Abstract
Obesity increases asthma prevalence and severity. However, the underlying mechanisms are poorly understood, and consequently, therapeutic options for asthma patients with obesity remain limited. Here we report that cholecystokinin—a metabolic hormone best known for its role in signaling satiation and fat metabolism—is increased in the lungs of obese mice and that pharmacological blockade of cholecystokinin A receptor signaling reduces obesity-associated airway hyperresponsiveness. Activation of cholecystokinin A receptor by the hormone induces contraction of airway smooth muscle cells. In vivo, cholecystokinin level is elevated in the lungs of both genetically and diet-induced obese mice. Importantly, intranasal administration of cholecystokinin A receptor antagonists (proglumide and devazepide) suppresses the airway hyperresponsiveness in the obese mice. Together, our results reveal an unexpected role for cholecystokinin in the lung and support the repurposing of cholecystokinin A receptor antagonists as a potential therapy for asthma patients with obesity.
Similar content being viewed by others
Introduction
Asthma is a major chronic lung disease that affects over 300 million people worldwide1. A key feature of asthma is airflow limitation, often caused by hyperactive contraction of airway smooth muscle (ASM) cells2. Consequently, a major therapeutic goal for asthma is to reduce ASM constriction2. β-agonists—a mainstay asthma therapy—work by relaxing ASM to relieve bronchospasm3,4. However, long-term use of β-agonists, especially long-acting β-agonists (LABAs), can lead to paradoxically adverse effects, including loss of asthma control and even death5,6. Furthermore, standard controller therapy that uses inhaled corticosteroid in combination with a β-agonist does not work well for certain patient populations, notably patients with obesity.
Obesity is a well-known risk factor for asthma: obesity increases both the prevalence and incidence of asthma and worsens asthma control7. It is estimated that in the United States alone, obesity leads to approximately 250,000 new cases of asthma each year8. In general, asthma patients with obesity have more symptoms, use more healthcare, and have worse quality of life7. Consistent with these human studies, obese mice exhibit innate airway hyperresponsiveness (AHR)9, a cardinal feature of asthma. Obesity also affects response to asthma therapy7. For example, corticosteroids are less effective in asthma patients with obesity10,11,12,13, for whom asthma control is more difficult to achieve14,15,16,17. There is also evidence that bronchodilators are less effective in asthma patients with obesity18. Despite the overwhelming evidence linking obesity to worsened asthma and to reduced response to therapy, the underlying mechanisms are not well understood. Consequently, there are few therapeutic options for asthma patients with obesity.
Cholecystokinin (CCK) is a peptide hormone found predominantly in the gastrointestinal (GI) tract and throughout the central nervous system (CNS)19. In the GI tract, CCK is released in response to food intake to regulate the motility and contraction of gall bladder and stomach20,21. In the nervous system, CCK acts as a neurotransmitter that signals satiation and modulates nociception22. While the function of CCK has been described mostly in the GI tract and in the CNS, CCK has also been shown to stimulate the secretion of calcitonin, insulin and glucagon and to act as a natriuretic kidney peptide23. CCK has two specific receptors, cholecystokinin A receptor (CCKAR) and cholecystokinin B receptor (CCKBR), which are expressed predominantly in the GI tract and nervous system to mediate the action of CCK24. In the GI tract, CCKAR is the predominant CCK receptor that induces the contraction of smooth muscle of gall bladder and stomach25,26. CCK receptors have been detected in the lung27,28,29,30. In isolated rat pulmonary interstitial macrophages, CCK receptors mediated the inhibitory effect of CCK on lipopolysaccharide-induced TNF-α and IL-1β mRNA expression31,32. Studies have also shown that CCK receptors are expressed by small cell lung cancer cells and that CCK elevates cytosolic calcium in these cells, suggesting a functional signaling pathway28,33,34,35. Intriguingly, Stretton and Barnes showed that a CCK octapeptide constricts both guinea pig and human airways36. However, the potential in vivo function of CCK in AHR or asthma was not further explored, and it was not known which CCK receptor mediates the CCK action in the airway.
