Abstract
Soluble sugars are the core components of fruit quality, and the degree of sugar accumulation is largely determined by tonoplast-localized sugar transporters. We previously showed that two classes of tonoplast sugar transporters, MdERDL6 and MdTST1/2, coordinately regulate sugar accumulation in vacuoles. However, the mechanism underlying this coordination remains unknown. Here we discovered that two transcription factors, MdAREB1.1/1.2, regulate MdTST1/2 expression by binding their promoters in apple. The enhanced MdAREB1.1/1.2 expression in MdERDL6-1-overexpression plants resulted in an increase in MdTST1/2 expression and sugar concentration. Further studies established that MdSnRK2.3, whose expression could be regulated by expressing MdERDL6-1, could interact with and phosphorylate MdAREB1.1/1.2, thereby promoting the MdAREB1.1/1.2-mediated transcriptional activation of MdTST1/2. Finally, the orthologous SlAREB1.2 and SlSnRK2.3 exhibited similar functions in tomato fruit as in their apple counterparts. Together, our findings provide insights into the regulatory mechanism of tonoplast sugar transport exerted by SnRK2.3-AREB1-TST1/2 for fruit sugar accumulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the main text or the Supplementary Information. Additional data related to this study are available from the corresponding author upon request. All biological materials used in this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Zhu, L. et al. MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato. Proc. Natl Acad. Sci. USA 118, e2022788118 (2021).
Ruan, Y. L. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 65, 33–67 (2014).
Wan, H., Wu, L., Yang, Y., Zhou, G. & Ruan, Y. L. Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends Plant Sci. 23, 163–177 (2018).
Braun, D. M. Phloem loading and unloading of sucrose: what a long, strange trip from source to sink. Annu. Rev. Plant Biol. 73, 553–584 (2022).
Wen, S., Neuhaus, H. E., Cheng, J. & Bie, Z. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 73, 2275–2289 (2022).
Lecourieux, F. et al. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 65, 821–832 (2014).
Wang, Z. et al. Heterologous expression of the apple hexose transporter MdHT2.2 altered sugar concentration with increasing cell wall invertase activity in tomato fruit. Plant Biotechnol. J. 18, 540–552 (2020).
Ren, Y. et al. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell 33, 1554–1573 (2021).
Jung, B. et al. Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat. Plants 1, 14001 (2015).
Deng, J. et al. The calcium sensor CBL2 and its interacting kinase CIPK6 are involved in plant sugar homeostasis via interacting with tonoplast sugar transporter TST2. Plant Physiol. 183, 236–249 (2020).
Wingenter, K. et al. A member of the mitogen-activated protein 3-kinase family is involved in the regulation of plant vacuolar glucose uptake. Plant J. 68, 890–900 (2011).
Vu, D. P. et al. Vacuolar sucrose homeostasis is critical for plant development, seed properties, and night-time survival in Arabidopsis. J. Exp. Bot. 71, 4930–4943 (2020).
Hedrich, R., Sauer, N. & Neuhaus, H. E. Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins. Curr. Opin. Plant Biol. 25, 63–70 (2015).
Rodrigues, C. M. et al. Vernalization alters sink and source identities and reverses phloem translocation from taproots to shoots in sugar beet. Plant Cell 32, 3206–3223 (2020).
Zhang, Q. et al. Evolutionary expansion and functional divergence of sugar transporters in Saccharum (S. spontaneum and S. officinarum). Plant J. 105, 884–906 (2020).
Aluri, S. & Büttner, M. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc. Natl Acad. Sci. USA 104, 2537–2542 (2007).
Poschet, G. et al. A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds. Plant Physiol. 157, 1664–1676 (2011).
Schneider, S. et al. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. 14, 325–336 (2012).
Klemens, P. A. et al. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 163, 1338–1352 (2013).
Guo, W. J. et al. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 164, 777–789 (2014).
Peng, Q. et al. The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple. BMC Plant Biol. 20, 191 (2020).
Furihata, T. et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl Acad. Sci. USA 103, 1988–1993 (2006).
Li, M. et al. Proteomic analysis reveals dynamic regulation of fruit development and sugar and acid accumulation in apple. J. Exp. Bot. 67, 5145–5157 (2016).
