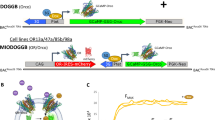
Abstract
Plant pathogen detection conventionally relies on molecular technology that is complicated, time-consuming and constrained to centralized laboratories. We developed a cost-effective smartphone-based volatile organic compound (VOC) fingerprinting platform that allows non-invasive diagnosis of late blight caused by Phytophthora infestans by monitoring characteristic leaf volatile emissions in the field. This handheld device integrates a disposable colourimetric sensor array consisting of plasmonic nanocolorants and chemo-responsive organic dyes to detect key plant volatiles at the ppm level within 1 min of reaction. We demonstrate the multiplexed detection and classification of ten individual plant volatiles with this field-portable VOC-sensing platform, which allows for early detection of tomato late blight 2 d after inoculation, and differentiation from other pathogens of tomato that lead to similar symptoms on tomato foliage. Furthermore, we demonstrate a detection accuracy of ≥95% in diagnosis of P. infestans in both laboratory-inoculated and field-collected tomato leaves in blind pilot tests. Finally, the sensor platform has been beta-tested for detection of P. infestans in symptomless tomato plants in the greenhouse setting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the paper and its Supplementary Information. All data generated or analysed are available from the corresponding authors on reasonable request.
References
Oerke, E. C. Crop losses to pests. J. Agric. Sci. 144, 31–43 (2006).
Pimentel, D., Lach, L., Zuniga, R. & Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 50, 53–65 (2000).
Nowicki, M., Foolad, M. R., Nowakowska, M. & Kozik, E. U. Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis. 96, 4–17 (2011).
Saville, A. C., Martin, M. D. & Ristaino, J. B. Historic late blight outbreaks caused by a widespread dominant lineage of Phytophthora infestans (Mont.) de Bary. PLoS ONE 11, e0168381 (2016).
Pennisi, E. Armed and dangerous. Science 327, 804–805 (2010).
Haverkort, A. J., Struik, P. C., Visser, R. G. F. & Jacobsen, E. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res. 52, 249–264 (2009).
Fry, W. E. et al. The 2009 late blight pandemic in the eastern United States—causes and results. Plant Dis. 97, 296–306 (2013).
Hussain, S., Lees, A. K., Duncan, J. M. & Cooke, D. E. L. Development of a species‐specific and sensitive detection assay for Phytophthora infestans and its application for monitoring of inoculum in tubers and soil. Plant Pathol. 54, 373–382 (2005).
Lees, A. K., Sullivan, L., Lynott, J. S. & Cullen, D. W. Development of a quantitative real‐time PCR assay for Phytophthora infestans and its applicability to leaf, tuber and soil samples. Plant Pathol. 61, 867–876 (2012).
Khan, M., Li, B., Jiang, Y., Weng, Q. & Chen, Q. Evaluation of different PCR-based assays and LAMP method for rapid detection of Phytophthora infestans by targeting the Ypt1 gene. Front. Microbiol. 8, 1920 (2017).
Hansen, Z. R. et al. Loop‐mediated isothermal amplification for detection of the tomato and potato late blight pathogen, Phytophthora infestans. J. Appl. Microbiol. 120, 1010–1020 (2016).
Bodrossy, L. & Sessitsch, A. Oligonucleotide microarrays in microbial diagnostics. Curr. Opin. Microbiol. 7, 245–254 (2004).
Wakeham, A. J., Keane, G. & Kennedy, R. Field evaluation of a competitive lateral-flow assay for detection of Alternaria brassicae in vegetable brassica crops. Plant Dis. 100, 1831–1839 (2016).
Harrison, J. G., Lowe, R. & Duncan, J. M. Use of ELISA for assessing major gene resistance of potato leaves to Phytophthora infestans. Plant Pathol. 40, 431–435 (1991).
Skottrup, P., Nicolaisen, M. & Justesen, A. F. Rapid determination of Phytophthora infestans sporangia using a surface plasmon resonance immunosensor. J. Microbiol. Methods 68, 507–515 (2007).
Ray, M. et al. Fungal disease detection in plants: traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron. 87, 708–723 (2017).
Koo, C. et al. Development of a real-time microchip PCR system for portable plant disease diagnosis. PLoS ONE 8, e82704 (2013).
Julich, S. et al. Development of a lab-on-a-chip device for diagnosis of plant pathogens. Biosens. Bioelectron. 26, 4070–4075 (2011).
Laothawornkitkul, J. et al. Volatile organic compounds as a diagnostic marker of late blight infected potato plants: a pilot study. Crop Prot. 29, 872–878 (2010).
Chen, G., Roy, I., Yang, C. & Prasad, P. N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 116, 2826–2885 (2016).
