Abstract
Cold stress negatively affects maize (Zea mays L.) growth, development and yield. Metabolic adjustments contribute to the adaptation of maize under cold stress. We show here that the transcription factor INDUCER OF CBF EXPRESSION 1 (ZmICE1) plays a prominent role in reprogramming amino acid metabolome and COLD-RESPONSIVE (COR) genes during cold stress in maize. Derivatives of amino acids glutamate/asparagine (Glu/Asn) induce a burst of mitochondrial reactive oxygen species, which suppress the cold-mediated induction of DEHYDRATION RESPONSE ELEMENT-BINDING PROTEIN 1 (ZmDREB1) genes and impair cold tolerance. ZmICE1 blocks this negative regulation of cold tolerance by directly repressing the expression of the key Glu/Asn biosynthesis genes, ASPARAGINE SYNTHETASEs. Moreover, ZmICE1 directly regulates the expression of DREB1s. Natural variation at the ZmICE1 promoter determines the binding affinity of the transcriptional activator ZmMYB39, a positive regulator of cold tolerance in maize, resulting in different degrees of ZmICE1 transcription and cold tolerance across inbred lines. This study thus unravels a mechanism of cold tolerance in maize and provides potential targets for engineering cold-tolerant varieties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The mass spectrometry raw data have been released in the Metabolights database (https://www.ebi.ac.uk/metabolights/MTBLS4404). Data sets including RNA-seq and ChIP-seq and detailed information can be viewed and downloaded from http://www.ncbi.nlm.nih.gov/sra/ under accession PRJNA779257. Source data are provided with this paper.
Code availability
The custom codes used in this study are deposited in GitHub (https://github.com/fudiyi/code-for-publication).
References
Ding, Y. L., Shi, Y. T. & Yang, S. H. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 222, 1690–1704 (2019).
Chinnusamy, V., Zhu, J. & Zhu, J. K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451 (2007).
Ma, Y. et al. COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221 (2015).
Guo, X. Y., Liu, D. F. & Chong, K. Cold signaling in plants: insights into mechanisms and regulation. J. Integr. Plant Biol. 60, 745–756 (2018).
Liu, J. Y., Shi, Y. T. & Yang, S. H. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 60, 780–795 (2018).
Shi, Y., Ding, Y. & Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637 (2018).
Song, Y. et al. The direct targets of CBFs: in cold stress response and beyond. J. Integr. Plant Biol. 63, 1874–1887 (2021).
Jia, Y. et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 212, 345–353 (2016).
Chinnusamy, V. et al. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17, 1043–1054 (2003).
Tang, K. et al. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 62, 258–263 (2020).
Ding, Y. et al. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 32, 278–289 (2015).
Li, H. et al. MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 43, 630–642 (2017).
Ye, K. et al. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 31, 2682–2696 (2019).
Zhang, C. Y. et al. OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev. Cell 43, 731–743 (2017).
Chen, X. X. et al. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 63, 53–78 (2021).
Allen, D. J. & Ort, D. R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42 (2001).
Riva-Roveda, L., Escale, B., Giauffret, C. & Perilleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 16, 212 (2016).
Sanchez, B., Rasmussen, A. & Porter, J. R. Temperatures and the growth and development of maize and rice: a review. Glob. Change Biol. 20, 408–417 (2014).
Fracheboud, Y., Ribaut, J. M., Vargas, M., Messmer, R. & Stamp, P. Identification of quantitative trait loci for cold-tolerance of photosynthesis in maize (Zea mays L.). J. Exp. Bot. 53, 1967–1977 (2002).
Presterl, T. et al. Quantitative trait loci for early plant vigour of maize grown in chilly environments. Theor. Appl. Genet. 114, 1059–1070 (2007).
Leipner, J., Jompuk, C., Camp, K. H., Stamp, P. & Fracheboud, Y. QTL studies reveal little relevance of chilling-related seedling traits for yield in maize. Theor. Appl. Genet. 116, 555–562 (2008).
Zhang, H. et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 20, 333 (2020).
Zeng, R. et al. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat. Commun. 12, 4713 (2021).
Qin, F. et al. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 45, 1042–1052 (2004).
