Abstract
Histone acetylation is a predominant active chromatin mark deposited by histone acetyltransferases (HATs) that transfer the acetyl group from acetyl coenzyme A (acetyl-CoA) to lysine ε-amino groups in histones. GENERAL CONTROL NON-REPRESSED PROTEIN 5 (GCN5) is one of the best-characterized HATs and functions in association with several adaptor proteins such as ADA2 within multiprotein HAT complexes. ADA2–GCN5 interaction increases GCN5 binding to acetyl-CoA and stimulates its HAT activity. It remains unclear whether the HAT activity of GCN5 (which acetylates not only histones but also cellular proteins) is regulated by acetyl-CoA levels, which vary greatly in cells under different metabolic and nutrition conditions. Here we show that the ADA2 protein itself is acetylated by GCN5 in rice cells. Lysine acetylation exposes ADA2 to a specific E3 ubiquitin ligase and reduces its protein stability. In rice plants, ADA2 protein accumulation reversely parallels its lysine acetylation and acetyl-CoA levels, both of which are dynamically regulated under varying growth conditions. Stress-induced ADA2 accumulation could stimulate GCN5 HAT activity to compensate for the reduced acetyl-CoA levels for histone acetylation. These results indicate that ADA2 lysine acetylation that senses cellular acetyl-CoA variations is a mechanism to regulate HAT activity and histone acetylation homeostasis in plants under changing environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are included in the main text. The structural models of ADA2 and E3-2 from the AlphaFold database and the modelling of the ADA2–E3-2 interaction are included in Supplementary Data 1. Source data are provided with this paper.
References
Nitsch, S., Zorro Shahidian, L. & Schneider, R. Histone acylations and chromatin dynamics: concepts, challenges, and links to metabolism. EMBO Rep. 22, e52774 (2021).
Shvedunova, M. & Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 23, 329–349 (2022).
Verdin, E. & Ott, M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 (2015).
Liu, X., Yang, S., Yu, C. W., Chen, C. Y. & Wu, K. Histone acetylation and plant development. Enzymes 40, 173–199 (2016).
Verdone, L., Caserta, M. & Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 83, 344–353 (2005).
Sivanand, S., Viney, I. & Wellen, K. E. Spatiotemporal control of acetyl-CoA metabolism in chromatin regulation. Trends Biochem. Sci. 43, 61–74 (2018).
Brownell, J. E. et al. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84, 843–851 (1996).
Grant, P. A. et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11, 1640–1650 (1997).
Kuo, M. H. et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 383, 269–272 (1996).
Morris, S. A. et al. Identification of histone H3 lysine 36 acetylation as a highly conserved histone modification. J. Biol. Chem. 282, 7632–7640 (2007).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Espinola-Lopez, J. M. & Tan, S. The Ada2/Ada3/Gcn5/Sgf29 histone acetyltransferase module. Biochim. Biophys. Acta Gene Regul. Mech. 1864, 194629 (2021).
Boyer, L. A. et al. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell 10, 935–942 (2002).
Grant, P. A. et al. Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 274, 5895–5900 (1999).
Balasubramanian, R., Pray-Grant, M. G., Selleck, W., Grant, P. A. & Tan, S. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277, 7989–7995 (2002).
Lee, K. K. & Workman, J. L. Histone acetyltransferase complexes: one size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 8, 284–295 (2007).
Vlachonasios, K. E. Histone acetylation: a requirement for petunia floral scent. J. Exp. Bot. 72, 3493–3495 (2021).
Sun, J. et al. Structural basis for activation of SAGA histone acetyltransferase Gcn5 by partner subunit Ada2. Proc. Natl Acad. Sci. USA 115, 10010–10015 (2018).
Mao, Y., Pavangadkar, K. A., Thomashow, M. F. & Triezenberg, S. J. Physical and functional interactions of Arabidopsis ADA2 transcriptional coactivator proteins with the acetyltransferase GCN5 and with the cold-induced transcription factor CBF1. Biochim. Biophys. Acta 1759, 69–79 (2006).
