Abstract
Properly patterned cell walls specify cellular functions in plants. Differentiating protoxylem and metaxylem vessel cells exhibit thick secondary cell walls in striped and pitted patterns, respectively. Cortical microtubules are arranged in distinct patterns to direct cell wall deposition. The scaffold protein MIDD1 promotes microtubule depletion by interacting with ROP GTPases and KINESIN-13A in metaxylem vessels. Here we show that the phase separation of MIDD1 fine-tunes cell wall spacing in protoxylem vessels in Arabidopsis thaliana. Compared with wild-type, midd1 mutants exhibited narrower gaps and smaller pits in the secondary cell walls of protoxylem and metaxylem vessel cells, respectively. Live imaging of ectopically induced protoxylem vessels revealed that MIDD1 forms condensations along the depolymerizing microtubules, which in turn caused massive catastrophe of microtubules. The MIDD1 condensates exhibited rapid turnover and were susceptible to 1,6-hexanediol. Loss of ROP abolished the condensation of MIDD1 and resulted in narrow cell wall gaps in protoxylem vessels. These results suggest that the microtubule-associated phase separation of MIDD1 facilitates microtubule arrangement to regulate the size of gaps in secondary cell walls. This study reveals a new biological role of phase separation in the fine-tuning of cell wall patterning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the supplementary material of this article. Source data are provided with this paper.
Code availability
The trained model parameters for our dataset and a sample code for the application of the model are available upon request.
References
Endler, A. & Persson, S. Cellulose synthases and synthesis in Arabidopsis. Mol. Plant 4, 199–211 (2011).
Paradez, A., Wright, A. & Ehrhardt, D. W. Microtubule cortical array organization and plant cell morphogenesis. Curr. Opin. Plant Biol. 9, 571–578 (2006).
Shaw, S. L., Kamyar, R. & Ehrhardt, D. W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003).
Dixit, R. & Cyr, R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell 16, 3274–3284 (2004).
Murata, T. et al. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat. Cell Biol. 7, 961–968 (2005).
Nakamura, M., Ehrhardt, D. W. & Hashimoto, T. Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 12, 1064–1070 (2010).
Elliott, A. & Shaw, S. L. Update: plant cortical microtubule arrays. Plant Physiol. 176, 94–105 (2018).
Gardiner, J. The evolution and diversification of plant microtubule-associated proteins. Plant J. 75, 219–229 (2013).
Hamada, T. Microtubule organization and microtubule-associated proteins in plant cells. Int. Rev. Cell Mol. Biol. 312, 1–52 (2014).
Oda, Y. Cortical microtubule rearrangements and cell wall patterning. Front. Plant Sci. 6, 236 (2015).
Kawamura, E. & Wasteneys, G. O. MOR1, the Arabidopsis thaliana homologue of Xenopus MAP215, promotes rapid growth and shrinkage, and suppresses the pausing of microtubules in vivo. J. Cell Sci. 121, 4114–4123 (2008).
Whittington, A. et al. MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613 (2001).
Lin, D. et al. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr. Biol. 23, 290–297 (2013).
Zhang, Q., Fishel, E., Bertroche, T. & Dixit, R. Microtubule severing at crossover sites by katanin generates ordered cortical microtubule arrays in Arabidopsis. Curr. Biol. 23, 2191–2195 (2013).
Ambrose, C. & Wasteneys, G. O. Cell edges accumulate γ tubulin complex components and nucleate microtubules following cytokinesis in Arabidopsis thaliana. PLoS ONE 6, e27423 (2011).
Eng, R. C. et al. KATANIN and CLASP function at different spatial scales to mediate microtubule response to mechanical stress in Arabidopsis cotyledons. Curr. Biol. 31, 3262–3274.e6 (2021).
Lindeboom, J. J. et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533 (2013).
Lindeboom, J. J. et al. CLASP stabilization of plus ends created by severing promotes microtubule creation and reorientation. J. Cell Biol. 218, 190–205 (2019).
Nakamura, M., Lindeboom, J. J., Saltini, M., Mulder, B. M. & Ehrhardt, D. W. SPR2 protects minus ends to promote severing and reorientation of plant cortical microtubule arrays. J. Cell Biol. 217, 915–927 (2018).
Feiguelman, G., Fu, Y. & Yalovsky, S. ROP GTPases structure–function and signaling pathways. Plant Physiol. 176, 57–79 (2018).
