Abstract
Highly sensitive rapid testing for COVID-19 is essential for minimizing virus transmission, especially before the onset of symptoms and in asymptomatic cases. Here, we report bioengineered enrichment tools for lateral flow assays (LFAs) with enhanced sensitivity and specificity (BEETLES2), achieving enrichment of SARS-CoV-2 viruses, nucleocapsid (N) proteins and immunoglobulin G (IgG) with 3-minute operation. The limit of detection is improved up to 20-fold. We apply this method to clinical samples, including 83% with either intermediate (35%) or low viral loads (48%), collected from 62 individuals (n = 42 for positive and n = 20 for healthy controls). We observe diagnostic sensitivity, specificity, and accuracy of 88.1%, 100%, and 91.9%, respectively, compared with commercial LFAs alone achieving 14.29%, 100%, and 41.94%, respectively. BEETLES2, with permselectivity and tunability, can enrich the SARS-CoV-2 virus, N proteins, and IgG in the nasopharyngeal/oropharyngeal swab, saliva, and blood serum, enabling reliable and sensitive point-of-care testing, facilitating fast early diagnosis.
Similar content being viewed by others
Introduction
Fast screening and testing for COVID-19 enables the identification of infected high-risk individuals and, consequently, reduces virus spread, providing better prevention and control of COVID-191,2. Rapid detection and treatment with proper quarantine are the best strategies for handling pandemics. For commercial rapid testing of COVID-19, 59 antigen diagnostic tests for SARS-CoV-2 are available under Emergency Use Authorization (EUA) (as of 1 Jan 2023)3. Notwithstanding the developed techniques with advanced diagnostic performance4,5,6,7,8,9,10,11,12,13, reverse transcription-quantitative polymerase chain reaction (RT–qPCR) tests are still considered the gold standard for COVID-19 diagnosis. Although a highly sensitive assay can be realized using RNA detection technologies, on-site frequent tests of COVID-19 are challenging to perform. RT-qPCR has high cost, long operation time (4–6 h), and long turnaround times (up to several days)14,15,16,17. Moreover, the highly sensitive and selective RT-qPCR technique requires expensive laboratory equipment and cumbersome RNA extraction steps, limiting its application in point-of-care tests (POCTs)18. Long turnaround times allow the infected people to spread the virus exponentially before getting results, limiting the impact of isolation and contact tracing8,19. Importantly, asymptomatic people spread the virus to communities with a similar viral load to those who develop symptoms20.
To handle COVID-19 spread, the best approach is fast, easy, on-site detection at the beginning of infection to stop onward spread with high-frequency tests. In contrast to routinely tested RT-qPCR, the high-frequency test should be low-cost and point-of-care. Mina et al. has claimed that the best COVID-19 filter can be achieved using frequent, low-cost, simple, and rapid tests because SARS-CoV-2 quickly grows and spreads out exponentially19. A one-time monitored highly sensitive RT-qPCR test can detect viral shedding long after the infectious period (about 9 days), up to 17 days21, i.e., the convalescence stage with less transmission.
Regarding high-frequency tests for decentralized local testing, the lateral flow assay (LFA) platform is confirmed as the best platform for point-of-care and self-testing as they provide convenient, easy-to-use, one-step, disposable, and fast results22. The LFA platforms are considered the best candidate as they meet the WHO (World Health Organization) “ASSURED” criteria (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end-users)23. The lower LFA performance limits the accuracy, increases the false negatives, and hampers the early diagnosis of COVID-19. Generally, the LFA platform shows good accuracy for high viral loads; however, accuracy decreases abruptly with low viral loads. In addition, immunoassay-based LFA tests are less sensitive than molecular-based RT-qPCR, generating more false negatives and increasing the risk of onward transmission of the virus. Recently, Chen et al. reported a quantitative and ultrasensitive in situ immunoassay using a nanoporous anodic aluminum oxide (AAO) membrane, showing the ability to enrich SARS-CoV-2 viruses9. Generally, AAO membranes have been considered a promising material to facilitate size-based separation and enrichment24,25,26.
Here, we develop bioengineered enrichment tools for LFA with enhanced sensitivity and specificity (BEETLES2) combined with a commercial COVID-19 LFA kit. A key component of BEETLES2 is a permselective tunable nanotrap that can enhance commercial LFA’s clinical performance. We prepare BEETLES2 with a combination of red blood cell membranes (RBCMs) and nanoporous anodic aluminum oxide (AAO). The combination of high AAO flux and an aquaporin (AQP) water channel, unlike size-based AAO membrane enrichment27,28, allows fast water transport with additional permselectivity within 3 min, achieving enrichment of SARS-CoV-2 viruses, nucleocapsid (N) proteins, and immunoglobulin G (IgG) antibodies. The limit of detection (LOD) is enhanced up to 20-fold. Moreover, we show enhanced diagnostic sensitivity, specificity, and accuracy when applied to patient samples.
Results and discussion
Nanotrap for enhancing COVID-19 diagnostics (BEETLES2)
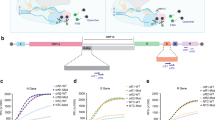
We demonstrate the key concept of BEETLES2, which enriches and separates biomolecules to sizes and charges, and its application for immuno and molecular assays (Fig. 1). A hybrid filter consisting of RBCM on an AAO membrane is called the BEETLES2 membrane. In the following sections, we describe more details about the permselective tunable enrichment of BEETLES2 with various criteria such as size, charge, and pressure. Figure 1a depicts a prototype for POCT sample preparation (details in Fig. S1). Equipped with the BEETLES2 membrane, we enriched the target samples and enhanced the assays for POCT and molecular diagnostics. For COVID-19 applications, we focused on the enrichment for N proteins, SARS-CoV-2 viruses, and IgG antibodies. The N protein, one of the four structural proteins of the SARS-CoV-2 virus, is the target protein for most COVID-19 Ag LFAs. The IgG antibodies are the target molecules for COVID-19 Ab LFA. Figure 1b shows the assay protocol from sampling to the assay. After sample collection, we enriched the target molecule via BEETLES2, applied a well-established diagnostic tool such as commercial LFA and RT-qPCR, and enhanced assay performance (Supplementary Video). Since BEETLES2 can enrich N proteins, SARS-CoV-2 viruses, and IgG antibodies, BEETLES2-assisted LFA enables the daily monitoring of infectious diseases.
a BEETLES2, a hybrid filter consisting of RBCM on an AAO membrane, has permselectivity and tunability that enable biomolecules to enrich with a separation function. b Assay with BEETLES2 for immunoassay and molecular assay. Cartoons in panels a, b were created with BioRender.com. BEETLES2, bioengineered enrichment tools for the LFA with enhanced sensitivity and specificity. AAO anodic aluminum oxide, LFA lateral flow assay, RT-qPCR reverse transcription quantitative polymerase chain reaction, RBCM red blood cell membrane.