Liu, Z. et al. Plasma membrane CRPK1-mediated phosphorylation of 14-3-3 proteins induces their nuclear import to fine-tune CBF signalling during cold response. Mol. Cell 66, 117–128 (2017).
Ruan, Y. L., Patrick, J. W. & Brady, C. Protoplast hexose carrier activity is a determinate of genotypic difference in hexose storage in tomato fruit. Plant Cell Environ. 20, 341–349 (1997).
Ren, Y. et al. A tonoplast sugar transporter underlies a sugar accumulation QTL in watermelon. Plant Physiol. 176, 836–850 (2018).
Wipf, D. et al. Identification of putative interactors of Arabidopsis sugar transporters. Trends Plant Sci. 26, 13–22 (2021).
Mou, W. et al. SlAREB1 transcriptional activation of NOR is involved in abscisic acid-modulated ethylene biosynthesis during tomato fruit ripening. Plant Sci. 276, 239–249 (2018).
Rook, F., Hadingham, S. A., Li, Y. & Bevan, M. W. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 29, 426–434 (2006).
Ma, Q. J. et al. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 174, 2348–2362 (2017).
Yakir, E. et al. MaMADS2 repression in banana fruits modifies hormone synthesis and signalling pathways prior to climacteric stage. BMC Plant Biol. 18, 267 (2018).
Fujita, Y. et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 50, 2123–2132 (2009).
Fujita, Y., Yoshida, T. & Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 147, 15–27 (2013).
Yang, J. et al. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 141, 164–171 (2019).
Wang, Z. et al. Variation in the promoter of the sorbitol dehydrogenase gene MdSDH2 affects binding of the transcription factor MdABI3 and alters fructose content in apple fruit. Plant J. 109, 1183–1198 (2022).
Fürtauer, L., Weckwerth, W. & Nagele, T. A benchtop fractionation procedure for subcellular analysis of the plant metabolome. Front. Plant Sci. 7, 1912 (2016).
Beshir, W. F. et al. Non-aqueous fractionation revealed changing subcellular metabolite distribution during apple fruit development. Hortic. Res. 6, 98 (2019).
Zhang, L. et al. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5). Plant Physiol. 188, 2059–2072 (2022).
Ma, S. et al. F-box protein MdAMR1L1 regulates ascorbate biosynthesis in apple by modulating GDP-mannose pyrophosphorylase. Plant Physiol. 188, 653–669 (2022).
Jia, M. et al. SnRK2 subfamily I protein kinases regulate ethylene biosynthesis by phosphorylating HB transcription factors to induce ACO1 expression in apple. New Phytol. 234, 1262–1277 (2022).
Liu, C., Yu, H., Rao, X., Li, L. & Dixon, R. A. Abscisic acid regulates secondary cell-wall formation and lignin deposition in Arabidopsis thaliana through phosphorylation of NST1. Proc. Natl Acad. Sci. USA 118, e2106367118 (2021).
Acknowledgements
We thank J. Zhang and J. Zhao (Horticulture Science Research Center, Northwest A&F University, Yangling, China) for providing professional technical assistance with GC–MS analysis; H. Zhao and F. Yuan (State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University, Yangling, China) and Y. Yuan and R. Chen (Horticulture Science Research Center, Northwest A&F University, Yangling, China) for providing confocal microscopy experimental assistance. This work was supported by the Program for the National Natural Science Foundation of China (31872043) to M.L., the Shaanxi Science and Technology Innovation Team Project (2022TD-18) to M.L., the Australian Research Council (DP180103834) to Y.-L.R., and the Earmarked Fund for the China Agriculture Research System (CARS-27) to F.M.