Fu, Y., Gu, Y., Zheng, Z., Wasteneys, G. & Yang, Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120, 687–700 (2005).
Fu, Y., Xu, T., Zhu, L., Wen, M. & Yang, Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 19, 1827–1832 (2009).
Lauster, T. et al. Arabidopsis pavement cell shape formation involves spatially confined ROPGAP regulators. Curr. Biol. 32, 532–544.e7 (2022).
Ren, H. et al. Spatio-temporal orientation of microtubules controls conical cell shape in Arabidopsis thaliana petals. PLoS Genet. 13, e1006851 (2017).
Ren, H. et al. SPIKE1 activates ROP GTPase to modulate petal growth and shape. Plant Physiol. 172, 358–371 (2016).
Wang, J. et al. Brassinosteroid signals cooperate with katanin-mediated microtubule severing to control stamen filament elongation. EMBO J. 42, e111883 (2023).
Yu, B. et al. Root twisting drives halotropism via stress-induced microtubule reorientation. Dev. Cell 57, 2412–2425.e6 (2022).
Zhang, C. et al. ROPGAP-dependent interaction between brassinosteroid and ROP2-GTPase signaling controls pavement cell shape in Arabidopsis. Curr. Biol. 32, 518–531 e516 (2022).
Malivert, A. et al. FERONIA and microtubules independently contribute to mechanical integrity in the Arabidopsis shoot. PLoS Biol. 19, e3001454 (2021).
Sampathkumar, A. et al. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3, e01967 (2014).
Takatani, S. et al. Microtubule response to tensile stress is curbed by NEK6 to buffer growth variation in the Arabidopsis hypocotyl. Curr. Biol. 30, 1491–1503.e2 (2020).
Tang, W. et al. Mechano-transduction via the pectin–FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 32, 508–517.e3 (2022).
Uyttewaal, M. et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149, 439–451 (2012).
Nagawa, S., Xu, T. & Yang, Z. RHO GTPase in plants: conservation and invention of regulators and effectors. Small GTPases 1, 78–88 (2010).
Wu, G., Gu, Y., Li, S. & Yang, Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13, 2841–2856 (2001).
Lavy, M. et al. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 17, 947–952 (2007).
Li, S., Gu, Y., Yan, A., Lord, E. & Yang, Z. B. RIP1 (ROP interactive partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol. Plant 1, 1021–1035 (2008).
Sugiyama, Y. et al. A Rho–actin signaling pathway shapes cell wall boundaries in Arabidopsis xylem vessels. Nat. Commun. 10, 468 (2019).
Turner, S., Gallois, P. & Brown, D. Tracheary element differentiation. Annu. Rev. Plant Biol. 58, 407–433 (2007).
Xu, H. et al. Secondary cell wall patterning-connecting the dots, pits and helices. Open Biol. 12, 210208 (2022).
Smertenko, T. et al. Brachypodium distachyon MAP20 functions in metaxylem pit development and contributes to drought recovery. N. Phytol. 227, 1681–1695 (2020).
Sasaki, T., Fukuda, H. & Oda, Y. CORTICAL MICROTUBULE DISORDERING1 is required for secondary cell wall patterning in xylem vessels. Plant Cell 29, 3123–3139 (2017).
Sugiyama, Y., Wakazaki, M., Toyooka, K., Fukuda, H. & Oda, Y. A novel plasma membrane-anchored protein regulates xylem cell-wall deposition through microtubule-dependent lateral inhibition of Rho GTPase domains. Curr. Biol. 27, 2522–2528.e4 (2017).
Oda, Y. & Fukuda, H. Rho of plant GTPase signaling regulates the behavior of Arabidopsis kinesin-13A to establish secondary cell wall patterns. Plant Cell 25, 4439–4450 (2013).
Oda, Y., Iida, Y., Kondo, Y. & Fukuda, H. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr. Biol. 20, 1197–1202 (2010).
Nagashima, Y. et al. A Rho-based reaction-diffusion system governs cell wall patterning in xylem vessels. Sci. Rep. https://doi.org/10.1038/s41598-018-29543-y (2018).
Oda, Y. & Fukuda, H. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337, 1333–1336 (2012).
Schneider, R. et al. Long-term single-cell imaging and simulations of microtubules reveal principles behind wall patterning during proto-xylem development. Nat. Commun. 12, 669 (2021).
Watanabe, Y. et al. Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 350, 198–203 (2015).