Li, Z. et al. The transcription factor bZIP68 negatively regulates cold tolerance in maize. Plant Cell 34, 2833–2851 (2022).
Guy, C., Kaplan, F., Kopka, J., Selbig, J. & Hincha, D. K. Metabolomics of temperature stress. Physiol. Plant. 132, 220–235 (2008).
Kaplan, F. et al. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 136, 4159–4168 (2004).
Maruyama, K. et al. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol. 164, 1759–1771 (2014).
Cook, D., Fowler, S., Fiehn, O. & Thomashow, M. F. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl Acad. Sci. USA 101, 15243–15248 (2004).
Zhu, J. H., Dong, C. H. & Zhu, J. K. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr. Opin. Plant Biol. 10, 290–295 (2007).
Kaplan, F. et al. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 50, 967–981 (2007).
Hannah, M. A. et al. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol. 142, 98–112 (2006).
Xu, G. H. et al. Evolutionary metabolomics identifies substantial metabolic divergence between maize and its wild ancestor, teosinte. Plant Cell 31, 1990–2009 (2019).
Yang, X. H. et al. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 28, 511–526 (2011).
Yang, N. et al. Genome wide association studies using a new nonparametric model reveal the genetic architecture of 17 agronomic traits in an enlarged maize association panel. PLoS Genet. 10, e1004573 (2014).
Li, Q. et al. Genome-wide association studies identified three independent polymorphisms associated with alpha-tocopherol content in maize kernels. PLoS ONE 7, e36807 (2012).
Feng, F. et al. OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell 30, 375–396 (2018).
Gaufichon, L., Reisdorf-Cren, M., Rothstein, S. J., Chardon, F. & Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 179, 141–153 (2010).
Martin, A. et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 18, 3252–3274 (2006).
Majumdar, R. et al. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front. Plant Sci. 7, 78 (2016).
Xiao, S. L. et al. Activation of mitochondrial orf355 gene expression by a nuclear-encoded DREB transcription factor causes cytoplasmic male sterility in maize. Mol. Plant 13, 1270–1283 (2020).
Li, H. et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 45, 43–50 (2013).
Zhang, Y. et al. Differentially regulated orthologs in sorghum and the subgenomes of maize. Plant Cell 29, 1938–1951 (2017).
Domon, J. M. et al. Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation. Phytochemistry 85, 51–61 (2013).
Dong, N. Q. & Lin, H. X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 63, 180–209 (2021).
Huang, J. L. et al. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 153, 1526–1538 (2010).
Forde, B. G. & Lea, P. J. Glutamate in plants: metabolism, regulation, and signalling. J. Exp. Bot. 58, 2339–2358 (2007).
Mazzucotelli, E., Tartari, A., Cattivelli, L. & Forlani, G. Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 57, 3755–3766 (2006).
Cao, J. J., Li, M. Y., Chen, J., Liu, P. & Li, Z. Effects of MeJA on Arabidopsis metabolome under endogenous JA deficiency. Sci. Rep. 6, 37674 (2016).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 3, 211–221 (2007).
Xia, J. G., Psychogios, N., Young, N. & Wishart, D. S. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37, W652–W660 (2009).
Fu, J. J. et al. RNA sequencing reveals the complex regulatory network in the maize kernel. Nat. Commun. 4, 2832 (2013).
Yu, J. M. et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38, 203–208 (2006).
Zhang, Z. W. et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42, 355–360 (2010).
Bradbury, P. J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007).
Ihaka, R. & Gentleman, R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5, 299–314 (1996).
Xing, H. L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Sidorov, V. & Duncan, D. Agrobacterium-mediated maize transformation: immature embryos versus callus. Methods Mol. Biol. 526, 47–58 (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{-{{\Delta\Delta}{c}{\rm{T}}}}\)2−ΔΔCT method. Methods 25, 402–408 (2001).
Mao, H. D. et al. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 6, 8326 (2015).
Yin, B. J., Li, T. T., Zhang, S. R., Li, Z. & He, P. L. Sensitive analysis of 33 free amino acids in serum, milk, and muscle by ultra-high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Anal. Methods 9, 2814–2823 (2016).
Tian, T. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129 (2017).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008).
Machanick, P. & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011).