Author information
Authors and Affiliations
Contributions
L.Z., M.L., F.M. and Y.-L.R. designed this research. L.Z., Y.L., C.W., Z.W., J.S., Y.P., B.L., W.C. and B.M. performed the experiments. L.Z., J.S., Y.L., M.L. and Y.-L.R. analysed the data. L.Z., M.L. and Y.-L.R. wrote the paper. M.L. and F.M. supervised the study. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Jintao Cheng, Li-Qing Chen and H. Ekkehard Neuhaus for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Relative distribution of compartment-specific marker enzyme activities and subcellular sugar concentration in MdERDL6-1 overexpressed plants using NAF method.
a, b, Relative distribution of compartment-specific markers in MdERDL6-1 overexpressed apple leaves (a) and tomato fruits (b). Tissues were fractionated using a NAF procedure and the activities of marker enzymes in the five fractions were determined. Data are expressed as percentage activities in each fraction. AGPase, UGPase, and ACP were used as plastidic, cytosolic, and vacuolar markers, respectively. c, d, Subcellular concentrations of Glc, Fru, and Suc in plastid, cytosol, and vacuole fractions of MdERDL6-1 overexpressed apple leaves (c) and tomato fruits (d). nd, no data. The bars represent the mean value ± SD (n = 3 independent biological replicates). The asterisks in (c,d) indicate significant differences as assessed by Student’s t-test (two-sided). ***P < 0.001, **P < 0.01, *P < 0.05, ns, not significant.
Extended Data Fig. 2 The screening of candidate transcription factors.
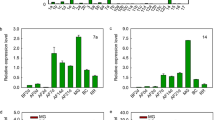
a, Differentially expressed transcription factors in the RNA-seq of MdERDL6-1 transgenic apples. The threshold was set as: RPKM > 1, fold change > 1.5. b, c, Predicted active transcription factors regulate MdTST1 (b) and MdTST2 (c) expression based on ATAC-seq in apple fruit. d, The ABRE element analysis on MdTST1/2 promoters. e, qRT-PCR validation of three differential transcription factors in the RNA-seq of MdERDL6-1 transgenic apples. The transcript levels were normalized with those of MdActin in transgenic apple. Relative expression levels for each gene were obtained via ddCT method, with the expression in wild type (WT) set as ‘1’.The bars represent the mean value ± SD (n = 3 seedlings). The asterisks in (e) indicate significant differences as assessed by Student’s t-test (two-sided). ***P < 0.001, **P < 0.01, ns, not significant.
Extended Data Fig. 3 The gene expression levels and promoter activities of MdSnRK2.3 and MdAREB1.1/1.2 after sugars feeding in apple calli.
a, b, The impacts of sugars feeding on MdSnRK2.3 and MdAREB1.1/1.2 expression levels. Apple calli was cultured on MS liquid medium with 2% different exogenous sugars (Water was used as control) and different concentrations of Glc for 24 h, and the gene expression levels were detected by qRT-PCR. The transcript levels were normalized with those of MdActin. Relative expression level was obtained via ddCT method, setting the expression level in water feeding as ‘1’. c, d, The impacts of sugars feeding on MdAREB1.1/1.2 promoter activities. Apple calli was cultured on MS liquid medium with different exogenous sugars and different concentrations of Glc for 24 h after infiltrating with Agrobacterium harboring MdAREB1.1/1.2pro-GUS plasmids. The treated and control apple calli were harvested for GUS activity assay after 24 h feeding. The 1924bp and 1937bp promoters of MdAREB1.1 and MdAREB1.2 were used in the GUS experiments. Man: mannose; Glc: glucose; Fru: fructose; Suc: sucrose; and Sor: sorbitol. Bars represent the mean value ± SD (n = 3 independent biological replicates). Different letters indicate significant differences as assessed by one-way ANOVA (Tukey’s test), respectively (P < 0.05).
Extended Data Fig. 4 Phylogenetic analysis, subcellular localization, self-activation assay and expression profiles of MdAREB1.1/1.2.