In this study we present evidence that CCK and its receptor CCKAR are functionally expressed in and induce contraction of ASM cells. In vivo, CCK is upregulated in the airways of diet-induced and genetically obese mice, suggesting that the hormone contributes to the heightened AHR associated with obesity. Our data using obese mouse models further demonstrate that antagonizing CCK/CCKAR in the lung attenuates obesity-induced AHR. Our studies provide critical preclinical evidence that supports the development of CCKAR antagonists into new therapeutics to treat asthma patients with obesity.
Results
CCKAR expression in ASM
A large family of proteins known as G-protein-coupled receptors (GPCR) are direct targets of about 35% of all modern pharmaceuticals37. Many existing asthma drugs such as β-agonists, anti-cholinergics, and leukotrienes work by targeting their cognate GPCRs in the airways9. To identify potential new therapeutic targets for asthma, we evaluated the expression of non-olfactory GPCRs (361 in total) in primary human ASM cells using our previously published RNA-seq-based transcriptomic dataset38. We assessed the expression of each non-olfactory GPCR based on the values of Fragments Per Kilobase of transcript per Million (FPKM) mapped reads. From this analysis, we identified 115 (~32%) non-olfactory GPCRs that are moderately-to-highly expressed (FPKM > 0.1) in ASM cells (Supplementary Data).
Among the top 5 expressed GPCRs, CCKAR has not been previously implicated in any aspect of ASM function. CCKAR expression in ASM cells has a high FPKM value of 49.63 (Fig. 1a and Supplementary Data). In comparison, the β-adrenergic receptor 2 (gene name ADRB2), which is the target of bronchodilator β-agonists, has an FPKM value of 0.27 (Fig. 1a and Supplementary Data). Interestingly, the other CCK receptor CCKBR is not expressed at all in the ASM cells (no reads in the RNA-seq data). The expression and function of CCKAR have been mostly investigated in extrapulmonary tissues such as the gallbladder and the sphincter of Oddi where it mediates CCK-induced contraction and relaxation, respectively39,40. However, CCKAR’s surprisingly high expression in ASM cells suggests a potential biological role for the receptor in the ASM and in airways.
a List of the top non-olfactory GPCRs expressed in ASM cells. b qRT measurement of CCKAR mRNA expression in different airways cell types. FIB: primary lung fibroblasts; HBE: human bronchial epithelial (HBE) cells: HBE(D0) day 0 of initiation of air-liquid interface (ALI) culture; HBE(D14): day 14 post-ALI; ASM: airway smooth muscle cells. Data are presented as mean ± SEM. n = 3 biologically independent samples per group. c Immunostaining of human lung tissue section for CCKAR (green). α-SMA (α-smooth muscle actin, red) was used as a marker of ASM cells. DAPI was used to stain the nuclei (blue). Scale bar = 25 µm. Shown is a representative image from two independent experiments with similar results. d OMTC-based measurement of cell stiffness in ASM cells treated with CCK agonist A71623 (10 nM), acetylcholine (Ach), β-agonist (Alb: albuterol) or PBS (vehicle). a.u.: arbitrary unit. e Representative TFM images in ASM cells treated with vehicle (Control), A71623 (10 nM), acetylcholine (Ach, 1 µM) or combination of A71623 (10 nM) and Ach (1 µM). Scale bar = 10 mm). f Quantification of TFM-based contractility measurement in ASM cells treated with vehicle (n = 4), A71623 (n = 8), Ach (n = 3), or combination A71623 and Ach (n = 8). Data are presented as mean ± SEM; biologically independent samples per group; A71623 vs. Control (P = 0.0020); Ach vs. Control (P = 0.0160); A71623 vs Ach + A71623 (P = 0.0222); Ach vs. Ach + A71623 (P = 0.0130); one-tailed, unpaired t-test (*P < 0.05). Source data are provided as a Source Data file.
