Abstract
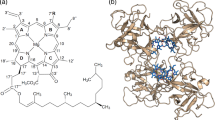
We altered the chlorophyll (Chl) binding sites in various versions of water-soluble chlorophyll protein (WSCP) by amino acid exchanges to alter their preferences for either Chl a or Chl b. WSCP is ideally suited for this mutational analysis since it forms a tetrameric complex with only four identical Chl binding sites. A loop of 4–6 amino acids is responsible for Chl a versus Chl b selectivity. We show that a single amino acid exchange within this loop changes the relative Chl a/b affinities by a factor of 40. We obtained crystal structures of this WSCP variant binding either Chl a or Chl b. The Chl binding sites in these structures were compared with those in the major light-harvesting complex (LHCII) of the photosynthetic apparatus in plants to search for similar structural features involved in Chl a/b binding specificity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available from the corresponding authors upon request. Crystal structures determined in this study have been deposited in the Protein Data Bank (http://www.rcsb.org), with accession code 6GIW (LvPCPS Chl a) and 6GIX (LvPCPS Chl b).
References
Mirkovic, T. et al. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 117, 249–293 (2017).
Remelli, R., Varotto, C., Sandonà, D., Croce, R. & Bassi, R. Chlorophyll binding to monomeric light-harvesting complex. A mutation analysis of chromophore-binding residues. J. Biol. Chem. 274, 33510–33521 (1999).
Formaggio, E., Cinque, G. & Bassi, R. Functional architecture of the major light-harvesting complex from higher plants. J. Mol. Biol. 314, 1157–1166 (2001).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A. C., Lamborghini, M. & Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005).
Hobe, S., Fey, H., Rogl, H. & Paulsen, H. Determination of relative chlorophyll binding affinities in the major light-harvesting chlorophyll a/b complex. J. Biol. Chem. 278, 5912–5919 (2003).
Kleima, F. J. et al. Decreasing the chlorophyll a/b ratio in reconstituted LHCII: structural and functional consequences. Biochemistry 38, 6587–6596 (1999).
Ikegami, I., Satoh, S. & Aoki, M. Binding affinity of Chl b for the Chl a-binding sites in PSI core complexes. Plant Cell Physiol. 48, 1092–1097 (2007).
Satoh, S., Ikeuchi, M., Mimuro, M. & Tanaka, A. Chlorophyll b expressed in cyanobacteria functions as a light-harvesting antenna in photosystem I through flexibility of the proteins. J. Biol. Chem. 276, 4293–4297 (2001).
Xu, H., Vavilin, D. & Vermaas, W. Chlorophyll b can serve as the major pigment in functional photosystem II complexes of cyanobacteria. Proc. Natl Acad. Sci. USA 98, 14168–14173 (2001).
Hirashima, M., Satoh, S., Tanaka, R. & Tanaka, A. Pigment shuffling in antenna systems achieved by expressing prokaryotic chlorophyllide a oxygenase in Arabidopsis. J. Biol. Chem. 281, 15385–15393 (2006).
Tanaka, A. et al. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc. Natl Acad. Sci. USA 95, 12719–12723 (1998).
Wang, P. & Grimm, B. Organization of chlorophyll biosynthesis and insertion of chlorophyll into the chlorophyll-binding proteins in chloroplasts. Photosynth. Res. 126, 189–202 (2015).
Dall’Osto, L., Bressan, M. & Bassi, R. Biogenesis of light harvesting proteins. Biochim. Biophys. Acta 1847, 861–871 (2015).
Ros, F., Bassi, R. & Paulsen, H. Pigment-binding properties of the recombinant photosystem II subunit CP26 reconstituted in vitro. Eur. J. Biochem. 253, 653–658 (1998).
Pagano, A., Cinque, G. & Bassi, R. In vitro reconstitution of the recombinant photosystem II light-harvesting complex CP24 and its spectroscopic characterization. J. Biol. Chem. 273, 17154–17165 (1998).
Croce, R. & Weiss, S. & Bassi, R. Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J. Biol. Chem. 274, 29613–29623 (1999).
Bassi, R., Croce, R., Cugini, D. & Sandonà, D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl Acad. Sci. USA 96, 10056–10061 (1999).