The primary advantage of BEETLES2 is that the viral protein and virus itself can be enriched under various buffers, including phosphate-buffered saline (PBS), saliva, serum, and viral transport medium (VTM). Moreover, abundant protein (albumin) and small inhibitors can be eliminated. Larger biomolecules, like IgG (~150 kDa), are enriched because larger proteins cannot freely pass through the BEETLES2 membrane. Interestingly, we observed the permselectivity of BEETLES2 membrane with the N proteins (~46 kDa) and bovine serum albumin (BSA) (~66.5 kDa) proteins. The N protein was positively charged under physiological conditions, whereas BSA was negatively charged. Therefore, we only enriched the N proteins while the negative charged BSA freely passed through the BEETLES2 membrane and was filtered out.
Physicochemical characterization of the nanotrap
Figure 2 presents the physical and chemical characterization of the BEETLES2 membrane. It consists of RBCM and AAO, which are key hybrid materials for enrichment. A honeycomb-like nanostructured AAO membrane with an aligned 20 nm pore was used as a substrate (Fig. 2a). The AAO membrane itself has no permselectivity and is responsible for size-based separation. To add tunable permselectivity, we functionalized RBCM onto the AAO membrane (Fig. 2b). Since the RBCM acts as a physical barrier, we observe no tunable permselectivity in the BEETLES2 membrane without pressure. However, we observed tunable permselectivity with an applied pressure >0.5 bar.
a AAO membrane with an aligned 20 nm pore as a substrate, showing no permselectivity. b Functionalization of RBCM onto the AAO membrane, showing permselectivity and tunability under applied pressure. c Fabrication and topological analysis of BEETLES2 membrane. d AFM images showing topography and cross-sectional profiles from the height map images of bare AAO and BEETLES2 membrane. (e, f) KPFM analysis of (e) surface potential images of bare AAO and BEETLES2 membrane and f Frequencies of surface potential depending on RBCM concentration (0-4% (v/v)). Data are from three pooled experiments (0% RBCM, n = 30; 0.1-4% RBCM, n = 50). g FRAP study of fluorescence intensity with time and images showing RBCM on the substrate is well preserved its lateral diffusivity and fluidity in 2D planar deposition and the recovery of ~80% of the original value was done in 150 s (spot radius: 20 μm). Error bars represent standard deviation from the mean. Cartoons in panels a–c were created with BioRender.com. BEETLES2, bioengineered enrichment tools for the LFA with enhanced sensitivity and specificity; AAO nanoporous anodic aluminum oxide, LFA lateral flow assay, RBCM red blood cell membrane, AFM atomic force microscopy, FRAP fluorescence recovery after photobleaching, KPFM Kelvin probe force microscopy.
We functionalized the AAO membrane with RBCM via the vesicle fusion method (Fig. 2c, Figs. S2 and 3). The conformational characteristics of the BEETLES2 membrane were characterized using scanning electron microscopy (SEM), atomic force microscopy (AFM), and fluorescent microscopy. The uncoated AAO membrane showed a mesh-like network, which aided water and protein penetration. Conversely, the BEETLES2 membrane had a smooth surface, indicating that RBCM suspension completely covered the porous structure and formed a multilayered RBCM structure (~1 μm on the AAO membrane, side view).
From the SEM images (Fig. S4), we observed conformal coating of RBCM with the least defect over 2% RBCM concentration. 4% RBCM also showed conformal coating; however, it slowed water transport compared to 2% RBCM. For additional topological analysis, we measured the surface roughness of the BEETLES2 membrane depending on the RBCM concentration (0 ‒ 4% (v/v)) via AFM (Fig. 2d, S5 and 6). Over the scanned 10 × 10 μm area, the surface root means square roughness (Rq) of each BEETLES2 membrane significantly decreased compared to that of a bare AAO membrane, indicating more conformal RBCM coating on the surface of AAO membrane.
Along with AFM and SEM analysis, we conducted Kelvin probe force microscopy (KPFM) (Fig. 2e, f and S7). Via KPFM, we measured the physicochemical characterizations of the BEETLES2 membrane, which was negatively charged due to the negatively charged phospholipids in RBCM29,30. In contrast, the BEETLES2 membrane with 4% RBCM showed a relatively positively charged surface compared to that with 2% RBCM. The flow of current between the KPFM cantilever tip and sample is speculated to have slowed down because the nonconductive RBCM was excessively wrapped in a dried state on the surface of the AAO membrane31. We selected 2% RBCM as the optimal condition because a negatively charged surface potential of the BEETLES2 membrane is significant for maximizing the electrostatic interaction with the positively charged nucleocapsid protein.
To cross-check the optimal RBCM concentration, we analyzed the fluorescent images with different RBCM concentrations (0–4% (v/v)). Fluorescent lipids (PE-CF) were intercalated into RBCM vesicles and coated on AAO membranes. Next, fluorescent images were measured (Fig. S8). Based on topological analysis, KPFM, and fluorescent data, 2% RBCM concentration was determined to be the best for the BEETLES2 membrane. Additionally, we validated fluorescence recovery after photobleaching (FRAP) and measured the lateral diffusivity and mobility of RBCM on the AAO membrane (Fig. 2g). Fluorescence recovery across the photobleached spots (spot radius: 20 μm) at the focal plane confirmed the formation of mobile and contiguous lipid bilayers on the AAO membrane. We calculated the mobile fraction (MF) and 2D diffusion coefficient (D) from fluorescence intensity recovery over time. The bleached fluorescence gradually restored up to approximately 80% of the original value within 150 s of laser removal. We determined that the cell membrane translational diffusion coefficient was 0.83 μm2 s−1, consistent with lipids in intact RBCs (D = 0.82 μm2 s−1)32,33. This result indicates that RBCM preserves its lateral diffusivity and fluidity in 2D planar deposition.