Hark, A. T. et al. Two Arabidopsis orthologs of the transcriptional coactivator ADA2 have distinct biological functions. Biochim. Biophys. Acta 1789, 117–124 (2009).
Anzola, J. M. et al. Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proc. Natl Acad. Sci. USA 107, 10308–10313 (2010).
Servet, C., Conde e Silva, N. & Zhou, D. X. Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol. Plant 3, 670–677 (2010).
Benhamed, M., Bertrand, C., Servet, C. & Zhou, D. X. Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18, 2893–2903 (2006).
Sieberer, T., Hauser, M. T., Seifert, G. J. & Luschnig, C. PROPORZ1, a putative Arabidopsis transcriptional adaptor protein, mediates auxin and cytokinin signals in the control of cell proliferation. Curr. Biol. 13, 837–842 (2003).
Bertrand, C., Bergounioux, C., Domenichini, S., Delarue, M. & Zhou, D. X. Arabidopsis histone acetyltransferase AtGCN5 regulates the floral meristem activity through the WUSCHEL/AGAMOUS pathway. J. Biol. Chem. 278, 28246–28251 (2003).
Vlachonasios, K. E., Thomashow, M. F. & Triezenberg, S. J. Disruption mutations of ADA2b and GCN5 transcriptional adaptor genes dramatically affect Arabidopsis growth, development, and gene expression. Plant Cell 15, 626–638 (2003).
Zhou, S. L. et al. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell 29, 1088–1104 (2017).
Xu, Q. et al. Histone deacetylases control lysine acetylation of ribosomal proteins in rice. Nucleic Acids Res. 49, 4613–4628 (2021).
Fournier, M. et al. KAT2A/KAT2B-targeted acetylome reveals a role for PLK4 acetylation in preventing centrosome amplification. Nat. Commun. 7, 13227 (2016).
Cieniewicz, A. M. et al. The bromodomain of Gcn5 regulates site specificity of lysine acetylation on histone H3. Mol. Cell Proteom. 13, 2896–2910 (2014).
Li, Y. et al. An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase–substrate interaction network. Nat. Commun. 8, 347 (2017).
Lu, Y. et al. Dynamics and functional interplay of histone lysine butyrylation, crotonylation, and acetylation in rice under starvation and submergence. Genome Biol. 19, 144 (2018).
Chen, C. et al. Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis. Nat. Plants 3, 814–824 (2017).
Cluntun, A. A. et al. The rate of glycolysis quantitatively mediates specific histone acetylation sites. Cancer Metab. 3, 10 (2015).
Hsieh, W. C. et al. Glucose starvation induces a switch in the histone acetylome for activation of gluconeogenic and fat metabolism genes. Mol. Cell 82, 60–74.e5 (2022).
Xu, Q. et al. ACL and HAT1 form a nuclear module to acetylate histone H4K5 and promote cell proliferation. Nat. Commun. 14, 3265 (2023).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Zhao, S. et al. ATP-citrate lyase controls a glucose-to-acetate metabolic switch. Cell Rep. 17, 1037–1052 (2016).
Marino, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014).
Pouikli, A. et al. Hypoxia promotes osteogenesis by facilitating acetyl-CoA-mediated mitochondrial–nuclear communication. EMBO J. 41, e111239 (2022).
Weinert, B. T. et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 11, 833 (2015).
Naquet, P., Kerr, E. W., Vickers, S. D. & Leonardi, R. Regulation of coenzyme A levels by degradation: the ‘Ins and Outs’. Prog. Lipid Res. 78, 101028 (2020).
Jiang, W. et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol. Cell 43, 33–44 (2011).
Liu, X. Y. et al. Histone deacetylase AtSRT1 links metabolic flux and stress response in Arabidopsis. Mol. Plant 10, 1510–1522 (2017).