Wightman, R. & Turner, S. Trafficking of the cellulose synthase complex in developing xylem vessels. Biochem. Soc. Trans. 38, 755–760 (2010).
Wightman, R. & Turner, S. R. The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J. 54, 794–805 (2008).
Derbyshire, P. et al. Proteomic analysis of microtubule interacting proteins over the course of xylem tracheary element formation in Arabidopsis. Plant Cell 27, 2709–2726 (2015).
Mao, G., Buschmann, H., Doonan, J. H. & Lloyd, C. W. The role of MAP65-1 in microtubule bundling during Zinnia tracheary element formation. J. Cell Sci. 119, 753–758 (2006).
Pesquet, E., Korolev, A. V., Calder, G. & Lloyd, C. W. The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr. Biol. 20, 744–749 (2010).
Schneider, R. et al. Two complementary mechanisms underpin cell wall patterning during xylem vessel development. Plant Cell 29, 2433–2449 (2017).
Feiguelman, G., Cui, X., Sternberg, H., Fu, Y. & Yalovsky, S. Microtubule-associated ROP interactors both delimit and transduce ROP signaling and regulate microtubule dynamics. Development 149, dev200811 (2022).
Ryu, K. H., Huang, L., Kang, H. M. & Schiefelbein, J. Single-cell RNA sequencing resolves molecular relationships among individual plant cells. Plant Physiol. 179, 1444–1456 (2019).
Mucha, E., Hoefle, C., Huckelhoven, R. & Berken, A. RIP3 and AtKinesin-13A—a novel interaction linking Rho proteins of plants to microtubules. Eur. J. Cell Biol. 89, 906–916 (2010).
Brembu, T., Winge, P. & Bones, A. M. The small GTPase AtRAC2/ROP7 is specifically expressed during late stages of xylem differentiation in Arabidopsis. J. Exp. Bot. 56, 2465–2476 (2005).
Kubo, M. et al. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 19, 1855–1860 (2005).
Yamaguchi, M. et al. VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 153, 906–914 (2010).
Gustafsson, M. G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D. & Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18, 715–730 (2006).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Meszaros, B., Erdos, G. & Dosztanyi, Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 46, W329–W337 (2018).
Romero, P., Obradovic, Z. & Dunker, A. K. Sequence data analysis for long disordered regions prediction in the calcineurin family. Genome Inform. Ser. Workshop Genome Inform. 8, 110–124 (1997).
Kroschwald, S., Maharana, S. & Simon, A. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters doi: 10.19185/matters.201702000010 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Cheng, X., Mwaura, B. W., Chang Stauffer, S. R. & Bezanilla, M. A fully functional ROP fluorescent fusion protein reveals roles for this GTPase in subcellular and tissue-level patterning. Plant Cell 32, 3436–3451 (2020).
Jacobs, B., Schneider, R., Molenaar, J., Filion, L. & Deinum, E. E. Microtubule nucleation complex behavior is critical for cortical array homogeneity and xylem wall patterning. Proc. Natl Acad. Sci. USA 119, e2203900119 (2022).
Jacobs, B., Molenaar, J. & Deinum, E. E. Robust banded protoxylem pattern formation through microtubule-based directional ROP diffusion restriction. J. Theor. Biol. 502, 110351 (2020).
Li, E. et al. A positive feedback circuit for ROP-mediated polar growth. Mol. Plant 14, 395–410 (2021).
Li, H. et al. The REN4 rheostat dynamically coordinates the apical and lateral domains of Arabidopsis pollen tubes. Nat. Commun. 9, 2573 (2018).
Emenecker, R. J., Holehouse, A. S. & Strader, L. C. Biological phase separation and biomolecular condensates in plants. Annu. Rev. Plant Biol. 72, 17–46 (2021).
Ong, J. Y. & Torres, J. Z. Phase separation in cell division. Mol. Cell 80, 9–20 (2020).
Sun, M. et al. NuMA regulates mitotic spindle assembly, structural dynamics and function via phase separation. Nat. Commun. 12, 7157 (2021).
Maan, R. et al. Multivalent interactions facilitate motor-dependent protein accumulation at growing microtubule plus-ends. Nat. Cell Biol. 25, 68–78 (2023).
Meier, S. M. et al. Multivalency ensures persistence of a +TIP body at specialized microtubule ends. Nat. Cell Biol. 25, 56–67 (2023).