Tunability and permselectivity of the BEETLES2 membrane
Figure 3 validates the performance of the BEETLES2 membrane in achieving fast permselective enrichment with the aid of fast water transport (flux: 221.61 Lm–2 h–1). Additionally, the AQP water-transporting proteins on RBCM provide a key route for permselective enrichment (Fig. 3a)34. We classified BEETLES2 membrane enrichment depending on pressure, molecular size, charge, and buffer conditions. The AAO membrane has a large pore size that allows various proteins such as albumin, viral protein, and immunoglobulin to pass through. However, the BEETLES2 membrane can selectively block these proteins because RBCM acts as a physical barrier. Interestingly, with external pressure, negatively charged and small molecules (e.g., albumin) can easily penetrate the BEETLES2 membrane owing to the high mobility of RBCM32,35.
a Flowchart showing the permselectivity and tunability of BEETLES2 membrane by pressure, molecular size, charge, and buffer conditions. b Pressure effect on the enrichment: negatively charged BSA with a strong dependency on pressure, showing tunable properties under pressure. c SDS-PAGE of BSA (MW: 66.5 kDa) d Size-based enrichment: Immunoglobulin G (IgG). e SDS-PAGE of IgG (MW: 150 kDa) and N Protein (MW: 46 kDa). f Surface charge-based enrichment (permselectivity): The enrichment with positively charged N proteins. g Surface charge effect (permselectivity): The enrichment with positively charged BSA (pI: 4.5~4.8) at lower pH, while no enrichment at higher pH (neutral and negatively charged conditions). (h, j) Enhanced sensitivity via BEETLES2 with (h) COVID-19 Ag test, (i) COVID-19 IgG rapid test, and (j) salivary COVID-19 Ag test. Error bars represent standard deviation from the mean. Panel a was created with BioRender.com. VTM viral transport medium, SDS-PAGE sodium dodecyl sulfate-polyacrylamide gel electrophoresis, BEETLES2 bioengineered enrichment tools for the LFA with enhanced sensitivity and specificity.
First, to elucidate the pressure effect on enrichment in terms of tunability, we operated BEETLES2 under various pressures (Fig. 3b–f). The error bar in Fig. 3 represents the run-to-run deviation (n = 3). We used NanoDrop and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to measure enrichment. Negatively charged BSA and positively charged N proteins were tested under a physiological condition. With positively charged N proteins, enrichment exceeded 24-fold measured by nanodrop regardless of pressure (0.1–3 bar). In contrast, negatively charged BSA showed strong dependency on pressure (Fig. 3b). Especially for BSA samples with lower pressure (0.1 bar), a significant increase in enrichment was observed due to no permeation of BSA. The most probable explanation is cell squeezing that enables delivery of a diversity of materials36, representing the need for applying pressure for negatively charged protein permeation. With increasing pressure, BSA freely passed through the BEETLES2’ membrane, without significant enrichment. Importantly, with tunable properties under pressure, we efficiently removed the most abundant protein, i.e., albumin, and small inhibitors. From SDS-PAGE, BSA was enriched under 0.1 bar while passing through over 1 bar, clearly confirming enrichment tunability under different pressures (Fig. 3c).
Second, we demonstrated the enrichment of IgG, with a molecular weight (MW) of 150 kDa, the most common antibody in blood and other body fluids (Fig. 3d). The daily monitoring of IgG could be a crucial test for convalescence in COVID-19. Owing to size filtration, we enriched IgG up to 18-fold regardless of the applied pressure (0.1–3 bar). To validate the size effect of IgG, we tested Human IgG Fc fragments (MW: 50 kDa) and observed no enrichment of IgG Fc fragments at a pressure of 3 bar, inferring the size effect of negatively charged IgG on the enrichments (Fig. S9). The pressure was controlled using regulator-controlled nitrogen gas. We enriched 3 mL of each sample into a final volume of 100 µL, theoretically corresponding to 30-fold enrichment. IgG enrichment was confirmed under a pressure of 3 bar (SDS-PAGE in Fig. 3e). Unlike an antigen or virus-based assay, for serological rapid diagnostic tests, only 1~2 drops (<100 µL) of blood was used. To handle tens of µL of samples, a new design is needed. One such prospective design could be the BEETLES2 membrane-integrated microfluidic system.
Third, we checked the surface charge effect on the permselectivity and enrichment of biomolecules (Fig. 3f). Under physiological conditions, N proteins (MW: 46 kDa) with isoelectric points (pI) of 10.3–10.7 exhibited an overall positive charge, whereas BSA (MW: 66.5 kDa) had a pI of 4.5–4.8, leading to a negative charge. The applied pressure was 3 bar. With a similar molecular weight, enrichment only with N proteins was approximately 24.16-fold, whereas BSA showed no significant enrichment. Unlike the negatively-charged BSA’s tunable properties between enrichment and separation with pressure, positively-charged N proteins showed only enrichment without tunability. These results indicate that the BEETLES2 membrane completely blocks the penetration of positively charged proteins through the filters via the electrostatic interaction of negatively charged phospholipids and positively charged viral proteins under physiological conditions37. The enriched N protein samples were acquired after 3 min of BEETLES2 operation (SDS-PAGE in Fig. 3e). In contrast, no enrichment from BSA in the enrichment zone was observed after 3 min.
To check the surface charge effect on enrichment, representing permselectivity, we controlled the surface charge of BSA (Fig. 3g). We conducted A280 absorbance measurements using NanoDrop to define the enrichment factor. Under a pH condition below the pI value of BSA (pI: 4.5–4.8), the overall surface charge was positive. At pH 3–4, under positively charged conditions, BSA was enriched up to 18.2-fold. Interestingly, the enrichment factor abruptly decreased near the pI value, where the overall charge seemed neutral. The most likely explanation for enrichment is the electrostatic force between the positively charged protein and negatively charged BEETLES2 membrane. At higher pH, the overall charge of BSA was negative. Thus, no significant decrease in the enrichment factor was observed.
