Abstract
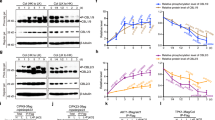
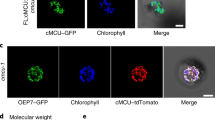
Potassium (K) is an essential nutrient, but levels of the free K ions (K+) in soil are often limiting, imposing a constant stress on plants. We have discovered a calcium (Ca2+)-dependent signalling network, consisting of two calcineurin B-like (CBL) Ca2+ sensors and a quartet of CBL-interacting protein kinases (CIPKs), which plays a key role in plant response to K+ starvation. The mutant plants lacking two CBLs (CBL2 and CBL3) were severely stunted under low-K conditions. Interestingly, the cbl2 cbl3 mutant was normal in K+ uptake but impaired in K+ remobilization from vacuoles. Four CIPKs—CIPK3, 9, 23 and 26—were identified as partners of CBL2 and CBL3 that together regulate K+ homeostasis through activating vacuolar K+ efflux to the cytoplasm. The vacuolar two-pore K+ (TPK) channels were directly activated by the vacuolar CBL–CIPK modules in a Ca2+-dependent manner, presenting a mechanism for the activation of vacuolar K+ remobilization that plays an important role in plant adaptation to K+ deficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author upon reasonable request.
Change history
19 May 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41477-020-0692-5
References
Luan, S., Lan, W. Z. & Lee, S. C. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL-CIPK network. Curr. Opin. Plant Biol. 12, 339–346 (2009).
Maathuis, F. J. M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258 (2009).
Ashley, M. K., Grant, M. & Grabov, A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J. Exp. Bot. 57, 425–436 (2006).
Wang, Y. & Wu, W. H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64, 451–476 (2013).
Rubio, F., Aleman, F., Nieves-Cordones, M. & Martinez, V. Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K+ uptake. Physiol. Plant. 139, 220–228 (2010).
Aleman, F., Nieves-Cordones, M., Martinez, V. & Rubio, F. Root K+ acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol. 52, 1603–1612 (2011).
Pyo, Y. J., Gierth, M., Schroeder, J. I. & Cho, M. H. High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 153, 863–875 (2010).
Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 14, 37–42 (2009).
Tang, R. J., Wang, C., Li, K. & Luan, S. The CBL-CIPK calcium signaling network: unified paradigm from 20 years of discoveries. Trends Plant Sci. (in the press).
Xu, J. et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125, 1347–1360 (2006).
Li, L. G., Kim, B. G., Cheong, Y. H., Pandey, G. K. & Luan, S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl Acad. Sci. USA 103, 12625–12630 (2006).
Ragel, P. et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 169, 2863–2873 (2015).
Li, J. et al. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 26, 3387–3402 (2014).
Scherzer, S. et al. Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. Proc. Natl Acad. Sci. USA 112, 7309–7314 (2015).
Gaymard, F. et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655 (1998).
Very, A. A. & Sentenac, H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54, 575–603 (2003).
Bassil, E. et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23, 3482–3497 (2011).
Barragan, V. et al. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24, 1127–1142 (2012).
Cherel, I., Lefoulon, C., Boeglin, M. & Sentenac, H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 65, 833–848 (2014).
Amtmann, A. & Armengaud, P. The role of calcium sensor-interacting protein kinases in plant adaptation to potassium-deficiency: new answers to old questions. Cell Res. 17, 483–485 (2007).
Moussavi-Nik, M. P. J., Hollamby, G. J. & Graham, R. D. Dynamics of nutrient remobilization during germination and early seedling development in wheat. J. Plant Nutr. 21, 421–434 (1998).
Tang, R. J. et al. Tonoplast calcium sensors CBL2 and CBL3 control plant growth and ion homeostasis through regulating V-ATPase activity in Arabidopsis. Cell Res. 22, 1650–1665 (2012).
Krebs, M. et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl Acad. Sci. USA 107, 3251–3256 (2010).
Cheong, Y. H. et al. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 52, 223–239 (2007).
Armengaud, P., Breitling, R. & Amtmann, A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 136, 2556–2576 (2004).
Pandey, G. K. et al. CIPK9: a calcium sensor-interacting protein kinase required for low-potassium tolerance in Arabidopsis. Cell Res. 17, 411–421 (2007).
Tang, R. J. et al. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl Acad. Sci. USA 112, 3134–3139 (2015).
Sze, H., Li, X. H. & Palmgren, M. G. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11, 677–689 (1999).