Passarini, F., Xu, P., Caffarri, S., Hille, J. & Croce, R. Towards in vivo mutation analysis: knock-out of specific chlorophylls bound to the light-harvesting complexes of Arabidopsis thaliana - the case of CP24 (Lhcb6). Biochim. Biophys. Acta - Bioenerg. 1837, 1500–1506 (2014).
Xu, P., Roy, L. M. & Croce, R. Functional organization of photosystem II antenna complexes: CP29 under the spotlight. Biochim. Biophys. Acta - Bioenerg. 1858, 815–822 (2017).
Mahboobe, J. et al. Structure-based exciton Hamiltonian and dynamics for the reconstituted wild-type CP29 protein antenna complex of the photosystem II. J. Phys. Chem. B 122, 4611–4624 (2018).
Eggink, L. L., Park, H. & Hoober, J. K. The role of chlorophyll in photosynthesis: hypothesis. BMC Plant Biol. 1, 1471 (2001).
Chen, M. & Cai, Z.-L. Theoretical study on the thermodynamic properties of chlorophyll d-peptides coordinating ligand. Biochim. Biophys. Acta - Bioenerg. 1767, 603–609 (2007).
Chen, M., Eggink, L. L., Hoober, J. K. & Larkum, A. W. D. Influence of structure on binding of chlorophylls to peptide ligands. J. Am. Chem. Soc. 127, 2052–2053 (2005).
Hoober, J. K., Eggink, L. L. & Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 94, 387–400 (2007).
Lutz, M. Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman spectroscopy. Biochim. Biophys. Acta 460, 408–430 (1977).
Horigome, D. et al. Structural mechanism and photoprotective function of water-soluble chlorophyll-binding protein. J. Biol. Chem. 282, 6525–6531 (2007).
Bednarczyk, D. et al. Chlorophyll fine tuning of chlorophyll spectra by protein-induced ring deformation. Angew. Chemie Int. Ed. 55, 1–6 (2016).
Satoh, H., Uchida, A., Nakayama, K. & Okada, M. Water-soluble chlorophyll protein in Brassicaceae plants is a stress-induced chlorophyll-binding protein. Plant Cell Physiol. 42, 906–911 (2001).
Murata, T., Toda, F., Uchino, K. & Yakushiji, E. Water-soluble chlorophyll protein of Brassica oleracea var. Botrys (cauliflower). Biochim. Biophys. Acta 245, 208–215 (1971).
Kamimura, Y., Mori, T., Yamasaki, T. & Katoh, S. Isolation, properties and a possible function of a water-soluble chlorophyll a/b-protein from Brussels sprouts. Plant Cell Physiol. 38, 133–138 (1997).
Shinashi, K. et al. Molecular characterization of a water-soluble chlorophyll protein from main veins of Japanese radish. J. Plant. Physiol. 157, 255–262 (2000).
Bektas, I., Fellenberg, C. & Paulsen, H. Water-soluble chlorophyll protein (WSCP) of Arabidopsis is expressed in the gynoecium and developing silique. Planta 236, 251–259 (2012).
Murata, T. & Murata, N. Water-soluble chlorophyll-proteins from Brassica nigra and Lepidium virginicum. Carnegie Inst. Wash. Yearb. 70, 504–507 (1971).
Takahashi, S. et al. Molecular cloning, characterization and analysis of the intracellular localization of a water-soluble chl-binding protein from Brussels sprouts (Brassica oleracea var. gemmifera). Plant Cell Physiol. 53, 879–891 (2012).
Takahashi, S. et al. Molecular cloning, characterization and analysis of the intracellular localization of a water-soluble chlorophyll-binding protein (WSCP) from Virginia pepperweed (Lepidium virginicum), a unique WSCP that preferentially binds chlorophyll b in vitro. Planta 238, 1065–1080 (2013).
Satoh, H., Nakayama, K. & Okada, M. Molecular cloning and functional expression of a water-soluble chlorophyll protein, a putative carrier of chlorophyll molecules in cauliflower. J. Biol. Chem. 273, 30568–30575 (1998).
Palm, D. M. et al. Water-Soluble Chlorophyll Protein (WSCP) stably binds two or four chlorophylls. Biochemistry 56, 1726–1736 (2017).
Schmidt, K., Fufezan, C., Krieger-Liszkay, A., Satoh, H. & Paulsen, H. Recombinant water-soluble chlorophyll protein from Brassica oleracea var. Botrys binds various chlorophyll derivatives. Biochemistry 42, 7427–7433 (2003).
