Abstract
Nitrogen is an essential macronutrient that is absorbed by roots and stored in leaves, mainly as ribulose-1,5-bisphosphate carboxylase/oxygenase1,2. During nitrogen deficiency (−N), plants activate leaf senescence for source-to-sink nitrogen remobilization for adaptative growth3,4,5,6. However, how −N signals perceived by roots are propagated to shoots remains underexplored. We found that ELF18-INDUCED LONG NONCODING RNA 1 (ELENA1) is −N inducible and attenuates −N-induced leaf senescence in Arabidopsis. Analysis of plants expressing the ELENA1 promoter β-glucuronidase fusion gene showed that ELENA1 is transcribed specifically in roots under −N. Reciprocal grafting of the wild type and elena1 demonstrated that ELENA1 functions systemically. ELENA1 dissociates the MEDIATOR SUBUNIT 19a–ORESARA1 transcriptional complex, thereby calibrating senescence progression. Our observations establish the systemic regulation of leaf senescence by a root-derived long non-coding RNA under −N in Arabidopsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed in this study are included in this Letter and its Supplementary Information files. The materials and transgenic plants generated in this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Have, M. et al. Nitrogen remobilization during leaf senescence: lessons from Arabidopsis to crops. J. Exp. Bot. 68, 2513–2529 (2017).
Feller, U., Anders, I. & Mae, T. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 59, 1615–1624 (2008).
Cheng, S. L. H. et al. Nutrient status regulates MED19a phase separation for ORESARA1-dependent senescence. N. Phytol. 236, 1779–1795 (2022).
Balazadeh, S. et al. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 62, 250–264 (2010).
Kim, J. H. et al. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057 (2009).
Rauf, M. et al. ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep. 14, 382–388 (2013).
Diaz, C. et al. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol. 147, 1437–1449 (2008).
Park, B. S. et al. Arabidopsis NITROGEN LIMITATION ADAPTATION regulates ORE1 homeostasis during senescence induced by nitrogen deficiency. Nat. Plants 4, 898–903 (2018).
Park, S. H. et al. Arabidopsis ubiquitin-specific proteases UBP12 and UBP13 shape ORE1 levels during leaf senescence induced by nitrogen deficiency. N. Phytol. 223, 1447–1460 (2019).
Qiu, K. et al. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Genet. 11, e1005399 (2015).
Seo, J. S. et al. ELF18-INDUCED LONG-NONCODING RNA associates with mediator to enhance expression of innate immune response genes in Arabidopsis. Plant Cell 29, 1024–1038 (2017).
Liu, J., Wang, H. & Chua, N. H. Long noncoding RNA transcriptome of plants. Plant Biotechnol. J. 13, 319–328 (2015).
Ma, L., Bajic, V. B. & Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 10, 925–933 (2013).
Statello, L. et al. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Yan, L. et al. High-efficiency genome editing in Arabidopsis using YAO promoter-driven CRISPR/Cas9 system. Mol. Plant 8, 1820–1823 (2015).
Seo, J. S. et al. ELF18-INDUCED LONG NONCODING RNA 1 evicts fibrillarin from mediator subunit to enhance PATHOGENESIS-RELATED GENE 1 (PR1) expression. N. Phytol. 221, 2067–2079 (2019).
Yang, L. et al. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 41, 958–967 (2023).
Liu, L. & Chen, X. Intercellular and systemic trafficking of RNAs in plants. Nat. Plants 4, 869–878 (2018).
Ham, B. K. et al. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21, 197–215 (2009).
Gruber, A. R. et al. The Vienna RNA websuite. Nucleic Acids Res. 36, W70–W74 (2008).
Durian, G. et al. Calcium-dependent protein kinase CPK1 controls cell death by in vivo phosphorylation of senescence master regulator ORE1. Plant Cell 32, 1610–1625 (2020).
Karimi, M., Inze, D. & Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Zhang, X. et al. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Zuo, J., Niu, Q. W. & Chua, N. H. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000).
Huang, C. H. et al. Early diagnosis and management of nitrogen deficiency in plants utilizing Raman spectroscopy. Front. Plant Sci. 11, 663 (2020).
Turnbull, C. G., Booker, J. P. & Leyser, H. M. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32, 255–262 (2002).