Sabela, M., Balme, S., Bechelany, M., Janot, J.-M. & Bisetty, K. A review of gold and silver nanoparticle-based colorimetric sensing assays. Adv. Eng. Mater. 19, 1700270 (2017).
Yu, T. & Wei, Q. Plasmonic molecular assays: recent advances and applications for mobile health. Nano Res. 11, 5439–5473 (2018).
Li, H. et al. A fluorescent chemodosimeter specific for cysteine: effective discrimination of cysteine from homocysteine. Chem. Commun. 45, 5904–5906 (2009).
Wang, W. et al. Detection of homocysteine and cysteine. J. Am. Chem. Soc. 127, 15949–15958 (2005).
De Lacy Costello, B. P. J. et al. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 50, 489–496 (2001).
Li, Z., Bassett, W. P., Askim, J. R. & Suslick, K. S. Differentiation among peroxide explosives with an optoelectronic nose. Chem. Commun. 51, 15312–15315 (2015).
Li, Z., Fang, M., LaGasse, M. K., Askim, J. R. & Suslick, K. S. Colorimetric recognition of aldehydes and ketones. Angew. Chem. Int. Ed. 56, 9860–9863 (2017).
Li, Z. & Suslick, K. S. Portable optoelectronic nose for monitoring meat freshness. ACS Sens. 1, 1330–1335 (2016).
Janata, J. Principles of Chemical Sensors 2nd edn (Springer, 2009).
Tabora, J. E. & Domagalski, N. Multivariate analysis and statistics in pharmaceutical process research and development. Annu. Rev. Chem. Biomol. Eng. 8, 403–426 (2017).
Meadows, I. Late blight detected in Haywood County—Aug. 20, 2018. NC State Extension Plant Pathology https://plantpathology.ces.ncsu.edu/2018/08/late-blight-detected-in-haywood-county-aug-20-2018/ (2018)
Jansen, R. M. C. et al. Induced plant volatiles allow sensitive monitoring of plant health status in greenhouses. Plant Signal. Behav. 4, 824–829 (2009).
Jansen, R. M. C. et al. Detection of diseased plants by analysis of volatile organic compound emission. Annu. Rev. Phytopathol. 49, 157–174 (2011).
Aksenov, A. A. et al. Volatile Organic Compounds (VOCs) for Noninvasive Plant Diagnostics (American Chemical Society, 2013).
Dudareva, N., Negre, F., Nagegowda, D. A. & Orlova, I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440 (2006).
Matsui, K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9, 274–280 (2006).
Holopainen, J. & Blande, J. Where do herbivore-induced plant volatiles go? Front. Plant Sci. 4, 185 (2013).
Paré, P. W. & Tumlinson, J. H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–332 (1999).
Marcel, D. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 32, 654–665 (2009).
Holopainen, J. K. & Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15, 176–184 (2010).
Scala, A., Allmann, S., Mirabella, R., Haring, M. & Schuurink, R. Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 14, 17781 (2013).
Erb, M. Volatiles as inducers and suppressors of plant defense and immunity—origins, specificity, perception and signaling. Curr. Opin. Plant Biol. 44, 117–121 (2018).
Laothawornkitkul, J. et al. Discrimination of plant volatile signatures by an electronic nose: a potential technology for plant pest and disease monitoring. Environ. Sci. Technol. 42, 8433–8439 (2008).
Wilson, A. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 13, 2295 (2013).
Cellini, A. et al. Potential applications and limitations of electronic nose devices for plant disease diagnosis. Sensors 17, 2596 (2017).
Li, Z., Jang, M., Askim, J. R. & Suslick, K. S. Identification of accelerants, fuels and post-combustion residues using a colorimetric sensor array. Analyst 140, 5929–5935 (2015).
Li, Z. & Suslick, K. S. A hand-held optoelectronic nose for the identification of liquors. ACS Sens. 3, 121–127 (2018).
Askim, J. R., Mahmoudi, M. & Suslick, K. S. Optical sensor arrays for chemical sensing: the optoelectronic nose. Chem. Soc. Rev. 42, 8649–8682 (2013).
Bingham, J. M., Anker, J. N., Kreno, L. E. & Van Duyne, R. P. Gas sensing with high-resolution localized surface plasmon resonance spectroscopy. J. Am. Chem. Soc. 132, 17358–17359 (2010).
Liu, N., Tang, M. L., Hentschel, M., Giessen, H. & Alivisatos, A. P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 10, 631 (2011).
Yang, Z., Sassa, F. & Hayashi, K. A robot equipped with a high-speed LSPR gas sensor module for collecting spatial odor information from on-ground invisible odor sources. ACS Sens. 3, 1174–1181 (2018).
Shang, L., Liu, C., Chen, B. & Hayashi, K. Plant biomarker recognition by molecular imprinting based LSPR sensor array: performance improvement by enhanced hotspot of Au nanostructure. ACS Sens. 3, 1531–1538 (2018).