Song, X. et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat. Cell Biol. 25, 79–91 (2023).
Tsukita, K. et al. Phase separation of an actin nucleator by junctional microtubules regulates epithelial function. Sci. Adv. 9, eadf6358 (2023).
Walczak, C. E., Gayek, S. & Ohi, R. Microtubule-depolymerizing kinesins. Annu. Rev. Cell Dev. Biol. 29, 417–441 (2013).
Deng, Z. Y. et al. OsKinesin-13A is an active microtubule depolymerase involved in glume length regulation via affecting cell elongation. Sci. Rep. 5, 9457 (2015).
Leong, S. Y., Edzuka, T., Goshima, G. & Yamada, M. Kinesin-13 and kinesin-8 function during cell growth and division in the moss Physcomitrella patens. Plant Cell 32, 683–702 (2020).
Desai, A., Verma, S., Mitchison, T. J. & Walczak, C. E. Kin I kinesins are microtubule-destabilizing enzymes. Cell 96, 69–78 (1999).
Feiguelman, G., et al. Microtubule-associated ROP interactors affect microtubule dynamics and modulate cell wall patterning and root hair growth. Development https://doi.org/10.1242/dev.200811 (2022).
Hasi, Q. & Kakimoto, T. ROP interactive partners are involved in the control of cell division patterns in Arabidopsis leaves. Plant Cell Physiol. 63, 1130–1139 (2022).
Fujikura, U. et al. Atkinesin-13A modulates cell-wall synthesis and cell expansion in Arabidopsis thaliana via the THESEUS1 pathway. PLoS Genet. 10, e1004627 (2014).
Lu, L., Lee, Y. R., Pan, R., Maloof, J. N. & Liu, B. An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol. Biol. Cell 16, 811–823 (2005).
Nakagawa, T. et al. Improved gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007).
Tsutsui, H. & Higashiyama, T. pKAMA-ITACHI vectors for highly efficient CRISPR/Cas9-mediated gene knockout in Arabidopsis thaliana. Plant Cell Physiol. 58, 46–56 (2017).
Shimada, T. L., Shimada, T. & Hara-Nishimura, I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 61, 519–528 (2010).
Falk, T. et al. U-Net: deep learning for cell counting, detection, and morphometry. Nat. Methods 16, 67–70 (2019).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. In Proc. International Conference on Learning Representations (ICLR, 2015).
Acknowledgements
We thank N.-H. Chua (Rockefeller University) for the pER8 vector; T. Nakagawa (Shimane University) for the pGWB vectors; S. Ide (National Institute of Genetics) for advice; K. Kobayashi (Nikon Imaging Center, Hokkaido University) for technical support; and R. Schneider (University of Potsdam) for discussion and technical advice. This work was supported by MEXT KAKENHI (grants 19H05670 and 19H05677 to Y.O.), JSPS KAKENHI (grants 21H02514, 20K21435 and 23K18126 to Y.O.), the Research Program for CORE lab of Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials in Network Joint Research Center for Materials and Devices from MEXT (grant 20186001 to Y.O.), the Mitsubishi Foundation, and the Naito Foundation to Y.O.
Author information
Authors and Affiliations
Contributions
Y.O. designed the research. T.H., S.K., T.S., S.T. and R.A. performed the experiments and analysed the data. Y.K. performed image analyses. M.W., M.S. and K.T. performed electron microscopy. Y.O., T.D. and H.F. contributed to material preparation. T.H. and Y.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Bo Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
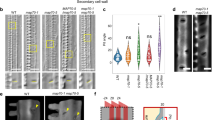
Extended Data Fig. 1 Secondary cell wall patterns of midd mutants.