We next validated the expression of CCKAR in ASM cells using qRT-PCR analysis. We found that CCKAR is highly expressed in ASM cells but is very lowly expressed in other cell types of airways, including lung fibroblasts, basal and differentiated bronchial epithelial cells (Fig. 1b). Moreover, immunostaining of human airway tissue sections supported the expression of CCKAR in the lung (Fig. 1c and Supplementary Fig. S1), specifically in ASM as shown by colocalization with the known ASM marker α-smooth muscle actin (αSMA) (Fig. 1c). Together these results demonstrate the expression of CCKAR in ASM cells.
Activation of CCKAR induces ASM contraction
CCKAR is known to mediate smooth muscle contraction in gallbladder and pyloric sphincter39,40; however, in the sphincter of Oddi, CCKAR mediates relaxation of the muscle tissue39,40. To test whether CCKAR evokes contraction or relaxation in ASM cells, we measured changes of cell stiffness using a biomechanical assay known as optical magnetic twisting cytometry (OMTC)41. Changes in cell stiffness measured in this way are tightly related to changes in contractile force42. Upon treatment with a highly specific CCKAR agonist A71623 (10 nM) the stiffness of ASM cells increased (Fig. 1d). Such increase in cell stiffness is comparable in magnitude and timing (~1.4 fold, ~50 s) to that induced by acetylcholine, a known ASM constrictor, and opposite to that induced by albuterol, a β-agonist that relaxes ASM cells. To assess more directly the effect of A71623 on ASM contractility, we used traction force microscopy (TFM), which measures the contractile forces exerted by cells on their surroundings (e.g. extracellular matrix)43. Using TFM, we measured contractile force in ASM cells pre- and post-treated with A71623 and found that A71623 increased ASM cell contraction by ~2.4 fold in the force response ratio compared to control (Fig. 1e, f). Moreover, A71623 enhanced acetylcholine-induced contraction of ASM cells as the effect of adding both compounds was greater than that induced by either compound alone (Fig. 1e, f). Together these biophysical measurements demonstrated that the CCKAR agonist A71623 induces contraction of ASM cells.
We next investigated whether ASM contraction by A71623 is specifically mediated by CCKAR. We first generated independent CCKAR-knockout ASM lines (CCKAR-KO1 and CCKAR-KO2) using the CRISPR/Cas9 technology with two different guide RNAs targeting distinct regions of the CCKAR gene (Fig. 2a). CRISPR-mediated knockout of CCKAR prevented A71623-induced contraction of ASM cells as measured by TFM (Fig. 2b). Similarly, there was no increase in cell stiffness upon A71623 treatment in the two CCKAR-KO ASM cell lines (Fig. 2c). Consistent with the results in CCKAR-KO cells, pretreatment with a pan-CCK antagonist lorglumide (Fig. 2d) or a highly specific CCKAR antagonist devazepide (Fig. 2e) completely abolished A71623-induced stiffening of ASM cells. Together, these data demonstrate that CCKAR is required for CCK agonist-induced contraction in ASM cells.
a T7E1 assay on CRISPR/Cas9-mediated CCKAR knockout ASM cells. CCKAR knockout (KO) cells were generated by lentiviral delivery of gRNAs targeting the CCKAR gene. DNA cleavage indicating mismatch and mutation induced by the two gRNAs targeting CCKAR was detected by T7 Endonuclease 1. Multiple bands in the DNA gel indicate mutation in the CCKAR gene in KO cells (KO1 and KO2). b TFM-based contractility measurement in control vs. CCKAR-KO ASM cells in response to A71623 treatment (10 nM). Data are presented as mean ± SEM; n = 4 biologically independent samples per group; sc vs. KO1 (P = 0.002); sc vs. KO2 (P = 0.0004); one-tailed, unpaired t-test (*P < 0.05). c OMTC-based measurement of cell stiffness in control vs. CCKAR-KO cells in response to A71623 treatment (100 nM). d, e OMTC-based measurement of cell stiffness in response to A71623 treatment (100 nM) and in the presence of devazepide (d) or lorglumide (e). Ethanol and PBS were used as vehicles for devazepide and lorglumide, respectively. sc Scrambled control, dvz Devazepide, lorg Lorglumide, EtOH Ethanol, a.u. Arbitrary unit. Source data are provided as a Source Data file.