Ozcan, A. Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools. Lab. Chip. 14, 3187–3194 (2014).
Contreras-Naranjo, J. C., Wei, Q. & Ozcan, A. Mobile phone-based microscopy, sensing, and diagnostics. IEEE J. Sel. Top. Quant. Electron. 22, 1–14 (2016).
Wei, Q. et al. Fluorescent imaging of single nanoparticles and viruses on a smart phone. ACS Nano 7, 9147–9155 (2013).
Wei, Q. et al. Imaging and sizing of single DNA molecules on a mobile phone. ACS Nano 8, 12725–12733 (2014).
Joh, D. Y. et al. Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood. Proc. Natl Acad. Sci. USA 114, E7054–E7062 (2017).
Kühnemund, M. et al. Targeted DNA sequencing and in situ mutation analysis using mobile phone microscopy. Nat. Commun. 8, 13913 (2017).
Hernández-Neuta, I. et al. Smartphone-based clinical diagnostics: towards democratization of evidence-based health care. J. Intern. Med. 285, 19–39 (2019).
Ninh, H. P., Tanaka, Y., Nakamoto, T. & Hamada, K. A bad-smell sensing network using gas detector tubes and mobile phone cameras. Sens. Actuator B Chem. 125, 138–143 (2007).
Azzarelli, J. M., Mirica, K. A., Ravnsbæk, J. B. & Swager, T. M. Wireless gas detection with a smartphone via rf communication. Proc. Natl Acad. Sci. USA 111, 18162–18166 (2014).
Salles, M. O., Meloni, G. N. & de Araujo, W. R. Paixão TRLC. Explosive colorimetric discrimination using a smartphone, paper device and chemometrical approach. Anal. Methods 6, 2047–2052 (2014).
Gahlaut, S. K., Yadav, K., Sharan, C. & Singh, J. P. Quick and selective dual mode detection of H2S gas by mobile app employing silver nanorods array. Anal. Chem. 89, 13582–13588 (2017).
Devadhasan, J. P., Kim, D., Lee, D. Y. & Kim, S. Smartphone coupled handheld array reader for real-time toxic gas detection. Anal. Chim. Acta 984, 168–176 (2017).
Small, I. M., Joseph, L. & Fry, W. E. Evaluation of the BlightPro decision support system for management of potato late blight using computer simulation and field validation. Phytopathology 105, 1545–1554 (2015).
Park, K. et al. Highly concentrated seed-mediated synthesis of monodispersed gold nanorods. ACS Appl. Mater. Interfaces 9, 26363–26371 (2017).
Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003).
FRENS, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973).
Zheng, Y., Xiao, M., Jiang, S., Ding, F. & Wang, J. Coating fabrics with gold nanorods for colouring, UV-protection, and antibacterial functions. Nanoscale 5, 788–795 (2013).
Ristaino, J. B., Groves, C. T. & Parra, G. R. PCR amplification of the Irish potato famine pathogen from historic specimens. Nature 411, 695 (2001).
Acknowledgements
This work was supported by the Chancellor’s Faculty Excellence Program, Kenan Institute for Engineering, Technology & Science and USDA AFRI grant (no. 2019-67030-29311). The authors also acknowledge the Analytical Instrumentation Facility at North Carolina State University for assistance in TEM characterization.
Author information
Authors and Affiliations
Contributions
Z.L., Q.W. and J.B.R. designed the experiments. Z.L. prepared the Au nanoplasmonic inks and the multiplexed chemical sensor array, carried out sensing experiments and analysed data. R.P. conducted the COMSOL simulation of gas flows. J.C.H. collected the fresh field samples for qPCR and VOC tests. R.P., T.Y., Z.L., A.C.S. and J.C.H. performed the leaf inoculation experiments and conducted PCR or qPCR analyses of the pathogens. Z.L. and T.B.T. designed and fabricated the smartphone imaging attachment. Z.L., Q.W. and J.B.R. wrote the manuscript. All authors contributed to the editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Plants thanks Alexander Aksenov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20 and Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Li, Z., Paul, R., Ba Tis, T. et al. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 5, 856–866 (2019). https://doi.org/10.1038/s41477-019-0476-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0476-y
This article is cited by
-
Diagnosis of fungi affected apple crop disease using improved ResNeXt deep learning model
Multimedia Tools and Applications (2024)
-
Precise in-field molecular diagnostics of crop diseases by smartphone-based mutation-resolved pathogenic RNA analysis
Nature Communications (2023)
-
Climate change impacts on plant pathogens, food security and paths forward
Nature Reviews Microbiology (2023)
-
A hybrid multifunctional physicochemical sensor suite for continuous monitoring of crop health
Scientific Reports (2023)