Enhanced assay performance using the BEETLES2 membrane
Figure 3h shows the enhanced sensitivity of COVID-19 Ag using BEETLES2. Compared to a commercial LFA (AllCheck COVID-19 Ag, Calth Inc., Republic of Korea) without BEETLES2 enrichment, all colorimetric signals via BEETLES2 increased, indicating higher detection sensitivity. We prepared seven different concentrations from by diluting N proteins with a PBS buffer and tested three samples for each point. The LOD was enhanced up to 20-fold after BEETLES2 enrichment.
To validate the versatility of the COVID-19 assay, we conducted the COVID-19 IgG rapid test (Fig. 3i). Similar to the COVID-19 Ag test, we observed enhanced sensitivity using the sample enrichment process. The test was performed thrice per point for seven different concentrations. Consequently, the LOD was enhanced up to 20-fold after BEETLES2 enrichment, showing great applicability in the LFA assay. We confirmed that BEETLES2 could enrich N proteins and IgG, which are the primary targets for the SARS-CoV-2 test. Moreover, it is likely that BEETLES2 could be applied to other target viruses, such as Influenza A/B since their N proteins and IgG properties are similar to SARS-CoV-2. To validate the applicability of BEETLES2 to other viruses, we performed an Influenza rapid test (Fig. S10). We used Influenza Antigen A (Shangdong/9/93(94/516, NIBSC, United Kingdom) and B (Wisconsin/1/2010 (cell derived) (12/110, NIBSC, United Kingdom) with their rapid kits (SGT i-flex Influenza A&B, Sugentech, Republic of Korea). Similar to the COVID-19 Ag test, we acquired an enhanced colorimetric signal using the sample enrichment process.
To demonstrate salivary assay feasibility, we performed the COVID-19 Ag assay using saliva samples (Fig. 3j). The N proteins spiked in artificial saliva for seven different concentrations. As commercial LFA was not optimized for salivary assay, the colorimetric data with commercial COVID-19 Ag LFA revealed lower sensing performance with higher LOD. With BEETLES2, we increased the sensing performance with increasing LOD up to 20-fold.
Enhanced clinical assay using the BEETLES2 membrane
Clinical samples (COVID-19 patients (n = 42) and healthy controls (n = 20)), including NP/OP swabs (n = 30) and saliva samples (n = 12), were used to validate BEETLES2’s clinical potential (Fig. 4). To clearly demonstrate performance enhancement, we included samples with intermediate (26 ≤ Ct <30, n = 15: 15/42, 35%) and low viral loads (Ct ≥ 30, n = 20: 20/42, 48%) where the sensitivity of commercial LFA abruptly dropped.
Clinical samples include COVID-19 patients (n = 42) and healthy controls (n = 20). We included low-viral patient samples (Ct ≥ 30, n = 20: 48%) to observe the enhancement in the case of low viral load. We performed the clinical test for each patient once (n = 1). a–f Sensitivity enhancement with BEETLES2 using (a) NP/OP samples, (b) saliva samples, (c) Delta variants, d Omicron variants, (e) samples from asymptomatic individuals, and (f) healthy controls. g–i Diagnostic accuracy of BEETLES2, including all the samples of NP/OP, saliva, asymptomatic, and various variants, shows that the overall sensitivity of BEETLES2 is significantly higher (88.1%) (P < 0.0001) than those using commercial LFA (14.29%) (P = 0.0041). Two-sided unpaired t-test was used for analysis and adjustments were not made for multiple comparisons. j Cross-reactivity using different respiratory viruses showing no cross-reactivity. k RT-qPCR with enrichment with AAO only and BEETLES2, showing that BEETLES2 can enhance molecular diagnostics even with VTM. l–n The feasibility of daily monitoring of COVID-19 represents the ability of frequent tests with significantly higher accuracy. With BEETLES2, we measured the Ct value over 37. Error bars represent standard deviation from the mean. VTM viral transport medium, BEETLES2 bioengineered enrichment tools for the LFA with enhanced sensitivity and specificity, AAO anodic aluminum oxide, LFA lateral flow assay, NP/OP nasopharyngeal/oropharyngeal.
Reportedly, the test accuracy must be understood for different groups and samples because of the heterogeneity of antigen tests and population groups8. We analyzed the most widely spread SARS-CoV-2, Delta and Omicron variants. Reportedly, the pooled percentage of asymptomatic infections was 32.40% for Omicron38. We also checked the ability to test asymptomatic individuals. Most samples were collected at Seoul St. Mary’s hospital. We collected clinical NP/OP in a viral transport medium (VTM) and assay prepared using the standard protocol according to the LFA manufacturer’s guidelines. We called the commercial LFA for control as w/o BEETLES2 and denoted BEETLES2-assisted commercial LFA as w/ BEETLES2. We acquired the cut-off value by maximizing the sum of sensitivity (true positive) and specificity (true negative) of COVID-19 tests.
First, we checked the sensitivity enhancement with BEETLES2 using NP/OP samples (Fig. 4a: n = 30). After loading, samples from NP/OP were diluted with a PBS buffer and injected into the stirred cell. A constant pressure (~3 bar) was then applied with a regulator. Generally, the sensitivity of commercial LFA abruptly decreased virus titers in intermediate and low viral loads (Ct ≥ 26). With NP/OP samples, the sensitivity of commercial LFA was 10% (3/30 = 10%). The sensitivity was then increased with BEETLES2 (27/30 = 90%).
Second, we validated sensitivity using saliva samples (Fig. 4b). An assay using saliva samples has significant advantages for easy and non-invasive sample collection39; however, salivary assays need additional sample collection steps such as prefiltration. For the prefiltration of cells and debris, we used a 450-nm filter membrane. Reportedly, tests using saliva samples have higher detection sensitivity in the first 10 days after infection40. Compared to the commercial LFA’s sensitivity before enrichment (3/12 = 25%), all the samples tested using BEETLES2 had higher sensitivity (10/12 = 83.3%), indicating performance enhancement postsample enrichment. For the practical use of salivary assays with BEETLES2, we suggest a simple saliva collection and purification system (Pure-SAL, Oasis Diagnostics) (Fig. S11a).