Chen, X. et al. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 26, 640–646 (2016).
Edwards, K., Johnstone, C. & Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19, 1349 (1991).
Fiil, B. K. et al. Coimmunoprecipitation (co-IP) of nuclear proteins and chromatin immunoprecipitation (ChIP) from Arabidopsis. CSH Protoc. 2008, pdb.prot5049 (2008).
Saleh, A., Alvarez-Venegas, R. & Avramova, Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 3, 1018–1025 (2008).
Acknowledgements
We thank X. Qi for the gift of pYAO CRISPR–Cas9 vectors and C. Xiangbin for guidance on Arabidopsis grafting. This work was supported by core funding from Temasek Life Sciences Laboratory and Disruptive & Sustainable Technology for Agricultural Precision (DiSTAP), an interdisciplinary research group (IRG) of the Singapore MIT Alliance for Research and Technology (SMART) Centre supported by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Author information
Authors and Affiliations
Contributions
S.L.H.C. and N.-H.C. designed the experiments. S.L.H.C., H.X. and J.H.T.N. executed the experiments. All the authors interpreted and discussed the data. S.L.H.C. and N.-H.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Yong Wang, Yasuhito Sakuraba, Martin Crespi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ELENA1 transcript levels in ELENA1 and ORE1 genotypes under nitrogen deficiency (-N).
RT-qPCR analysis of ELENA1 transcript levels in WT (Nitrogen sufficient; +N), WT (-N), EL-KD#10, EL-KD#20, EL-OE#16, EL-OE#29, ore1 and ORE1 OE on -N. The value of WT ( + N) was set to 1. Data are means ± SD. n = 3 (biologically independent repeats) and individual data points as overlays. ns, no statistical difference. Asterisks indicate statistically significant difference compared with WT (-N). **P < 0.01; one-way ANOVA, Dunnett’s multiple comparison analysis.
Extended Data Fig. 2 ELENA1 does not influence growth, nitrate content and expression of ORE1 target genes under nitrogen sufficient (+N) conditions.
(a) Average fresh weight of shoots and roots in the indicated genotypes grown under +N conditions. (b) Shoot-to-root ratio of fresh weight in the indicated genotypes. (c) Total nitrate content in shoots and roots of the indicated genotypes grown under +N conditions. (d) Shoot-to-root ratio of total nitrate content in the indicated genotypes. (e) Transcript analysis of ORE1 target genes BFN1, RNS3, SAG29, SINA1 and VNI2 in the indicated genotypes treated on +N medium. ns indicated not statistically significant, P > 0.05; two-way ANOVA, multiple comparison with Dunnett post hoc analysis. Value of each gene in WT ( + N) was set to 1. (a, b, c, d, e) Data are means ± SD. n = 3 and individual data points as overlays. ns, no statistical difference; one-way ANOVA (a,b,c,d), two-way anova (e) Dunnett’s multiple comparison analysis.
Extended Data Fig. 3 ELENA1 transcripts are bona fide lncRNAs under nitrogen deficiency (-N).
(a) -N induced leaf senescence phenotype of empty vector transgenic plant, EL-OE#29, EL5M-OE #1 and EL8M-OE #1. Scale bar represents 1 cm. (b) Total chlorophyll content in different leaf groups of the indicated genotypes treated on -N medium. Leaf number, L. FW, fresh weight. (c) Expression level of ELENA1 in the indicated genotypes treated on -N medium. Value of EV ( + N) was set to 1. (b, c) Data are means ± SD. n = 3 (biologically independent samples). Each sample contained 20 seedlings and individual data points as overlays. ns, no statistical difference; one-way ANOVA, Dunnett’s multiple comparison analysis.
Extended Data Fig. 4 Time course analysis of ELENA1 and ORE1 transcripts in individual leaf during nitrogen deficiency (-N) in WT plants.
RT-qPCR analysis of ELENA1 and ORE1 transcripts in various leaves of WT plants Data are means ± SD. n = 3 (biologically independent samples) and individual data points as overlays. The expression level of L1 + N at each indicated day was set at 1.
Extended Data Fig. 5 Genetic information of elena1 mutant.