(a) Density of secondary cell wall pits in roots (n = 30 cells, one cell/plant). (b) Density of secondary cell wall pits when pairs of distinct small pits (DPs) were counted as one pit (n = 30 cells, one cell/plant). (c) Areas of secondary cell wall pits without DPs in WT, midd1, midd2, midd1 midd2, midd1 midd2/pMIDD1:GFP–MIDD1, kinesin-13a, and kinesin-13a/pKinesin-13A:Kinesin-13A-tagRFP plants (n = 100 pits from 15 plants for WT, 13 plants for midd1, 22 plants for midd2, 14 plants for midd1 midd2, 15 plants for midd1 midd2/pMIDD1:GFP-MIDD1, 12 plants for kinesin-13a, 15 plants for kinesin-13a/pKinesin-13A:Kinesin-13A-tagRFP). (DP) indicates the area of DPs. (d) Electron micrographs of protoxylem vessels in roots. Red arrowheads indicate the ingrowth of secondary cell walls. Scale bar = 2 μm. Data are representative of three independent experiments. (e) Three replicated measurements of stripe densities (sample number of upper left, upper right and lower left panels: n = 20, 20, and 20 cells for WT; 21, 20, and 15 cells for midd1 midd2; 16, 20, and 18 cells for midd1 midd2/pMIDD1:GFP-MIDD1; 19, 21, and 21 cells for kinesin-13a; 21, 20, and 19 cells for kinesin-13a/pKinesin-13A:Kinesin-13A-tagRFP; 20, 19, and 21 cells for midd1 midd2 kinesin-13a; 20, 20, and 20 cells for rop7 rop8 rop11; 19, 20, and 20 cells for rop7 rop8 rop11/pROP7:ROP7; 20, 20, and 19 cells for rop7 rop8 rop11/pROP8:ROP8; and 20, 20, 20 cells for rop7 rop8 rop11/pROP11:ROP11; one cell/plant). Letters indicate statistically significant differences (p < 0.05) determined using one-way ANOVA, followed by Tukey’s test. Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD (A-C, and E). (f) T-DNA insertions of midd1 and midd2 mutants.
Extended Data Fig. 2 Observation of patterns of secondary cell walls and microtubules in different stages and in ROP-overexpressing (ROPox) plants.
(A and B) DIC (a) and stripe density (b) of protoxylem vessels at the early and late stages of secondary cell wall deposition in WT and midd1 midd2 roots. M, metaxylem; P, protoxylem. Scale bar = 5 μm (n = 8 cells from 8 plants). Letters indicate statistically significant differences (p < 0.05) determined by one-way ANOVA, followed by Tukey’s test. (C and D) DIC (c) and length (d) of protoxylem cells of WT and midd1 midd2 roots at the earliest stage of secondary cell wall deposition indicated by magenta dotted lines. The cell edges were determined based on the differences in the extent of secondary cell wall deposition under DIC. n = 8 cells from 8 plants for WT and 18 cells from 18 plants for midd1 midd2, p = 0.45, two-sided t-test. (e) DIC of protoxylem vessels in WT, ROP11ox, ROP11T22Nox, and ROP11G17Vox roots. (f) Density of stripe patterns (n = 15 cells for WT, 15 cells for ROP11ox, 14 cells for ROP11T22Nox, and 15 cells for ROP11G17Vox, one cell/plant). Letters indicate statistically significant differences (p < 0.05) determined by one-way ANOVA, followed by Tukey’s test. Scale bars = 5 μm. Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD (B, D, and F). (G and H) Microtubules (labeled with EYFP-TUB6) in early- and late-stage metaxylem (g) and protoxylem (h) cells in the roots of WT, midd1 midd2, and kinesin-13a plants. Cell walls were stained with propidium iodide (PI). Images were obtained by spinning disk confocal microscopy. Experiments were repeated twice with similar results. Scale bars = 5 μm.
Extended Data Fig. 3 Signal transition in trans-differentiating protoxylem cells.
(A and B) Representative images of YFP-labeled microtubules in VND7GR hypocotyl cells of WT (a) and midd1 midd2 (b) plants, without projection (see Fig. 2a and b for projected images). Images were obtained by spinning disk confocal microscopy. (C and D) Magnification of the boxed regions shown in A (c) and B (d). Experiments were repeated twice with similar results. (e) Mean intensity of 3–5 future band regions and 3–5 future gap regions of eight WT (cell1 to cell8) and eight midd1 midd2 cells (cell1 to cell8). The representative results (cell1) of bands or gaps are shown in Fig. 2g and h. (f) Histogram showing the signal intensities of cell cortex including bands and gaps at the first and final frames of time-lapse images. (g) Skewness of histograms of signal intensities. Letters indicate statistically significant differences (one-way ANOVA and Tukey’s test; n = 8 cells from 8 plants; p < 0.05). Scale bars = 5 μm (A–D). Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD. Images in (A–D) were obtained by spinning disk confocal microscopy.
Extended Data Fig. 4 Microtubule dynamics in gap regions of differentiating cells.