Third, we observed the sensitivity using two variants (Fig. 4c, d, respectively). Generally, the LFA of the COVID-19 Ag test targets N proteins. Unlike RT-qPCR, which strongly depends on the variant, such as Delta and Omicron41, the N proteins targeted by the COVID-19 Ag test are less dependent on mutations. Both for Delta (n = 10, Fig.4c) and Omicron (n = 32, Fig. 4d), all the samples were tested using BEETLES2 and commercial LFA. Consequently, we acquired higher sensitivity for Delta (10/10 = 100%) and Omicron (27/32 = 84.38%) with BEETLES2 than with commercial LFA: 20% (2/10 = 20%) and 12.5% (4/32 = 12.5%) for Delta and Omicron, respectively.
Fourth, we considered asymptomatic cases (Fig. 4e). Fast identification of asymptomatic cases is a key strategy for reducing onward transmission of COVID-19 viruses42. The true positive values, i.e., sensitivity, using BEETLES2 was higher (3/4 = 75%) than the commercial LFA (controls: 0/4 = 0%). We elucidated data points near the cut-off line with LFA test results (Fig. S12). Although the number of asymptomatic samples was not sufficient, BEETLES2 might provide earlier and better detection of asymptomatic individuals.
Fifth, to check the specificity, we tested the colorimetric signals from healthy controls (n = 20) showing 100% specificity (true negatives = 20/20) (Fig. 4f). Specificity is known as the true negative rate. Therefore, we increased the specificity by reducing false positives.
Overall, we assessed the diagnostic accuracy of BEETLES2, considering all samples of NP/OP, saliva, asymptomatic, and various variants (Fig. 4g and h). Figure 4g shows the assay with BEETLES2, and Fig. 4h shows the assay with commercial LFA. The overall true positive values, i.e., sensitivity, using BEETLES2 is significantly higher (37/42 = 88.1%) than those using commercial LFA without BEETLES2 (6/42 = 14.29%) (Fig. 4i). Our proposed BEETLES2 system meets the World Healthcare Organization’s (WHO) ‘desirable criteria’ guidelines for POCT (sensitivity: >90%, specificity: >99%, Ct ≥ 30, and assay time: <20 min) beyond the ‘acceptable criteria’ (sensitivity: >80%, specificity: >97%, 26 ≤ Ct ≤35, and assay time: <40 min)43.
Another important parameter for COVID-19 diagnostics is cross-reactivity (Fig. 4j). We evaluated cross-reactivity using different respiratory viruses such as respiratory syncytial virus A (RSV A), RSV B, Influenza A(H1N1), Influenza B, and Human coronavirus-OC43 (HCoV-OC43) (n = 3 for each sample) and observed no cross-reactivity.
Both the LFA test and BEETLES2 enrichment could contribute to cross-reactivity and specificity. However, since the LFA manufacturer strictly controlled cross-reactivity and specificity (>99%), we expect that the results of cross-reactivity and specificity are primarily due to BEETLES2 enrichment (Table. S1). We observed no cross-reactivity and specificity problems for other viruses, demonstrating a high potential to meet WHO criteria.
An AAO membrane has been used to develop an immunoassay for SARS-CoV-2 detection in saliva by enriching SARS-CoV-2 viruses9. Following that study, we prepared only an AAO membrane and observed virus purification and enrichment via RT-qPCR (Fig. 4k). In PBS buffers, the intact viruses could be enriched through the AAO membrane, as presented. However, we could not enrich virus particles using the AAO membrane in a lysate buffer (VTM) due to a lack of VTM lysates viruses for enrichment. Therefore, the Ct value using the AAO membrane did not change significantly. In many real clinical cases, samples are delivered with inactivated lysate (i.e., VTM). The Ct value was improved using BEETLES2, even with VTM, up to 5.1, theoretically corresponding to 34.3 enrichment concentrations.
Sample-to-answer platform for daily monitoring
To achieve the daily monitoring feasibility for COVID-19, we collected daily samples of NP/OP and saliva from infected individuals with symptoms (Fig. 4l–m). The day of onset (day 0) was defined as the first day of symptoms. All the patients were RT-qPCR positive on the first day of symptoms. We acquired daily data via RT-qPCR and LFA with BEETLES2 enrichment. As RT-qPCR can detect viral shedding long after the infectious period (~9 days), the positive result from RT-qPCR might include unnecessary quarantine. Conversely, frequent highly sensitive LFA testing (2–3 daily tests) can provide changes in the on/off signal, even the quantitative data. Daily monitoring data revealed that the LFA assay with BEETLES2 is highly correlated with the viral load (Ct value), representing that our technique evaluates frequent tests with significantly higher accuracy than the commercial LFA and RT-qPCR. With BEETLES2, we could measure the Ct value over 37, which the commercial LFA could not detect (Fig. 4n).
Compared with other existing virus isolation and enrichment techniques, our BEETLES2 has advantages in the permselectivity, tunability, powerful enrichment capability for both intact viruses and their N proteins, and applicability as a hand-held device (see Table. S2 for the comparison of different virus isolation and enrichment focusing techniques). To commercialize BEETLES2, we should address the following issues: 1) pressure endurable design. Since the BEETLES2 assay is performed under applied pressure, no liquid sample leakage can be tolerated. Especially when handling infectious samples, a leak-free design is needed for safety. 2) The volume issue in serological rapid diagnostic tests. For serological rapid diagnostic tests, 1–2 drops (<100 µL) a typically acquired from a finger prick; therefore, we need to develop devices for blood-based tests with small sample volumes. A microfluidic device integrated with the BEETLES2 membrane is an ideal candidate for serological assays, 3) Shelf life is a key parameter to commercialize BEETLES2. We obtained stability, for up to 15 days, under room temperature. However, the expected shelf life can be extended further with additional tests (Fig. S13).
In conclusion, BEETLES2’s sample preparation with its powerful permselectivity and tunability provides more sensitive binary response than commercial LFA in 3 min. It has two significant practical advantages: first, the system can be easily coupled with well-established commercial LFAs and increase its clinical sensitivity (>88%) and accuracy (>91%) for low titer clinical samples including Delta and Omicron variants, compared with commercial LFA (14.29% and 41.94%, respectively). Second, BEETLES2 can selectively enrich the SARS-CoV-2 virus, N proteins, and IgG from the nasal swab, saliva, and blood serum, showing its versatility and applicability to COVID-19 Ag and IgG testing.