Bednarczyk, D., Takahashi, S., Satoh, H. & Noy, D. Assembly of water-soluble chlorophyll-binding proteins with native hydrophobic chlorophylls in water-in-oil emulsions. BBA - Bioenerg. 1847, 307–313 (2015).
Takahashi, S., Ono, M., Uchida, A., Nakayama, K. & Satoh, H. Molecular cloning and functional expression of a water-soluble chlorophyll-binding protein from Japanese wild radish. J. Plant. Physiol. 170, 406–412 (2013).
Agostini, A. et al. An unusual role for the phytyl chains in the photoprotection of the chlorophylls bound to water-soluble chlorophyll-binding proteins. Sci. Rep. 7, 7504 (2017).
Ramachandran, G. N., Lakshminarayanan, A. V., Balasubramanian, R. & Tegoni, G. Studies on the conformation of amino acids XII. Energy calculations on prolyl residue. Biochim. Biophys. Acta 221, 165–181 (1970).
Horn, R. & Paulsen, H. Early steps in the assembly of light-harvesting chlorophyll a/b complex. J. Biol. Chem. 279, 44400–44406 (2004).
Di Valentin, M. et al. Triplet-triplet energy transfer in the major intrinsic light-harvesting complex of Amphidinium carterae as revealed by ODMR and EPR spectroscopies. Biochim. Biophys. Acta - Bioenerg. 1797, 1759–1767 (2010).
Iriyama, K., Ogura, N. & Takamiya, A. A simple method for extraction and partial purification of chlorophyll from plant material, using dioxane. J. Biochem. 76, 901–904 (1974).
Booth, P. J. & Paulsen, H. Assembly of light-harvesting chlorophyll a/b complex in vitro. Time-resolved fluorescence measurements. Biochemistry 35, 5103–5108 (1996).
Wintermans, J. F. G. M. & De Mots, A. Spectoscophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109, 448–453 (1965).
Hobe, S., Niemeier, H., Bender, A. & Paulsen, H. Carotenoid binding sites in LHCIIB: relative affinities towards major xanthophylls of higher plants. Eur. J. Biochem. 267, 616–624 (2000).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry land cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
CCP4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. D50, 760–763 (1994).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 60, 2126–2132 (2004).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994).
Acknowledgements
We thank T. Hares for WSCP mutant preparation and S. Müller for preliminary experiments. Moreover, we thank E. Hofmann for providing definition files for Chl ligands and helpful discussion. We are grateful to D. Noy for valuable discussion. This work has been funded by a grant from the Deutsche Forschungsgemeinschaft to H.P. (Pa 324/10-1). P.G. thanks the Studienstiftung des deutschen Volkes for support.
Author information
Authors and Affiliations
Contributions
H.P. designed the research. S.T. and H.S. designed the WSCP mutants. P.G. developed the reconstitution method. D.M.P., A.A. and V.A. prepared the WSCP samples and performed and analysed the spectroscopic measurements. E.J. resolved the crystal structures. A.A. analysed the crystal structures. D.M.P., A.A., M.W. and H.P. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–6, Supplementary Tables 1–4 and Supplementary References.
Rights and permissions
About this article
Cite this article
Palm, D.M., Agostini, A., Averesch, V. et al. Chlorophyll a/b binding-specificity in water-soluble chlorophyll protein. Nature Plants 4, 920–929 (2018). https://doi.org/10.1038/s41477-018-0273-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0273-z
This article is cited by
-
Comparative genomics analysis provides insights into evolution and stress responses of Lhcb genes in Rosaceae fruit crops
BMC Plant Biology (2023)
-
Signatures of intramolecular vibrational and vibronic Q\(_{\mathrm{x}}\)–Q\(_{\mathrm{y}}\) coupling effects in absorption and CD spectra of chlorophyll dimers
Photosynthesis Research (2023)
-
Excessive composite pollution carbon sources enhance the bio-fertilizer efficiency of Tetradesmus obliquus: focused on cultivation period
Environmental Science and Pollution Research (2023)
-
Controlling synthetic membraneless organelles by a red-light-dependent singlet oxygen-generating protein
Nature Communications (2022)
-
Integration of transcriptome and proteome analysis reveals the mechanism of freezing tolerance in winter rapeseed
Plant Growth Regulation (2022)