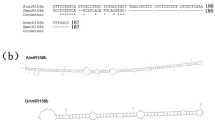
(a) Schematic of ELENA1 genomic loci in WT and elena1. In elena1, the genomic region between -297 and -7 upstream of transcriptional start site of ELENA1 was excised by CAS9. (b) DNA sequencing result of indicated genomic loci in (A) of elena1 aligned to that of WT. (c) ELENA1 expression level of WT and elena1 treated on nitrogen deficient (-N) medium. Data are means ± SD. n = 3 (biologically independent samples). Each sample contained 20 seedlings and individual data points as overlays. Expression level in day 0 WT was set as 1. Asterisks indicate statistically significant difference compared with WT. **P < 0.01; one-way ANOVA, Dunnett’s multiple comparison analysis.
Extended Data Fig. 6 Transcript analysis of ORE1 target genes in shoots of graft chimeras under -N.
RT-qPCR analysis of ORE1 target genes BFN1, RNS3, SAG29, SINA1 and VNI2 in the indicated genotypes treated on +N or -N medium. Data are means ± SD. n = 3 (biologically independent samples). Value of each gene transcript in WT ( + N) was set to 1. Each sample contained 20 seedlings and individual data points as overlays. Asterisks indicate statistically significant difference compared with WT/WT ( + N). ns, no statistical difference.**P < 0.01; two-way ANOVA, Dunnett’s multiple comparison analysis.
Extended Data Fig. 7 Transgenic ELENA1 transcripts are root-to-shoot mobile under nitrogen deficient (-N) condition.
(a) Schematic of primer design for specific detection of transgenic ELENA1. (b) ELENA1 transcripts root-to-shoot mobility assay. Indicated graft chimeras were generated and treated on -N. Shoot and root RNA and gDNA of the graft chimeras were analysed by RT-PCR, no RT PCR, gDNA-PCR and ACT2 correspondingly for 35x PCR cycles. Numbers indicate biological repeat sample. (c) DNA sequencing of the PCR amplicon in RT-PCR reaction in (b).
Extended Data Fig. 8 Comprehensive genetic analysis of MED19a/ORE1/ELENA1 under nitrogen deficient (-N) condition.
(a)-N induced leaf senescence phenotype of the indicated genotypes. Scale bar represents 1 cm. (b) Total chlorophyll content in different leaf groups of the indicated genotypes treated on -N medium. Leaf number, L. FW, fresh weight. Data are means ± SD. n = 3 (biologically independent samples). Each sample contained 20 seedlings and individual data points as overlays. Alphabets indicate statistically significant groups with P < 0.05. One-way ANOVA, Tukey’s multiple comparison analysis.
Extended Data Fig. 9 ELENA1 transcripts show systemic root-to-shoot movement under nitrogen deficiency (-N) is independent of MED19a and ORE1.
ELENA1 expression levels in shoots and roots of WT ( + N), WT (-N), med19a-2 (-N), and ore1 (-N). Data are means ± SD. n = 3 (biologically independent samples). Value of WT ( + N) shoot was set to 1. Each sample contained 20 seedlings and individual data points were shown. ns, no statistical difference; one-way ANOVA, Dunnett’s multiple comparison analysis.
Extended Data Fig. 10 Effects of increasing concentrations of ELENA1 and antisense ELENA1 transcripts on MED19a-ORE1 complex in vitro.
Left panel, sense ELENA1 RNA; Right panel, antisense ELENA1 RNA. Experiment was repeated 3 times with similar results.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and source data for Supplementary Fig. 5.
Supplementary Table
List of primers.
Source data
Source Data Fig. 1
Source data and P values.
Source Data Fig. 2
Source data and P values.
Source data Fig. 3
Source data and P values.
Source Data Fig. 4
Source data and P values.
Source Data Fig. 4
Unmodified gel.
Source Data Extended Data Fig. 1
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Source Data Extended Data Fig. 5
Source data.
Source Data Extended Data Fig. 6
Source data.
Source Data Extended Data Fig. 8
Source data and P values.
Source Data Extended Data Fig. 9
Source data.
Source Data Extended Data Fig. 10
Unmodified gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, S.L.H., Xu, H., Ng, J.H.T. et al. Systemic movement of long non-coding RNA ELENA1 attenuates leaf senescence under nitrogen deficiency. Nat. Plants 9, 1598–1606 (2023). https://doi.org/10.1038/s41477-023-01521-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01521-x