Combining sample enrichment of BEETLES2 with commercial LFA represents an advance in POCT detection since it meets all the requirements stated by WHO for POCT, i.e., ease of use, low cost, and accuracy44. We expect BEETLES2 to be commercialized at a nominal additional cost, thereby meeting the inexpensive criteria. Moreover, the combination of AI-assisted smartphone applications with BEETLES2 could precisely predict on/off switching and perform quantitative classification, demonstrating great potential for the REASSURED (Real-time connectivity, Ease of specimen collection, Affordable, Sensitive, Specific, User friendly, Rapid, Equipment free and Delivered)45, a new criterion for digital connectivity, and possibly detect asymptomatic transmission with simple frequent test strategies8,42, which is difficult with RT-qPCR testing.
Methods
Ethical statement
Respiratory samples were prospectively collected from patients diagnosed with COVID-19 infection at Seoul St. Mary’s Hospital from April 2021 until May 2022. This study was approved by the institutional review board (KC21TIDI0134K) at Seoul St. Mary’s Hospital, and informed consent was obtained from the participants.
BEETLES2 membrane: preparation of RBCM
We extracted RBCM from K2EDTA anticoagulated human whole blood and removed the plasma and buffy coat using an 800-g centrifuge for 5 min to isolate red blood cells. After removing the supernatant, we washed the remaining red blood cells thrice with ice-cold 1×PBS with gentle handshaking. Next, we hemolyzed the red blood cells by suspending them in ice-cold 0.25×PBS for 30 min. Finally, we eliminated free hemoglobin using a 20,000-g centrifuge for 30 min. After three wash-outs, a light pink RBCM pellet was obtained and stored at −80 °C for future use. The extracted RBCMs were in the form of a negatively charged liposome of 200 nm size (Fig. S2). The sonicated solution was dropped on the AAO membrane (6809-6002, Whatman, UK) and incubated for 30 min at 50 °C to form multiple supported lipid bilayers. To investigate the microstructure of the BEETLES2 membrane, we conducted structural analysis using SEM, AFM, and fluorescent microscopy.
BEETLES2 membrane: property analysis
AAO-RBCM membranes were sampled for KPFM imaging on a p-type silicon wafer (ePAK International, USA), a substrate with electrical conductivity. The silicon wafers were immersed in piranha solution (H2O2 and H2SO4) for 15 min, washed with distilled water, and dried with N2 gas (Sejong Industrial Gas Co., Korea). A MultiMode VIII atomic force microscope was used to analyze the AAO-RBCM membranes electrically and topologically in amplitude-modulated KPFM mode (Bruker, USA). KPFM measurements were performed at 23 °C in air using the lift scan mode based on the tapping mode. Conductive AFM tips coated with Pt (SCM-PIT-V2; Bruker, USA) were used to analyze the nano-electrical properties of the samples. A topological AFM image was captured in the first scan using the tapping mode with a zero-tip bias. To detect the surface potentials, the AFM tip was lifted 20 nm above the sample surface with an applied sample bias voltage during interleave scanning. The mechanical drive to the cantilever was turned off during interleave scanning, and a bias voltage of alternating current (AC) at 1000 mV was applied to the probe at the mechanical resonance (ω) of the cantilever. The VAC causes the cantilever to oscillate owing to attractive and repulsive electrostatic interactions (Fes) between the probe and sample, defined as follows:
where VDC is the direct current (DC) bias voltage, and VCPD is the contact potential difference between the probe and sample.
Applying a compensating VDC to the probe to eliminate electrostatic forces (i.e., Fes) between the probe and sample establishes a proportional-integral-derivative feedback loop that monitors and controls the amplitude of cantilever oscillations. These depend on the capacitance C and height z of the probe and sample. The KPFM scan rate was 0.6 Hz, the scan size and amplitude setpoint at 12 nm was 4 μm × 4 μm at 512 × 512 pixels, respectively. The MountainsSPIP software was used to level, process, and analyze the AFM images (version 9; Digital Surf, France). In addition, using Gwyddion, surface roughness studies were carried out (version 2.60).
FRAP was used to validate the SLB formation of RBCMs for assessing their fluidity and diffusivity. In all experiments, 1 wt% PE-CF was intercalated into RBCMs as the fluorescent probe. We bleached a 20 μm diameter spot at the z-plane of the RBCM layers via a 488-nm optically pumped semiconductor laser (Coherent, Inc.) at 150 mW for 5 s. Fluorescence recovery after photobleaching at the desired spot was monitored for 5 min via a Zeiss LSM-800 confocal microscope. Each image reference spot served as a standard for measuring and normalizing spot fluorescence intensity. The normalized fluorescence intensity against time fit with a Bessel function was reported by Soumpasis et al.26. Next, the diffusion coefficient (D) of the dye in each SLB composition was calculated using the following expression: D = w2/4t1/2, where w is the radius of the photobleached spot and t1/2 is the time required to achieve half of the maximum recovery of fluorescence intensity.
Assay: Reagents and analysis
We used commercial COVID-19 Ag rapid kits (Calth Inc., Republic of Korea) and tested the enhanced performance via BEETLES2. We prepared a COVID-19 N protein recombinant antigen (45 kDa, FPZ0516, Fapon Biotech Inc., China), considered the best COVID-19 Ag test target, using 1×PBS buffers (LB004, DUKSAN, Republic of Korea) to test sensitivity and LOD. Bovine serum albumin (BSA) (A7030, Sigma-Aldrich, USA), Human IgG (I4506, Sigma-Aldrich, USA) and Human IgG Fc fragments (401104, Sigma-Aldrich, USA) were purchased from Sigma-Aldrich. Subsequently, we prepared seven concentrations of the COVID-19 N protein sample with 1xPBS. We added ~3 mL of each sample onto a polycarbonate stirred cell (341000, STERLITECH, USA) and operated for 3 min. Next, the 100 μL of the enriched sample was recovered with a pipette. NanoDrop spectrophotometers (Thermo Scientific, USA) and SDS-PAGE (KOMA precast gel BC type, Koma Biotech, Republic of Korea) were used to analyze and quantify proteins.