Galdieri, L. & Vancura, A. Acetyl-CoA carboxylase regulates global histone acetylation. J. Biol. Chem. 287, 23865–23876 (2012).
Xu, Q. et al. ROS-stimulated protein lysine acetylation is required for crown root development in rice. J. Adv. Res. 48, 33–46 (2022).
Zhang, H., Zhao, Y. & Zhou, D. X. Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 45, 12241–12255 (2017).
Shi, L. & Tu, B. P. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 33, 125–131 (2015).
Shi, L. & Tu, B. P. Protein acetylation as a means to regulate protein function in tune with metabolic state. Biochem. Soc. Trans. 42, 1037–1042 (2014).
Shen, Y., Wei, W. & Zhou, D. X. Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci. 20, 614–621 (2015).
Lu, Y. et al. Metabolic regulation of the plant epigenome. Plant J. 114, 1001–1013 (2023).
Hu, Y., Lu, Y., Zhao, Y. & Zhou, D. X. Histone acetylation dynamics integrates metabolic activity to regulate plant response to stress. Front. Plant Sci. 10, 1236 (2019).
Fatland, B. L., Nikolau, B. J. & Wurtele, E. S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 17, 182–203 (2005).
Fatland, B. L. et al. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 130, 740–756 (2002).
Kafkia, E. et al. Operation of a TCA cycle subnetwork in the mammalian nucleus. Sci. Adv. 8, eabq5206 (2022).
Kim, J. M. et al. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 3, 17097 (2017).
He, Y. et al. Self-cleaving ribozymes enable the production of guide RNAs from unlimited choices of promoters for CRISPR/Cas9 mediated genome editing. J. Genet. Genomics 44, 469–472 (2017).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Zhao, H. et al. PaACL silencing accelerates flower senescence and changes the proteome to maintain metabolic homeostasis in Petunia hybrida. J. Exp. Bot. 71, 4858–4876 (2020).
Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Nicholas, K.B., & Nicholas, H.B. GeneDoc: a tool for editing and annotating multiple sequence alignments. Embnew News 4, 1–4 (1997).
Wang, F. et al. Biochemical insights on degradation of Arabidopsis DELLA proteins gained from a cell-free assay system. Plant Cell 21, 2378–2390 (2009).
Wang, W. et al. SnRK1 stimulates the histone H3K27me3 demethylase JMJ705 to regulate a transcriptional switch to control energy homeostasis. Plant Cell 33, 3721–3742 (2021).
Schneider, C., Rasband, W. & Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
We thank Q. Zhang and X. Li for technical assistance, H. Song for help with the confocal experiments and X. Zhang for advice on figure enhancement. Computation resources were provided by the high-throughput computing platform of the National Key Laboratory of Crop Genetic Improvement at Huazhong Agricultural University and supported by H. Liu. The work was supported by National Natural Science Foundation of China (grant no. 32070563, D.-X.Z.), the Fundamental Research Funds for the Central Universities (grant no. 2662015PY228, D.-X.Z.) and the French Agence Nationale de Recherche project (grant no. REPHARE ANR-19-CE12-0027, D.-X.Z.).
Author information
Authors and Affiliations
Contributions
Y. Yu did the experimental work and data analysis. F.Z. participated in the construction of transgenic plants. Y. Yue and Y.Z. contributed to the experimentation. D.-X.Z. designed and supervised the work and wrote the paper with input from Y. Yu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Chenlong Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1. Representative MS/MS spectra of 7 acetylpeptides of ADA2.
SPSHIAGGANK(ac)K, ASNVGQFK(ac)DGANVAK, DGANVAK(ac)VEDGHVDR,SIGVK(ac)KPR, YSADEGPSLTELSGYNSK(ac)R, ESGQLLSNTK(ac)VVHK, IESDGNLDQK(ac)K, with an acetylation site at K of ADA2 respectively. The x axis represents the mass charge ratio (m/z), and the y axis represents the relative abundance of ions.