CCK expression and induction by free fatty acids in ASM cells
Our in vitro biophysical experiments so far have used a synthetic analogue of CCK hormone (A71623) that was given exogenously to ASM cell cultures. Studies have shown that cells that express CCK receptors often also express endogenous CCK, which provides evidence for autocrine activation and signaling of CCKAR44,45. We thus examined whether ASM cells also express CCK. Our qRT-PCR measurements (Fg. 3a) showed that CCK is expressed in ASM cells at a level much higher than in other airway cells such as lung fibroblasts, basal airway epithelial cells, or differentiated airway epithelial cells. Immunostaining experiment also showed that CCK is expressed in the ASM cells (marked by colocalization with α-SMA) in the mouse airways (Fig. 3b).
a qRT measurement of CCK mRNA expression in airway cells. FIB: lung fibroblasts; HBE: human bronchial epithelia cells; HBE(D0): day 0 of initiation of air-liquid interface (ALI) culture; HBE(D14): day 14 post-ALI; ASM: airway smooth muscle cells. (n = 3 biologically independent samples per group). b Immunostaining of mouse lung tissue section for CCK (green). α-SMA (α-smooth muscle actin, red) was used as a marker of ASM. Scale bar = 25 µm. Shown is a representative image from three independent experiments with similar results. c qRT measurement of CCK mRNA expression in ASM cells after treatment with indicated free fatty acids (250 μM) or control (isopropanol). n = 4 biologically independent samples per group; hexanoic vs. control (P = 0.0772); 9-decenoic vs. control (P < 0.0001); dodecanoic vs. control (P = 0.0533); palmitoleic vs. control (P < 0.0001); myristoleic vs. control (P = 0.0007). Data represent the mean ± SEM; two-tailed, unpaired t-test (*P < 0.05). d ELISA measurement of CCK in ASM cell culture medium after free fatty acid treatment (n = 4 biologically independent samples per group); palmitoleic acid vs. control (P = 0.0019); myristoleic acid vs. control (P = 0.0280). Data represent the mean ± SEM; one-tailed, unpaired t-test (*P < 0.05). e qRT measurement of CCK mRNA expression in the lungs of HFD-fed mice vs. regular chow-fed mice. n = 6 mice per group. Data represent the mean ± SEM; P = 0.0132; one-tailed, unpaired t-test (*P < 0.05). f CCK protein expression in the lungs of HFD-fed mice vs. regular chow-fed mice. n = 4 mice per group. Data represent the mean ± SEM; P < 0.0001; one-tailed, unpaired t-test (*P < 0.05). g qRT measurement of CCK mRNA expression in the airways (lungs and trachea) db/db mice compared to wild type control (WT). n = 4 mice per group; Data represent the mean ± SEM; P = 0.0152; one-tailed, unpaired t-test (*P < 0.05). h CCK protein expression in the lungs of db/db mice (n = 4) vs. wild-type control (WT). n = 4 mice per group; Data represent the mean ± SEM; P = 0.0353; one-tailed, unpaired t-test (*P < 0.05). Actin or 18 s rRNA was used as reference gene. Source data are provided as a Source Data file.