For the COVID-19 Ab test (Fig. 3i), we prepared seven concentrations of the COVID-19 antibody (sodium citrate plasma, Trina Bioreactives AG, Switzerland) sample with 1xPBS. We added 3 mL of each sample into a polycarbonate stirred cell (341000, STERLITECH, USA) mounted with an RBCM-coated AAO membrane and operated it for 3 min. Next, the enriched sample was recovered via a pipette with 100 μL LFA extraction buffer. Subsequently, we used the enriched sample in the COVID-19 Ab LFA kit (AllCheck COVID-19 IgG/IgM, Calth Inc., Korea) and noted the result at 10 min.
Similarly, for the salivary COVID-19 Ag test (Fig. 3j), we diluted seven concentrations of the COVID-19 N protein sample with artificial saliva (A7990, Solarbio, China). First, we added 3 mL of each sample into a polycarbonate stirred cell mounted with an RBCM-coated AAO membrane and operated it for 3 min. Next, the enriched sample was recovered via a pipette with 100 μL of LFA extraction buffer. Subsequently, we used the enriched sample in the COVID-19 Ag LFA kit and noted the result at 10 min.
We analyzed the color intensities of the commercial LFA kit and BEETLES2-assisted LFA using a custom-made National Instrument (NI) controlled optical system coupled with LabVIEW v 2019 SP1 (National Instruments Co., USA). To set the LOD (Fig. 3) and cut-off values (Fig. 4), we first measured color signals using the commercialized reader and custom-made NI-controlled optical system with LabVIEW. Next, five individually trained engineers (Calth Inc. http://www.thecalth.com) observed the colorimetric signal using the standard color chart and manufacturer’s guidelines to determine the LOD. Meanwhile, we set the cut-off values to minimize false positives for acquiring higher specificity under the manufacturer’s guidelines.
Assay: clinical validation
We collected clinical samples of NP/OP and saliva from COVID-19 patients at Seoul St. Mary’s hospital with appropriate Institutional Review Board Committee approval (KC21TIDI0134K). Although NP/OP swabs were contained in the viral transport medium (VTM), saliva samples were collected in a sterilized tube and diluted with PBS.
To test the performance in low-viral targets, for high viral load (Ct <26), we spiked NP/OP samples into PBS buffers and prepared them as low-viral samples. For saliva samples with a high viral load, we spiked the samples with PBS buffer. Finally, we prepared low-viral samples with Ct ≥ 30. For healthy controls, we collected (or purchased) samples from healthy volunteers and stored them at −20 °C. The obtained results were compared with RT-qPCR SARS-CoV-2 tests using AccuPower® SARS-CoV-2 variants ID2 kit (BIONEER, Republic of Korea), according to the manufacturer’s protocol. The cut-off value for on-site COVID-19 diagnostics was acquired by maximizing the sum of sensitivity (true positive) and specificity (true negative) of COVID-19 tests.
To realize the prototype for POCT sample preparation, we fabricated a hand-powered portable gadget integrated with the BEETLES2 membrane (See Fig. S11). We designed two reservoirs: sample and commercially available running buffer reservoirs. Micro milling was used for the prototype. We calculated the average hand-powered pressure from five individuals (4 men and 1 woman) with BEETLES2 as 2.3 ± 0.2 bar (Fig. S14). We showed that the permselectivity of BSA and N proteins was maintained under hand-powered pressure. Hand-powered portable syringes are low-cost alternatives for point-of-care diagnostics, which can be directly applicable to commercial LFA. We first connected the BEETLES2 membrane to the sample reservoir and pushed the plunger for enrichment. After 3 min of operation, we reversed the direction of BEETLES2 membrane attachment to the running buffer reservoir. Since the commercial running buffer included a surfactant, we could successfully demonstrate the N protein assay regardless of the electrostatic force between the N protein and RBCM layer. Subsequently, we assayed using commercial LFA following the manufacturer’s guidelines. With further investigation, we are developing a simplified second prototype wherein a vacuum chamber is used for applying pressure.
Statistics and reproducibility
The error bars presented in the figures represent the mean ± SD. We repeated all the experiments at least thrice per point and analyzed the data using Microsoft Exel, Prism v 7.0. Biorender, 3D MAX v 2020, V-Ray v 4.0, Adobe Photoshop v 2020, and Adobe Illustrator v 2020 software were used for graphical analyses. No statistical method was used to predetermine sample size. To determine the significance of the clinical validation, we analyzed its data with a two-sided unpaired t-test to examine the differences between the groups. A P value below 0.05 was considered statistically significant.
Data availability
Source data are provided with this paper.
References
Watson, J., Whiting, P. F. & Brush, J. E. Interpreting a covid-19 test result. Bmj 369, m1808 (2020).
Vandenberg, O., Martiny, D., Rochas, O., Belkum, A. V. & Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 19, 171–183 (2021).
Food U., Administration D. In vitro diagnostics EUAs—antigen diagnostic tests for SARS-CoV-2. FDA, Silver Spring, MD https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2[Google Scholar], (2021).
Qiu, G. et al. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. Acs. Nano 14, acsnano.0c02439 (2020).
Macchia, E. et al. A handheld intelligent single-molecule binary bioelectronic system for fast and reliable immunometric point-of-care testing. Sci. Adv. 8, eabo0881 (2022).
Lee, J. H. et al. A rapid quantitative on-site coronavirus disease 19 serological test. Biosens. Bioelectron. 191, 113406 (2021).
Fozouni, P. et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 184, 323–333.e329 (2021).
Crozier, A., Rajan, S., Buchan, I. & McKee, M. Put to the test: use of rapid testing technologies for covid-19. Bmj 372, n208 (2021).
Chen, Y., Liu, F. & Lee, L. P. Quantitative and ultrasensitive in situ immunoassay technology for SARS-CoV-2 detection in saliva. Sci. Adv. 8, eabn3481 (2022).
Seo, G. et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. Acs. Nano 14, acsnano.0c02823 (2020).