a, Phylogenetic tree of MdAREBs from apple and the orthologous genes from Arabidopsis and tomato. The AREB orthologous sequences were collected to build an un-rooted phylogenetic tree using the neighbour-joining method of the MEGA7 software. A 1000 trial of bootstrap analysis was used to provide confident estimates for phylogenetic tree topology. The colored circles are studied genes in this research. The scale bar corresponds to 0.05. b, Nuclear localization of MdAREB1.1/1.2. 35Spro:MdAREB1.1/1.2-GFP were transiently expressed in protoplasts of Arabidopsis leaves. DAPI served as a nuclear dye. The GFP signals of MdAREB1.1/1.2 overlapped with DAPI, indicating that MdAREB1.1/1.2 localized in the nuclear. Scale bars, 20 μm. c, The transcriptional self-activation assay of MdAREB1.1/1.2. The pGBKT7-MdAREB1.1/1.2 and empty pGBKT7 vector (negative control) were introduced into the yeast strain Y2H respectively, and the transformed cells were spotted on SD-Trp and SD-Trp-His-Leu+x-α-Gal medium. The plates were incubated at 30 °C for 3 d. d, The heat map of MdAREBs expression levels based on RNA-seq in mature leaves (ML) and five stages of developing fruits (S1-S5). Fold difference is designated as a log2 value, with the data in S1 set as 1. The experiments in (b, c) were repeated independently at least three times, with similar results.
Extended Data Fig. 5 Impacts of altering MdAREB1.1 or MdAREB1.2 expression on MdTST1/2 expression levels and sugar concentrations in ‘Orin’ apple calli.
a, The complete coding sequences of MdAREB1.1 or MdAREB1.2 were inserted into the pMDC83 vector for gene stable overexpression (OE-MdAREB1.1#1, #2; OE-MdAREB1.2#1, #2), while the specific cDNA fragments were cloned into pK7GWIWG2 vector for gene stable silencing (PK7-MdAREB1.1#1, #2; PK7-MdAREB1.2#1, #2). The empty vectors pMDC83 and PK7 served as controls respectively. b, The mRNA relative expression levels of MdAREB1.1/1.2 and MdTST1/2 in transgenic apple calli, setting that from pMDC83 as ‘1’. c, The sugar concentrations (Fru, fructose; Glc, glucose; Suc, sucrose) in transgenic apple calli. The bars represent the mean value ± SD (n = 3 independent biological replicates). Different letters indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (P < 0.05).
Extended Data Fig. 6 Phylogenetic tree, expression profiles, and subcellular localization analysis of MdSnRK2s.
a, Phylogenetic analysis of MdSnRK2s from apple and the orthologous genes from Arabidopsis and tomato. The SnRK2s orthologous sequences were collected to build an un-rooted phylogenetic tree using the neighbour-joining method of the MEGA7 software, which was obviously clustered into three subclasses. A 1000 trial of bootstrap analysis was used to provide confident estimates for phylogenetic tree topology. The colored circles are studied genes in this research. The scale bar corresponds to 0.05. b, The heat map of MdSnRK2s expression levels based on RNA-seq in MdERDL6-1 overexpressing apple lines. Fold difference is designated as a log2 value, with the data in WT set as 1. c, The protein abundances (quantified proteins) of MdSnRK2.1/2.3/2.5 are extracted from tandem mass tags (TMT) quantitative proteomics of five developing stages of apple fruits. The bars represent the mean value ± SD (n = 3 independent biological replicates). d, Subcellular localization of MdSnRK2.3-GFP fusion protein in the protoplasts from Arabidopsis leaves. GFP, green fluorescent protein. DAPI (4’,6-diamidino-2-phenylindole) was used to stain the nucleus. Auto, the red auto fluorescence of chloroplasts. Bar = 20 μm. The experiments in (d) were repeated independently at least three times, with similar results.
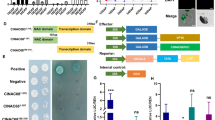
Extended Data Fig. 7 Y1H and dual-luciferase assay analysis of MdAREB1.1/1.2 and SlAREB1.2 binding to their own promoters.
a, b, Yeast one-hybrid assays showed that MdAREB1.1/1.2 and SlAREB1.2 bound to their own promoters containing ABRE cis-elements. Schematic diagram of P1 and P2 truncation promoters with or without ABRE cis-elements, the vertical lines represent ABRE cis-elements (a). The positive controls were pAbAi-P53 and pGADT7-53. The screening concentration of AbA were 150 ng/mL, 180 ng/mL, and 200 ng/mL of MdAREB1.1, MdAREB1.2, and SlAREB1.2 promoters respectively. Each colony was dissolved in 5 μL sterile NaCl and then diluted to 10−1 to 10−3. c, Dual-luciferase assays in tobacco (N. benthamiana) leaves revealed that MdAREB1.1/1.2 and SlAREB1.2 binding to their own promoters. The left are the dual-luciferase signal imagings, and the right are dual-luciferase activity assays. The 1924bp and 1937bp promoters of MdAREB1.1 and MdAREB1.2, 1911bp promoter of SlAREB1.2 were used in this assay. The positive controls are pGreenII 0800-LUC-P53 and pGreenII 62-SK-53. The experiments were repeated independently at least three times, with similar results.