(a) Microtubule dynamics at the early stage of differentiation observed by spinning disk confocal microscopy. Future band region is surrounded by a yellow dotted line. Images were obtained at 6 h after the DEX treatment. Representative microtubule ends are highlighted using red, blue, or green dots. Experiments were repeated twice with similar results. Scale bar = 5 μm. Images were obtained by spinning disk confocal microscopy. (b) Shrinking speed of microtubules (n = 104 microtubules from EB of 2 cells, 114 microtubules from EG of 2 cells, and 106 microtubules from LG of 2 cells for WT, n = 125 microtubules from EB of 3 cells, 112 microtubules from EG of 3 cells, and 112 microtubules from LG of 3 cells for midd1 midd2). EB, EG, and LG indicate early band, early gap, and late gap regions, respectively. Letters indicate statistically significant differences (one-way ANOVA and Tukey’s test; p < 0.05). Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD. (c) Histograms showing the orientation of microtubules at late gap regions in WT and midd1 midd2 (n = 100 microtubules were measured in 2 cells from 2 plants). (d) Histograms showing the length of microtubules just before the catastrophe at late gap regions in WT and midd1 midd2 (n > 150 microtubules were measured in 2 cells from 2 plants).
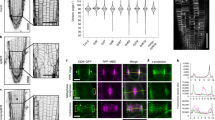
Extended Data Fig. 5 MIDD1 speckle formation in gap regions.
(a) Time-course analysis of the area of microtubules (red) and MIDD1 speckles (green) in gap regions relative to the area of gaps. Mean ± standard deviation (SD) of five gaps in a VND7-induced cell were plotted. Results from the two VND7GR-induced cells are shown. The result obtained from another cell is shown in Fig. 4f. (b) Catastrophe of the microtubule (pIRX3:mRFP-TUB6) upon the collision with MIDD1 speckle (pMIDD1:GFP-MIDD1). White arrowheads indicate the MIDD1 speckle which microtubule tip collides. Experiments were repeated twice with similar results. Scale bar = 2 μm. See also Supplementary Video 6. (c) Dynamics of GFP-MIDD1 (pMIDD1:GFP-MIDD1) in VND7GR-induced cell. MIDD1 speckle (Orange arrowheads) forms after depolymerization of microtubules labelled with GFP-MIDD1. Red and green arrowheads indicate growth and shrinkage of microtubules, respectively. Blue arrowheads indicate a new MIDD1-labelled microtubule that undergoes catastrophe upon collision with the MIDD1 speckle. Experiments were repeated twice with similar results. Scale bar = 2 μm. See also Supplementary Video 7. (d) Signal intensity ratio of GFP-MIDD1 relative to the associated EYFP-TUB6-labeled microtubules at band and gap regions at the late stages of differentiation (n = 400 filaments were measured in 6 cells from 3 plants). Asterisks indicate statistically significant differences (***p < 0.001; two-sided Student’s t-test). Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD. Images in (B) and (C) were obtained by spinning disk confocal microscopy.
Extended Data Fig. 6 Kinesin-13A speckles in gap regions.
(a) Microtubule (pIRX3:EYFP-TUB6) and Kinesin-13A-tagRFP (pKinesin-13A:Kinesin-13A-tagRFP) in VND7GR hypocotyl epidermal cells of WT or midd1 midd2 plants. Images were obtained between 8 and 9 h after the onset of DEX treatment. White arrowheads indicate speckles of Kinesin-13A. Experiments were repeated twice with similar results. (b) Microtubules (pIRX3:EYFP-TUB6) and Kinesin-13A-tagRFP (pKinesin-13A:Kinesin-13A-tagRFP) or GFP-MIDD1 (pMIDD1:GFP-MIDD1) and Kinesin-13A-tagRFP in protoxylem cells in the WT root. White arrowheads indicate speckles of Kinesin-13A. Experiments were repeated twice with similar results. (c) Microtubules (pIRX3:EYFP-TUB6) and mScarlet-MIDD1 (pMIDD1:mScarlet-i-MIDD1) in metaxylem and protoxylem cells in the roots of kinesin-13a plants. White arrowheads indicate speckles of MIDD1 in gaps. Experiments were repeated three times with similar results. (d) GFP-MIDD1 and Kinesin-13A-tagRFP in VND7GR hypocotyl epidermal cells after treatment with 1% and 1.5% 1,6-HD at 6–12 h after the onset of DEX treatment. Red arrowheads indicate speckles of MIDD1 or Kinesin-13A. (E and F) Area of MIDD1 speckles (e) and Kinesin-13A speckles (f) relative to the gap region. Data represent the mean of all gaps included in the image (n = 8 cells from 6 plants for ‘0%’, 8 cells from 6 plants for ‘1%’, 8 cells from 6 plants for ‘1.5%’). Asterisks indicate statistically significant differences (***p < 0.001, **p < 0.01, *p < 0.05; one-way ANOVA and Tukey’s test). Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD. All of images were obtained by spinning disk confocal microscopy. Scale bars = 5 μm.