Extended Data Fig. 2 Lysine acetylation effects on ADA2 function.
(a) Protein interaction between ADA2 full-length, truncated fragments, or the K263R point mutation version and GCN5 in yeast two hybrid assays. (b) Effects of the point mutations (K263R and K263Q) for ADA2 nucleus localization in rice protoplasts. At least two repetitions were performed. (c) Tests of ADA2 function to promote the acetyltransferase activity of GCN5. In vitro HAT assay was performed using recombinant GCN5-His as acetyltransferases and recombinant H3.3-His as substrates with or without ADA2-His recombinant protein in 50 µM acetyl-CoA. GCN5E233AD274A-His is GCN5 catalytically dead mutant, GCN5Y485A-His is a bromodomain mutation that disrupts acetyl-lysine binding. (d) Tests of effect of the ADA2 7KR point mutations on acetyltransferase activity of GCN5. In vitro HAT assay was performed using recombinant GST-GCN5 as acetyltransferases and recombinant H3.3-His as substrates with or without ADA2-His or ADA27KR-His recombinant protein in 50 µM Acetyl-CoA. The assays in c and d were analyzed by immunoblotting with anti-H3, anti-pan-H3 acetylation, anti-H3K9 acetylation, anti-H3K14 acetylation, anti-acetyl-lysine and anti-ADA2 antibody. Data in c and d are the two repetitions (except the controls used in c and d are different).
Extended Data Fig. 3 Detrimental effect of ADA2 acetylation on its stability.
The two other replicates of Fig. 2a.
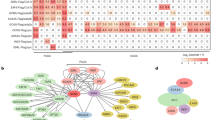
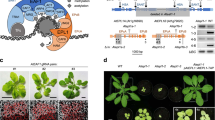
Extended Data Fig. 4 Identification of a specific E3 ligase that targets acetylated ADA2 in rice.
(a) Network view of predicted E3-substrate interactions in UbiBrowser web services. In order to found the candidate E3 ligase we used ADA2 homologs gene TADA2b (Homo sapiens) as queried substrate to predicted potential E3 ligase. In network view, the central node is the queried substrate, and the surrounding nodes are the predicted E3 ligases. The width of the edge reflects the confidence of the interaction. (b) Predicted high confidence (green background) and middle confidence (yellow background) interacting E3 proteins in Homo sapiens, and their homologs in rice. (c) Co-localization of E3-2 (fused to RFP) with wild type and mutant ADA2 proteins (fused to GFP) in transiently transfected rice protoplasts. (d) Visualization of the ZDOCK predicted ADA2 and E3-2 interacting residues. The distances between E3-2 and ADA2 or ADA2 K263Q mutant residues were measured using pymol software. (e) Production of rice E3-2 CRISPR/Cas9 mutants. sgRNA position in the gene and decoded sequence mutations of the E3-2 gene are shown. (f) Phenotypes of e3-2-1 and e3-2-2 plants in comparison with a control line (with null mutation) at seedling stage. (g) ADA2 protein levels in e3-2 mutants relative to the control line. Nuclear proteins were tested by immunoblotting with anti-ADA2 and anti-H3 antibodies. Band intensities were quantified using ImageJ software, relative to the control line, and indicated below the blots.
Extended Data Fig. 5 ADA2 protein is accumulated during the dark period.
(a) Nuclear proteins were extracted from rice seedlings (14 d after germination), grown on a 12-h diurnal light/dark cycle sampled at 6-h intervals from ZT12 to ZT6 for 2 days or with continuous darkness as indicated, followed by immunoblotting with anti-ADA2 and anti-H3 antibodies. (b) Endogenous ADA2 was immunoprecipitated from the nuclear proteins prepared as in (a) with anti-ADA2 antibody, followed by immunoblotting with anti-acetyl-lysine (anti-Lys-Ac) antibody to detect ADA2 acetylation. (c) The other replicate of Fig. 3A. (d) ADA2 transcript levels in rice seedlings indicated in (a). Transcript levels (n = 3, mean values from 3 measurements are presented) were measured by reverse transcription quantitative polymerase chain reaction (qPCR) and normalized using Actin as the reference gene.