Acknowledgements
We thank J Zuo, F Qin and C Jiang for helpful discussion. The transgenic seeds of maize were created by the Center for Crop Functional Genomics and Molecular Breeding of China Agricultural University. This work was supported by grants from the National Key Research and Development Project (2020YFA0509902), the National Natural Science Foundation of China (32022008, 31700214, 31921001) and the National Science Foundation of Tianjin (19JCYBJC29500).
Author information
Authors and Affiliations
Contributions
S.Y. conceived the project. Y.T.S. and S.Y. designed the experiments. H.J., Y.T.S. and J.Y.L. performed most of the experiments. Z.L. and Z.Y. performed amino acid measurement. Y.L.S. performed the maize transformation. X.Y., D.F., S.W. and M.L. performed GWAS, RNA-seq and ChIP-seq analyses. X.Y. and J.S.L. provided the inbred population. All authors analysed the data. Y.T.S., J.H. and S.Y. wrote the manuscript with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Baoxing Song and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Analysis of metabolic profiling under cold stress in maize young seedlings.
a, Heatmap displaying the log10 value of differentially accumulated metabolites before or after cold treatment (4 °C, 12 h) in maize varieties. b, Metabolic pathway analysis plot created using MetaboAnalyst 4.0. Plots depict several metabolic pathway alterations induced by cold treatment. The x-axis represents the pathway impact value computed from pathway topological analysis, and the y-axis is the -log10 of the P-value obtained from pathway enrichment analysis. The pathways that were most substantially changed are characterized by both a high -log(P) value and high impact value (top right region). c, Distribution of mGWAS signals across the maize genome under warm temperature.
Extended Data Fig. 2 Characterization of ZmICE1-overexpression transgenic plants and zmice1 mutants.
a, Schematics of mutations in the coding region of ZmICE1. CRISPR/Cas9 technology was used to generate two mutant alleles, zmice1-1 (deleting 19 bp) and zmice1-2 (deleting 44 bp). Dots indicate deleted bases. b, The calculation method of relative injury percentage of the maize seedlings after cold treatment. c-d, Cold phenotypes (c) and leaf injury (d) of transgenic lines of zmice1-1×zmice1-2 (F1 plants generated by crossing zmice1-1 with zmice1-2). V2 seedlings were treated with or without at 4 °C for 4 d. Shown in (d) are means ± s.d. from 3 biological replicates (n = 5 plants for each replicate). P-values are from two-sided t-test. e, Growth phenotype of the zmice1-1 mutant. WT and zmice1-1 were grown at green house for 4 weeks. f, Relative expression levels of ZmICE1 in WT and ZmICE1-overexpression seedlings. Total RNAs were extracted from 12-day-old seedlings. g, ZmICE1 gene expression level in various tissues. h, ZmICE1 expression in V2-stage maize seedlings under cold stress. Data are means ± s.d. from 3 biologically independent samples. P-values shown are from two-sided t-test. i, Subcellular localization of ZmICE1-GFP in maize protoplasts and ZmICE1-GFP plants. ZmICE1-GFP was transformed into maize protoplasts, and GFP was used as a control. The roots of ZmICE1-GFP plants were treated with or without 4 °C. GFP signals were visualized by laser confocal-scanning microscope Leica sp5. Scale bars, 10 μm. j, Immunoblotting analysis of ZmICE1-GFP nuclear protein in maize protoplasts with or without 4 °C treatment. ZmICE1 was detected with anti-GFP antibody (1:3000). Anti-H3 antibody (1:10000) was used as a loading control. Relative binding intensity was quantified with ImageJ software. Data in f, g and h are mean values ± s.d. from 3 biologically independent samples; P-values are from two-sided t-test. The experiments in f, g, h, i and j were independently performed three times with similar results.