Extended Data Fig. 7 Localization of IDR-truncated MIDD1 in root xylem vessels.
(a) Three-dimensional structure of MIDD1 predicted by AlphaFold2. Unstructured IDR is indicated at the N-terminus. (b) Microtubules labeled with EYFP-TUB6 (pIRX3:EYFP-TUB6); full-length mScarlet-i-MIDD1(pMIDD1:mScarlet-i-MIDD1) or truncated mScarlet-i-MIDD1 lacking 1–20 amino acids (MIDD1Δ20), 1–40 amino acids (MIDD1Δ40), 1–59 amino acids (MIDD1Δ59), or 1–20/40–59 amino acids (MIDD1Δ1–20/40–59) labeled with mScarlet-i expressed in protoxylem and metaxylem cells. White arrowheads indicate MIDD1 speckles. Experiments were repeated twice with similar results. Scale bar = 5 μm. (c) Density of stripe patterns (n = 12 cells for WT, 16 cell for midd1 midd2, 13 cells for midd1 midd2/pMIDD1:mScarlet-i-MIDD1, 13 cells for midd1 midd2/pMIDD1:mScarlet-i-MIDD1Δ20, 12 cells for midd1 midd2/pMIDD1:mScarlet-i-MIDD1Δ40, 12 cells for midd1 midd2/pMIDD1:mScarlet-i-MIDD1Δ59, 13 cells for midd1 midd2/pMIDD1:mScarlet-i-MIDD1Δ1-20/40-59, each cell was measured from 1 plant). Letters indicate statistically significant differences (p < 0.05) determined by one-way ANOVA, followed by Tukey’s test. Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD. Images were obtained by spinning disk confocal microscopy. Scale bar = 10 μm.
Extended Data Fig. 8 Localization of IDR-truncated MIDD1 in tobacco leaves.
MIDD1 (pLexA:EYFP-MIDD1) and microtubules (pUBQ10:tagRFP-TUB6) transiently expressed in Nicotiana benthamiana leaves. Experiments were repeated three times with similar results. Images were obtained by spinning disk confocal microscopy. Scale bar = 10 μm.
Extended Data Fig. 9 Localization patterns of ROP in VND7GR cells.
(a) Microtubules (pIRX3:mRFP-TUB6) and GFP-MIDD1ΔN (pMIDD1:GFP-MIDD1ΔN) at the midplane of the differentiating cell. Images were obtained between 6 and 10 h after the onset of DEX treatment. (b) Kymograph along the white dotted lines shown in A. Experiments were repeated three times with similar results. (c) Profiles of mRFP and GFP signal intensities along the yellow dotted lines shown in the rightmost panels of A. (d) ROP2-GFP and PI-stained cell walls in the differentiating protoxylem cell. Images were obtained at 8 h after the DEX treatment. Kymograph along the white dotted line is shown in the right panel. Experiments were repeated twice with similar results. (e) ROP2-GFP in the differentiating protoxylem cell. Images are projection of Z-stack images. Kymograph along red dotted lines are shown on the right side of each time point. Images in the lower panel indicate horizontally averaged signal intensity of GFP-ROP2. Experiments were repeated twice with similar results. (f) Kymographs along the red dotted line shown in the lower panel of (E). Images were obtained by spinning disk confocal microscopy (A, B, and D–F). Scale bars = 5 μm.
Extended Data Fig. 10 MIDD1 localization in the rop mutant.