Ferreira, A. L. et al. Low-Cost Optodiagnostic for Minute-Time Scale Detection of SARS-CoV-2. Acs. Nano 15, 17453–17462 (2021).
Mei, X. et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 26, 1224–1228 (2020).
Cheong, J. et al. Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nat. Biomed. Eng. 4, 1159–1167 (2020).
Teymouri, M. et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 221, 153443 (2021).
Beeching, N. J., Fletcher, T. E. & Beadsworth, M. B. J. Covid-19: Testing times. BMJ 369, m1403 (2020).
Lai, C.-C., Wang, C.-Y., Ko, W.-C. & Hsueh, P.-R. In vitro diagnostics of coronavirus disease 2019: Technologies and application. J. Microbiol. Immunol. Infect. 54, 164–174 (2021).
Pokharel, S. et al. Two-test algorithms for infectious disease diagnosis: Implications for COVID-19. PLoS Global Public Health 2, e0000293 (2022).
Santiago, I. Trends and Innovations in Biosensors for COVID-19 Mass Testing. ChemBioChem 21, 2880–2889 (2020).
Mina, M. J., Parker, R. & Larremore, D. B. Rethinking Covid-19 Test Sensitivity — A Strategy for Containment. New Engl. J. Med. 383, e120 (2020).
Cevik, M., Kuppalli, K., Kindrachuk, J. & Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. Bmj 371, m3862 (2020).
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet. Microbe. 2, e13–e22 (2021).
Singh, N. K. et al. Hitting the diagnostic sweet spot: Point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. Biosens. Bioelect. 180, 113111 (2021).
Mabey, D., Peeling, R. W., Ustianowski, A. & Perkins, M. D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2, 231–240 (2004).
Woo, H.-K. et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. Acs Nano 11, 1360–1370 (2017).
Chen, Y. et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 18, 212–218 (2021).
Jani, A. M. M., Losic, D. & Voelcker, N. H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 58, 636–704 (2013).
Yang, J., Lin, G.-S., Mou, C.-Y. & Tung, K.-L. Mesoporous silica thin membrane with tunable pore size for ultrahigh permeation and precise molecular separation. ACS Appl. Mater. Interf. 12, 7459–7465 (2020).
Lee, C. S., Kim, I. & Jang, J. W. Yoon Ds, Lee YJ. Aquaporin‐Incorporated Graphene‐Oxide Membrane for Pressurized Desalination with Superior Integrity Enabled by Molecular Recognition. Adv. Sci. 8, 2101882 (2021).
Jang, J. W. et al. Surface Functionalization of Enzyme-Coronated Gold Nanoparticles with an Erythrocyte Membrane for Highly Selective Glucose Assays. Anal. Chem. 94, 6473–6481 (2022).
Fernandes, H. P., Cesar, C. L. & Barjas-Castro, M. D. L. Electrical properties of the red blood cell membrane and immunohematological investigation. Revista brasileira de hematologia e hemoterapia 33, 297–301 (2011).
Kalinin, S. V. & Balke, N. Local electrochemical functionality in energy storage materials and devices by scanning probe microscopies: status and perspectives. Adv. Mater. 22, E193–E209 (2010).
Bloom, J. A. & Webb, W. W. Lipid diffusibility in the intact erythrocyte membrane. Biophys. J. 42, 295–305 (1983).
Filippov, A., Orädd, G. & Lindblom, G. The Effect of Cholesterol on the Lateral Diffusion of Phospholipids in Oriented Bilayers. Biophys. J. 84, 3079–3086 (2003).
Li, Y. et al. Water-ion permselectivity of narrow-diameter carbon nanotubes. Sci. Adv. 6, eaba9966 (2020).
Joo, B., Hur, J., Kim, G.-B., Yun, S. G. & Chung, A. J. Highly Efficient Transfection of Human Primary T Lymphocytes Using Droplet-Enabled Mechanoporation. Acs. Nano 15, 12888–12898 (2021).
Ding, X. et al. High-throughput nuclear delivery and rapid expression of DNA via mechanical and electrical cell-membrane disruption. Nat. Biomed. Eng. 1, 0039 (2017).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Shang, W. et al. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines (Basel) 10, 1049 (2022).
Soares Nunes, L. A., Mussavira, S. & Sukumaran Bindhu, O. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem. Med. 25, 177–192 (2015).
Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. New Engl. J. Med. 383, 1283–1286 (2020).
Ascoli, C. A. Could mutations of SARS-CoV-2 suppress diagnostic detection? Nat. Biotechnol. 39, 274–275 (2021).
Quilty, B. J. et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Heal 6, e175–e183 (2021).
Organization W. H. COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic v. 1.0.). World Health Organization (2020).
Straightforward, inexpensive and sensitive. Nat. Biomed. Eng. 6, 923–924 (2022).
Land, K. J., Boeras, D. I., Chen, X.-S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019).
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2021M3E5E3080743).
Author information
Authors and Affiliations
Contributions
S.J.P., S.L., D.L. (Dongtak Lee), D.S.Y., Y.K.Y., and J.H.L. conceived and designed the study. N.E.L., J.S.P., and J.H.H. conducted the PCR experiments. D.L. (Dongtak Lee), J.W.J., and H.K. performed the extraction of the red blood cell membrane and material characterization. S.R. and G.L. conducted Kelvin probe force microscopy-based material characterization. D.L. (Dongho Lee), S.C., C.P., D.L. (Dong-Gun Lee), R.L., and D.N. provided clinical samples and interpreted the results. S.J.P., S.L., D.L. (Dongtak Lee), Y.K.Y., and J.H.L. drafted the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Olivier Vandenberg and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, S.J., Lee, S., Lee, D. et al. PCR-like performance of rapid test with permselective tunable nanotrap. Nat Commun 14, 1520 (2023). https://doi.org/10.1038/s41467-023-37018-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-37018-6
This article is cited by
-
Biporous silica nanostructure-induced nanovortex in microfluidics for nucleic acid enrichment, isolation, and PCR-free detection
Nature Communications (2024)
-
Integration of an Aptamer-Based Signal-On Probe and a Paper-Based Origami Preconcentrator for Small Molecule Biomarkers Detection
BioChip Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.