Extended Data Fig. 3 Identification of direct targets of ZmICE1 by RNA-seq and Chip-seq.
a, Functional classification of putative ZmICE1 target genes using gene ontology (GO) annotations. The color scale represents the −Log10 of false discovery rate (FDR) values. b, Distributions of ZmICE1 binding sites for COR genes-associated loci, as shown in Integrated Genome Browser. Blue lines (WT) or red lines (ZmICE1-GFP) indicate notable peaks calculated by MACS2. The bottom track indicates the gene’s direction on the chromosome. Blue bars indicate peaks of ZmICE1. c, Transcript abundance of COR genes in wild type and zmice1-1 in FPKM. d, Expression of ZmDREB1s in WT and zmice1 mutants treated at 4 °C for 0 h and 12 h. Data are means ± s.d. from 3 biologically independent samples; P-values shown are from two-sided t-test. The experiments were independently performed three times with similar results.
Extended Data Fig. 4 ZmICE1 participates in the regulation of amino acid metabolism pathway.
a, The GO terms and KEGG pathway enrichment analysis of ZmICE1-regulated metabolism under normal condition. Cutoff value is FDR < 0.05. b, Analysis of free amino acid contents in the leaves of V2-stage young WT and zmice1-2 seedlings with or without cold treatment at 4 °C for 12 h. Data are means ± s.d. from 4 biologically independent samples. Different letters represent differences (P < 0.05) determined by one-way ANOVA with Tukey’s multiple comparisons test. The experiment were independently performed twice with similar results.
Extended Data Fig. 5 The effect of cold stress on the content of endogenous amino acids in maize.
a, The -log10 (peak area) value of free amino acids evaluated by LC-MS/MS before or after cold treatment (4 °C, 12 h) in maize association panel. The plots shown are means ± s.d. (n is shown in figures; two-sided t-test). b, Relative expression of ZmAS3 and ZmGS1-3 in WT, ZmAS3- and ZmGS1-3-overexpression seedlings. Data are means ± s.d. from 3 biologically independent samples. P-values shown are from two-sided t-test. The experiments were independently performed three times with similar results. c, Relative leaf injury of cold-treated seedlings without or with application of Leu or Val. Maize seedlings at V2 stage were treated with 20 mM Leu or Val for 12 h, followed by 4 °C treatment for 2 d. Data are means ± s.d. from 3 biological replicates (n = 5 plants for each replicate). P-values are from two-sided t-test. d, Relative amount of endogenous amino acids in maize seedlings with exogenously applied Asn and Glu at 20 mM. Data are means ± s.d. from 4 biologically independent samples. P-values shown are from two-sided t-test.
Extended Data Fig. 6 SNP sites associated with amino acid content and evolution of haplotypes of maize inbred lines.
a, Quantile-quantile (Q–Q) plots with -log10 P-values of Thr, Asn, Lys and Ser, 95% confidence interval (blue shading) for linear regression. b, Association plot for Ser content is shown for the region at 156.0–158.0 Mb on chromosome 3 (x axis). Negative log10-transformed P values from the compressed mixed linear model are plotted on the y axis. The horizontal dashed line indicates the genome-wide significance threshold. The locations of the predicted open reading frame in B73 genome are indicated by color boxes. The 9 annotated genes associated with Ser content are listed in the left panel. c, Conditional association analysis. Top is the non-conditioned plot and the bottom is the association plot after conditioning on the lead SNP. d, Phylogenetic tree of inbred lines. All SNPS in the ZmICE1 gene were used to construct the maximum-likelihood tree by MEGA5.0. Bootstrap value is indicated on the tree. The HapA inbred lines (12 total) were highlighted in red. Others (177 lines) are HapB. e, Violin plot of expression levels of ZmDREB1s in maize inbred lines of HapA and HapB. Shown are the log2 of FPKM values from RNA-seq data in developing kernels. P-values are from two-sided student t-test.
Extended Data Fig. 7 ROS burst in zmice1 mutant, ZmAS3-OE and ZmGS1-3-OE with and without cold treatment.
a-c, Histochemical staining by NBT (nitroblue tetrazolium) and DAB (3′,3′-diaminobenzidine) of leaves of WT, zmice1-1 (a), ZmAS3-OE (b), and ZmGS1-3-OE (c) seedlings with or without cold treatment for 12 h. Representative images of leaf histochemical staining are shown from three independent experiments. Scale bars, 1 mm.