(A and C) GFP-MIDD1 (pMIDD1:GFP-MIDD1) in early- and late-stages of metaxylem cells in the roots of WT (A) and rop7 rop8 rop11 (C) plants observed by spinning disk confocal microscopy. Cell walls are stained with PI. White arrowheads indicate MIDD1 speckles. Experiments were repeated twice with similar results. Scale bars = 5 μm. (B and D) Intensity profiles of GFP and PI along the white dotted line shown in the rightmost panels of (A) and (C). Note that the peaks of GFP are located at the gaps in WT (B) while they located at bands in the rop mutant (D). (E) Signal intensity ratio of GFP-MIDD1 in the gap region relative to that in the band region in differentiating protoxylem cells of WT and rop7 rop8 rop11 plants (n = 5 cells from 5 plants). Asterisks indicate statistically significant differences (*p < 0.05; two-sided Student’s t-test). Box plot indicates the median (horizontal line) and the interquartile range from 25 to 75%, and whiskers indicate SD.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2: statistical analysis of rescue frequency (Table 1) and primers used for plasmid construction (Table 2).
Supplementary Video 1
Long-term time-lapse imaging of microtubules (EYFP–TUB6) in VND7GR hypocotyl epidermal cells. Each image is the projection of 61 images obtained at 3-s intervals at every 15-min time point between 6 and 10 h after the onset of DEX treatment. Scale bar, 5 μm.
Supplementary Video 2
Long-term time-lapse imaging of microtubules (EYFP–TUB6) in midd1 midd2 hypocotyl epidermal cells expressing VND7GR. Each image is the projection of 61 images obtained at 3-s intervals at every 15-min time point between 7 and 11 h after the onset of DEX treatment. Scale bar, 5 μm.
Supplementary Video 3
Growing and shrinking microtubules at gap regions in VND7GR-induced cells. Images were obtained at 10 h after the onset of DEX treatment. Microtubule ends are highlighted using red, blue and green dots. Scale bar, 5 μm.
Supplementary Video 4
Growing and shrinking microtubules at the gap region in midd1 midd2 cells expressing VND7GR. Images were obtained at 11 h after the onset of DEX treatment. Microtubule ends are highlighted using red, blue and green dots. Scale bar, 5 μm.
Supplementary Video 5
Long-term time-lapse imaging of microtubules (mRFP–TUB6) and GFP–MIDD1 in VND7GR-induced cells. Each image is the projection of 61 images obtained at 3-s intervals at every 15-min time point between 6 and 9 h after the onset of DEX treatment. Scale bar, 5 μm.
Supplementary Video 6
Microtubules (mRFP–TUB6) exhibited catastrophe after collision with speckles of GFP–MIDD1 (yellow arrowhead). Images were obtained every 3 s at 10 h after the onset of DEX treatment. Scale bar, 2 μm.
Supplementary Video 7
Microtubule labelled with GFP–MIDD1 exhibited catastrophe immediately after collision with the MIDD1 speckle (yellow arrowhead). Images were obtained every 3 s starting at 10 h after the onset of DEX treatment. Scale bar, 2 μm.
Supplementary Video 8
SIM of microtubules (mRFP–TUB6) and GFP–MIDD1 at the early stage of protoxylem differentiation. Images were obtained every 10 s starting at 6 h after the onset of DEX treatment. White arrowheads indicate the shrinking of microtubules after collision with MIDD1 speckles. Scale bar, 2 μm.
Supplementary Video 9
Formation of MIDD1 speckles (pMIDD1:GFP–MIDD1) after microtubule (pIRX3:mRFP–TUB6) depolymerization. Images were obtained every 3 s at 10 h after the onset of DEX treatment. White arrowheads indicate MIDD1 droplets formed from shrinking microtubules. Scale bar, 2 μm.
Supplementary Video 10
Fusion of MIDD1 speckles observed by SIM. Images were obtained every 10 s starting at 10 h after the onset of DEX treatment. White arrowheads indicate the fusion of MIDD1 droplets. Scale bar, 2 μm.
Supplementary Video 11
FRAP experiment of GFP–MIDD1. Images were obtained every 0.5 s. A pulse laser was applied to the area indicated by the white circle. Scale bar, 10 μm.
Supplementary Data 1
Results of statistical analyses shown in Figs. 1b, 1d, 1f, 2i–m, 3e–h, 5h and 5j and Extended Data Figs. 1a–c, 1e, 2b, 2d, 2f, 3g, 4b, 5d, 6e, 6f, 7e and 10e.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Higa, T., Kijima, S.T., Sasaki, T. et al. Microtubule-associated phase separation of MIDD1 tunes cell wall spacing in xylem vessels in Arabidopsis thaliana. Nat. Plants 10, 100–117 (2024). https://doi.org/10.1038/s41477-023-01593-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01593-9