As the ligand binding site of CCKAR is located extracellularly46, we next tested whether endogenous CCK is secreted by ASM cells. In vivo, the CCK hormone is induced and secreted by cells lining the small intestine in response to ingestion of food, especially those with high fat content47. Because free fatty acids have been shown to induce CCK secretion in culture47 and in vivo48, we next sought to determine if CCK may likewise be induced by free fatty acids in ASM cells. We treated ASM cells with various types of free fatty acids and assessed their effects on CCK expression and secretion. As shown in Fig. 3c, treatment with long-chain free fatty acids (palmitoleic and myristoleic acids) increased CCK mRNA by ~3.5 fold in ASM cells, whereas treatment with free fatty acids with shorter carbon chains (hexanoic acid, decenoic acid, or dodecanoic acid) did not significantly increase CCK mRNA expression. Consistent with the mRNA expression data, the CCK hormone level increased by ~30 and ~15 fold in ASM cells treated with palmitoleic and myristoleic acids, respectively, as detected by ELISA (Fig. 3d). Together these data show that expression of CCK hormone is induced by free fatty acids in ASM cells.
Elevated CCK levels in the lungs of obese mice
Because circulating free fatty acids are elevated in obesity49,50, which is associated with the development of AHR9, and because our data revealed that fatty acids induce CCK expression in ASM cells, we asked whether CCK expression is elevated concomitantly with an increased level of fatty acids in vivo. We examined the lungs of both high-fat-diet (HFD)-induced obese and genetically obese (db/db) mice. We found that the levels of free fatty acids (FFA) in both serum and lungs of HFD-fed obese mice were elevated compared to regular chow-fed lean mice (Supplementary Fig. S2a, b). Importantly, there were concomitant increases in both CCK mRNA and CCK protein levels in the lungs of HFD-fed mice compared to regular chow-fed mice (Fig. 3e, f). Similarly, the levels of FFA in the lungs and serum of db/db mice were elevated compared to wild type (WT) mice (Supplementary Fig. S2c, d), and the levels of CCK mRNA and protein were elevated in the lungs of db/db mice compared to WT mice (Fig. 3g, h). Together these data reveal elevated CCK levels in the lungs of obese mice.
Antagonizing CCK/CCKAR attenuates the innate AHR in obese mice
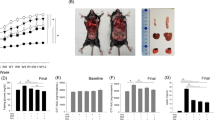
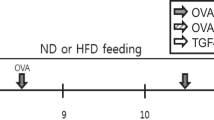
Obese mice exhibit innate AHR, a clinical feature of asthma9. Our data showing elevated CCK in the lungs of obese mice suggest that a heightened CCK/CCKAR signaling and subsequent ASM contraction may contribute to the development of obesity-associated AHR. We therefore directly tested whether antagonizing CCKAR counteracts AHR in obese mouse models. Mice fed with high-fat diet (60% kcal/fat) gain weight very rapidly and develop AHR, which becomes pronounced at 21–24th weeks of age compared to the regular-chow-fed, lean controls51. We administered CCKAR antagonists into HFD-obese and control mice and then assessed AHR in response to increasing doses of methacholine (Fig. 4a). CCKAR antagonists were administered via intranasal delivery, which may avoid undesirable side effects and limit the gastrointestinal-hepatic first-pass metabolism that can contribute to loss of administered drugs52,53. Consistent with the known effects of HFD on airway function51, in the absence of CCKAR antagonists, we observed an increased AHR in HFD-fed mice as compared to the regular chow mice (Fig. 4b, c). Importantly, intranasal administration of either proglumide (a pan-CCK antagonist) or devazepide (a potent and highly selective CCKAR antagonist) significantly reduced AHR in HFD-fed obese mice, as shown by the decrease in airway resistance (RL) to a level similar to that of lean controls (Fig. 4b, c). The antagonist treatments did not affect the airway resistance in regular-chow-fed mice (Fig. 4b, c).