Extended Data Fig. 6 Time course of ADA2 protein accumulation under stress.
(a) ADA2 protein and transcript levels under PEG stress. Upper panels: Immunoblotting analysis of ADA2 protein levels in rice seedlings treated with or without PEG during the day time, harvested at 3 h intervals. Anti-H3 was used as loading controls. Lower part: relative ADA2 transcript levels during PEG treatment. ADA2 transcript levels in rice seedlings (n = 3, mean values from 3 measurements are presented) were measured by reverse transcription quantitative polymerase chain reaction (qPCR) and normalized using Actin as the reference gene. (b) ADA2 protein and transcript levels in seedlings treated with 20% PEG6000 for 24 h with or without ActD (40 µM) treatment. Nuclear proteins were analyzed by immunoblotting with anti-ADA2 and anti-H3 antibodies. Band intensities were quantified using ImageJ software, relative to CK, and indicated below the blots. Two replicates (rep 1 &2) are shown. ADA2 transcript levels in rice seedlings (bars indicate the mean ± SE of three replicates) were measured by reverse transcription quantitative polymerase chain reaction (qPCR) and normalized using Actin as the reference gene. (c) ADA2 protein levels in rice seedling (10 days after germination) under submergence. Rice plant samples were harvested at ZT6 every 24 hours during 7 days, for acetyl-CoA and nuclear protein extraction, followed by immunoblotting with anti-ADA2 and anti-H3 acetylation antibodies. ADA2 transcript levels in rice seedlings (bars indicate the mean ± SE of three replicates) were measured by reverse transcription quantitative polymerase chain reaction (qPCR) and normalized using Actin as the reference gene.
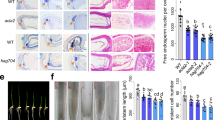
Extended Data Fig. 7 ADA2 is required for PEG stress tolerance and expression and histone acetylation of stress responsive genes.
(a) Drought sensitive phenotypes of wild-type and ada2 mutant plants. Rice seedlings of the same size/length were dehydrated for 4 days and then rehydrated for 2 days. The average percentage of survival were calculated from two independent experiments with at least 13 plants of each genotype in each replicate. (b) WT and ada2-1 mutant seedlings were treated with or without 20% PEG6000 for 24 h. The H3K14 acetylation levels of the stress mark genes were measured by Cut&tag-qPCR, enrichment values represent the relative fold change of H3K14 acetylation antibody to IgG antibody. Transcript levels of the stress mark genes in rice seedlings were measured by reverse transcription quantitative polymerase chain reaction (qPCR) and normalized using actin as a reference gene. SUS4 was used as negative control. For all data, bars indicate the mean ± SE of three replicates (Unpaired and two-tailed t test, the significance level was p < 0.05). (c) The WT seedlings were treated with or without 20% PEG6000 in the absence of MG132 for 24 h. H3K14 acetylation and transcript levels of the stress-responsive genes were measured as in B, and the p-value between the two groups is shown above the column, bars indicate the mean ± SE of three replicates (Unpaired and two-tailed t test, the significance level was p < 0.05).
Supplementary information
Supplementary Information
The primer sequences used in this study.
Supplementary Data 1
The structural models of ADA2 and E3-2 from the AlphaFold database and the modelling of the ADA2–E3-2 interaction.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Figs. 2–5 and Extended Data Figs. 5–7
Statistical source data for Figs. 2–5 and Extended Data Figs. 5–7.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Zhao, F., Yue, Y. et al. Lysine acetylation of histone acetyltransferase adaptor protein ADA2 is a mechanism of metabolic control of chromatin modification in plants. Nat. Plants 10, 439–452 (2024). https://doi.org/10.1038/s41477-024-01623-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01623-0