = 8). j, Histogram of crossover number in Col × Ler (blue, n = 240) and meiMIGS-HSP70-1 × Ler (red, n = 96) F2 individuals. Dotted blue and red lines indicate mean values. k, As for (j) but showing normalized crossover frequencies (cM/Mb) along chromosome arms from the telomere (TEL) to the centromere (CEN). Black (g,i) or coloured (h) dots indicate cM values of individual plants. Red (g,i) or black (h) dots and horizontal lines represent mean ± s.d. of cM values from individual plants (two-sided Welch’s t-test). (g,h,i) n = the number of biologically independent plants.
Extended Data Fig. 9 Ubiquitination modification and proteasome-dependent degradation of HEI10 in Arabidopsis protoplasts.
a, Immunoprecipitation and immunoblot analysis of ubiquitin (Ub)-conjugated HEI10-HA in Arabidopsis protoplasts. The plasmid constructs for either FLAG-tagged ubiquitin (FLAG-Ub) or HEI10-HA, or both were co-transfected into protoplasts. IP, immunoprecipitation. IB, immunoblot. Coomassie blue (CB) stained membrane is shown as a loading control. Experiments were performed at least three times. b,c, Immunoblot analysis (b) and quantification plot (c) of HEI10-HA protein upon treatment of translation elongation (cycloheximide, CHX) and proteasome (MG132) inhibitors in Arabidopsis protoplasts. Coloured dots indicate the normalized intensities of immunoblots. Black dots and horizontal lines indicate mean ± s.d. of values of the normalized immunoblot intensities at 0h (CHX, n = 3, CHX+MG132, n = 3, P = 0.47), 0.5h (CHX, n = 3, CHX+MG132, n = 3, P = 4.5 × 10−2), 1h (CHX, n = 3, CHX+MG132, n = 3, P = 7.3 × 10−3) and 2h (CHX, n = 3, CHX+MG132, n = 3, P = 2.9 × 10−2). Significance between time points was examine using a two-sided Welch’s t-test. n = the number of independent experiments.
Extended Data Fig. 10 HCR1 and HCR3 are required for restricting class I crossovers.
a, Crossover frequencies of CTL1.26 in Col (n = 8), hcr1 (n = 8), hcr3 (n = 7) and hcr1 hcr3 (n = 7) (Supplementary Table 27). Black dots indicate cM values of individual plants. Red dots and horizontal lines represent mean ± s.d. of cM values from individual plants. Significance between genotypes was tested by a two-sided Welch’s t-test (Col vs hcr1, P = 1.18 × 10−7, Col vs hcr3, P = 4.00 × 10−10, Col vs hcr1 hcr3, P = 2.73 × 10−7, hcr1 vs hcr1 hcr3, P = 6.28 × 10−6, hcr3 vs hcr1 hcr3, P = 1.39 × 10−3). n = the number of biologically independent plants. b, Immunoblot analysis of HEI10 in Col, hcr1, hcr3 and hcr1 hcr3. Coomassie blue-stained membrane and tubulin protein blot were shown as a loading control. Experiments were performed at least three times.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–28, references and source data for Supplementary Figs. 5 and 6.
Supplementary Data
Statistical source data for Supplementary Fig. 6.
Source data
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots and gels.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Source Data Extended Data Figs. 1, 3, 6, 8 and 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Kim, J., Son, N. et al. Control of meiotic crossover interference by a proteolytic chaperone network. Nat. Plants 10, 453–468 (2024). https://doi.org/10.1038/s41477-024-01633-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01633-y