Hedrich, R. Ion channels in plants. Physiol. Rev. 92, 1777–1811 (2012).
Hedrich, R., Mueller, T. D., Becker, D. & Marten, I. Structure and function of TPC1 vacuole SV channel gains shape. Mol. Plant 11, 764–775 (2018).
Gobert, A., Isayenkov, S., Voelker, C., Czempinski, K. & Maathuis, F. J. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl Acad. Sci. USA 104, 10726–10731 (2007).
Shaner, N. C., Steinbach, P. A. & Tsien, R. Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005).
Zhang, H. W. et al. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E2036–E2045 (2017).
Voelker, C., Schmidt, D., Mueller-Roeber, B. & Czempinski, K. Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 48, 296–306 (2006).
Hohner, R. et al. Photosynthesis in Arabidopsis is unaffected by the function of the vacuolar K+ channel TPK3. Plant Physiol. 180, 1322–1335 (2019).
Walker, D. J., Leigh, R. A. & Miller, A. J. Potassium homeostasis in vacuolate plant cells. Proc. Natl Acad. Sci. USA 93, 10510–10514 (1996).
Martinoia, E. Vacuolar transporters—companions on a longtime journey. Plant Physiol. 176, 1384–1407 (2018).
Jaslan, D. et al. Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat. Commun. 10, 2659 (2019).
Kleist, T. J., Spencley, A. L. & Luan, S. Comparative phylogenomics of the CBL-CIPK calcium-decoding network in the moss Physcomitrella, Arabidopsis, and other green lineages. Front. Plant Sci. 5, 187 (2014).
Gomez-Porras, J. L. et al. Phylogenetic analysis of K+ transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Front. Plant Sci. 3, 167 (2012).
Yang, Y. et al. Calcineurin B-like proteins CBL4 and CBL10 mediate two independent salt tolerance pathways in Arabidopsis. Int. J. Mol. Sci. 20, 2421 (2019).
Behera, S. et al. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 213, 739–750 (2017).
Hedrich, R. & Neher, E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329, 833–836 (1987).
Ward, J. M. & Schroeder, J. I. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6, 669–683 (1994).
Pan, Y. et al. Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 48, 710–725 (2019).
Tian, W. et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572, 131–135 (2019).
Wang, Y. et al. Golgi-localized cation/proton exchangers regulate ionic homeostasis and skotomorphogenesis in Arabidopsis. Plant Cell Environ. 42, 673–687 (2019).
Murashige, T. & S., F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–495 (1962).
Acknowledgements
We thank the Arabidopsis Biological Resource Center for providing Arabidopsis thaliana seed stocks. This work was supported by the National Science Foundation (grant nos. MCB-1714795 and ISO-1339239 to S.L.), the Innovative Genomics Institute of the University of California (to S.L.) and the National Natural Science Foundation of China (grant no. 31770266 to F.-G.Z.). C.W. is sponsored by the Tang Distinguished Scholarship of the University of California at Berkeley.
Author information
Authors and Affiliations
Contributions
R.-J.T. and S.L. conceived and designed the experiments. R.-J.T. performed most of the molecular, genetic and physiological experiments. F.-G.Z. conducted the electrophysiological experiments. Y.Y., C.W. and K.L. assisted in some of the molecular experiments and subcellular localization. T.J.K. and P.G.L. provided some tools and reagents. R.-J.T. and S.L. wrote the manuscript. All the authors discussed the results and commented on the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Ingo Dreyer, Enrico Martinoia and the other, anonymous, reviewer for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19 and Table 1.
Rights and permissions
About this article
Cite this article
Tang, RJ., Zhao, FG., Yang, Y. et al. A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments. Nat. Plants 6, 384–393 (2020). https://doi.org/10.1038/s41477-020-0621-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0621-7
This article is cited by
-
Accelerated germination of aged recalcitrant seeds by K+-rich bulk oxygen nanobubbles
Scientific Reports (2023)
-
Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis
Nature Communications (2023)
-
Genome-Wide Identification of CIPK Genes in Sugar Beet (Beta vulgaris) and Their Expression Under NaCl Stress
Journal of Plant Growth Regulation (2023)
-
Dealing with Environmental Fluctuations: Diversity of Potassium Uptake Systems Across the Three Domains of Life
Journal of Plant Growth Regulation (2023)
-
The combination of nanotechnology and potassium: applications in agriculture
Environmental Science and Pollution Research (2023)