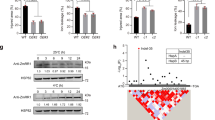
Extended Data Fig. 8 Co-expression analysis identifies ZmMYB39 as a positive regulator of ZmICE1.
a, Transient dual-LUC expression assay in maize protoplasts. The LUC reporter was driven by 1.5-kb ZmICE1 promoter fragments from inbred lines SK (HapA) and two mutated forms (substitution of the nucleotide at position −576 from T to A or C. Data are means ± s.d. from 4 biological replicates. P-values shown are from two-sided t-test. b, Alignment of ZmICE1 promoter sequences from Zheng58 and SK. The positions of SNP were labelled with blue arrows. MRE indicates MYB Recognition Element (AACCTAA). c, Interaction network of ZmICE1-related genes in maize. d, The maximum-likelihood tree based on protein sequences of ZmMYB39 homologues in plant species. e, Expression of ZmMYB39, ZmMYB41 and ZmICE1 in WT under cold stress. f-g, Expression of ZmMYB39 and ZmMYB41 in WT and overexpression seedlings. h, Cumulative germination rates of ZmMYB39-OE seeds when subjected to cold stress. Data are means ± s.d. from 3 biological replicates (n = 32/34/35 seeds in in each independent experiment; * P < 0.05, two-sided t-test). i, Leaf injury of ZmMYB41-OE plants. Data are means ± s.d. from 3 biological replicates (n = 5 plants for each replicate). The P-values are from two-sided t-test. j, Expression of ZmICE1 in WT and ZmMYB41-OE lines. k, Schematics of mutation in the coding region of ZmMYB39 generated by CRISPR/Cas9. (l-m) Cold tolerance phenotype (l) and leaf injury percentage (m) of zmmyb39. Shown in (m) are means ± s.d. from 3 biological replicates (n = 5 plants for each replicate). P-values are from two-sided t-test. (n) EMSA showing the association of ZmMYB39 with the ZmICE1 promoter. The intensity of the shifted band was quantified by ImageJ software, and the relative intensity is shown below the quantified band. Three independent experiments were done with the similar results. In e, f, g and j, data are means ± s.d. from 3 biologically independent samples. The P-values shown are from two-sided t-test. These experiments were independently performed three times with similar results.
Extended Data Fig. 9 Kernel phenotypes of ZmICE1-overexpression lines.
Maize plants were grown under optimal nutrition conditions and harvested at maturity in three locations. 5 biological replicates were performed with similar results. n is shown in figures, The P-values shown are from two-sided t-test.
Extended Data Fig. 10 Effect of Glu and Arg on cold tolerance differ in plant species.
a, Freezing phenotypes of 12-day-old Arabidopsis seedlings treated with amino acids. The seedlings were grown under a 16-h-white light/8-h-dark photoperiod at 22 °C and then sprayed with 20 mM amino acids for 12 h before being exposed to freezing treatment. At least three plates were treated in each treatment. Representative photographs were taken after a 3-day recovery period. b, Cold tolerance phenotypes in different species with exogenous application of 20 mM Glu. c, Expression of DREB1B and DREB1A genes in Arabidopsis treated at 4 °C for 3 h. 2-week-old plants grown on 1/2 MS plates at 22 °C were sprayed with 20 mM Glu and Arg for 6 h followed by a 4 °C treatment of 3 h. The qPCR data were normalized to the mock. Data are means ± s.d. from 3 biologically independent samples. P-values shown are from two-sided t-test. ACTIN gene was used as internal reference. The experiments were independently performed three times with similar results. d, Relative amount of endogenous amino acids in Arabidopsis seedlings after sprayed with 20 mM Glu. The 12-day-old plants grown on 1/2 MS plates at 22 °C were sprayed with 20 mM Glu. Materials were collected at 12 h after spray to detected free amino acid content. Data are means ± s.d. from 3 biologically independent samples. P-values shown are from two-sided t-test. Experiments were independently performed twice with similar results. (e) Difference of Glu metabolic pathway in Arabidopsis and maize.
Supplementary information
Supplementary Table
List of primer sequences used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data and unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 8
Statistical source data and unprocessed western blots for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Statistical source data for Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Statistical source data for Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, H., Shi, Y., Liu, J. et al. Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat. Plants 8, 1176–1190 (2022). https://doi.org/10.1038/s41477-022-01254-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01254-3