Extended Data Fig. 8 Impacts of altering SlAREB1.2 expression on SlTST1/2 expression levels and sugar concentrations in tomato fruits.
a, The fruits of transgenic tomato lines (overexpression lines: OE#2, OE#5; silence lines: PK7#1, PK7#3). b, c, The relative expression levels of SlAREB1.2 and SlTST1/2 in the transgenic tomato measured using qRT-PCR and normalized to those of SlActin, setting that from WT as ‘1’. d, e, The soluble solids contents and carbohydrate levels in ripening fruits of transgenic tomato. Glc, glucose; Fru, fructose; Suc, sucrose. The bars represent the mean value ± SD (n = 3 independent biological replicates). The asterisks indicate significant differences as assessed by Student’s t-test (two-sided). ***P < 0.001, **P < 0.01, *P < 0.05.
Extended Data Fig. 9 Impacts of VIGS-induced gene silencing of SlAREB1.2 on SlTST1/2 expression levels and sugar concentrations in MdERDL6-1 overexpressing tomato fruits.
a, The Agrobacterium containing the resulting vectors pTRV2-SlAREB1.2 and pTRV1 were mixed in equal proportions, which were used for injection in two MdERDL6-1 overexpressing tomato lines (OE1-pTRV#SlAREB1.2 and OE2-pTRV#SlAREB1.2), with empty pTRV2 and pTRV1 mixture for injection as controls (WT, OE-1, and OE-2). The arrows indicate the injection sites in fruits. b, The mRNA relative expression levels of SlAREB1.2 and SlTST1/2 in transgenic tomato fruits of wild-type and MdERDL6-1 overexpressed background, setting that from WT as ‘1’. c, The sugar concentrations (Fru, fructose; Glc, glucose; Suc, sucrose) in transgenic tomato fruits of wild-type and MdERDL6-1 overexpressed background. The bars represent the mean value ± SD (n = 3 independent biological replicates). Different letters indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (P < 0.05).
Extended Data Fig. 10 Impacts of VIGS-induced gene silencing of SlSnRK2.3 on SlTST1/2 expression levels and sugar concentrations in MdERDL6-1 overexpressing tomato fruits.
a, The Agrobacterium containing the resulting vectors pTRV2-SlSnRK2.3 and pTRV1 were mixed in equal proportions, which were used for injection in two MdERDL6-1 overexpressing tomato lines (OE1-pTRV#SlSnRK2.3 and OE2-pTRV#SlSnRK2.3), with empty pTRV2 and pTRV1 mixture for injection as controls (WT, OE-1, and OE-2). The arrows indicate the injection sites in fruits. b, The mRNA relative expression levels of SlSnRK2.3, SlAREB1.2 and SlTST1/2 in transgenic tomato fruits of wild-type and MdERDL6-1 overexpressed background, setting that from WT as ‘1’. c, The sugar concentrations (Fru, fructose; Glc, glucose; Suc, sucrose) in transgenic tomato fruits of wild-type and MdERDL6-1 overexpressed background. The bars represent the mean value ± SD (n = 3 independent biological replicates). Different letters indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (P < 0.05).
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1–6.
Source data
Source Data Fig. 1
Statistical source data and unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots and gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, L., Li, Y., Wang, C. et al. The SnRK2.3-AREB1-TST1/2 cascade activated by cytosolic glucose regulates sugar accumulation across tonoplasts in apple and tomato. Nat. Plants 9, 951–964 (2023). https://doi.org/10.1038/s41477-023-01443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01443-8
This article is cited by
-
Theme and variations
Nature Plants (2023)