a Scheme of CCKAR antagonist treatment in HFD or regular chow-fed mice. Eight-week old C57BL/J6 mice were fed with HFD or regular chow for at least 13 weeks. Mice were then given proglumide (50 mg/kg) or devazepide (25 μg/kg) intranasally and AHR was assessed by Flexivent. b Pulmonary resistance in HFD-fed mice treated with proglumide (HFD_prog, n = 11) or vehicle (HFD_veh, n = 12) and regular chow-fed mice treated with proglumide (chow_veh, n = 8) or vehicle (chow veh, n = 9). *P = 0.0070 (HFD_prog vs. HFD_veh); F values: treatment (F = 5.664, P = 0.0009); methacholine (F = 63.60, P < 0.0001); interaction (F = 0.7942, P = 0.6835). c Pulmonary resistance in HFD-fed mice treated with devazepide (HFD_dvz, n = 9) or vehicle (HFD_veh, n = 9) and regular chow-fed mice treated with devazepide (chow_dvz, n = 5) or vehicle (chow_veh, n = 6). *P < 0.0001 (HFD_dvz vs. HFD_veh); F values: treatment (F = 2.934, P = 0.0354); methacholine (F = 50.13, P < 0.0001); interaction (F = 1.645, P = 0.0684). d Scheme of CCKAR antagonist treatment in db/db or WT control mice. Mice were given proglumide (50 mg/kg) or devazepide (25 μg/kg) intranasally and AHR was assessed by Flexivent. e Pulmonary resistance in db/db mice treated with proglumide (db/db_prog, n = 8) or vehicle (db/db_veh, n = 9) and WT mice treated with proglumide (WT_prog, n = 5) or vehicle (WT_veh, n = 6). *P = 0.0184 (db/db_prog vs. db/db_veh); F values: treatment (F = 16.73, P < 0.0001); methacholine (F = 39.54, P < 0.0001); interaction (F = 2.993, P = 0.0003). f Pulmonary resistance in db/db mice treated with devazepide (db/db_dvz, n = 11) or vehicle (db/db_veh, n = 12) and WT mice treated with proglumide (WT_prog, n = 6) or vehicle (WT_veh, n = 6). *P = 0.0074 (db/db_dvz vs. db/db_veh); F values: treatment (F = 25.61, P < 0.0001); methacholine (F = 29.68, P < 0.0001); interaction (F = 3.544, P < 0.0001). g Possible mechanism of obesity-associated AHR. Obesity may induce CCK in ASM cells through circulating FFA or independent of FFA. This leads to secretion and binding of CCK to CCKAR on ASM cells, which in turn results in ASM contraction and AHR. Antagonizing CCKAR blocks CCK-induced ASM contraction and reduces obesity-associated AHR. Source data are provided as a Source Data file. Figure 4a and d were created by Biorender.com. Results represent the mean ± SEM. P and F values were determined using two-way ANOVA with Bonferroni correction.
We likewise tested the effect of antagonizing CCKAR on AHR in genetically induced obese mice (Fig. 4d). Db/db mice, which lack the receptor for leptin, rapidly develop obesity on the standard chow diet and exhibit AHR starting at 8 weeks, even in the absence of any inciting exposure9,54. Consistent with the known effects of genetic obesity on airway function9, in the absence of CCKAR antagonists, we observed an increased AHR in db/db mice as compared to the WT mice (Fig. 4e, f). Similar to the effect of antagonizing CCKAR in HFD-fed mice, intranasal administration of either proglumide or devazepide significantly reduced AHR in the db/db obese mice (Fig. 4e, f).
Because inflammation may contribute to the development of AHR, we investigated whether blocking CCKAR affects airway inflammation. We found that the bronchoalveolar lavage (BAL) cell counts were not changed by CCKAR antagonist treatments in either HFD-fed (Supplementary Fig. S3a, b) or db/db obese mice (Supplementary Fig. S3c). We also assessed the levels of a panel of BAL inflammatory molecules/cytokines. We found that the majority of cytokines were not altered in obese mice or by CCKAR antagonists (Supplementary Fig. S4). We observed two cytokines (eotaxin and MIP-1α) that were increased in BAL of db/db mice. However, CCKAR antagonist treatment did not alter the levels of these two cytokines. Together these results suggest that antagonizing CCKAR attenuates the innate AHR in obese mice without affecting airway inflammation.
Discussion
Obesity is a significant co-morbidity that associates with increased prevalence and severity of asthma7. The molecular mechanisms underlying the obesity-asthma association remain poorly understood. The lack of concrete knowledge of the pathophysiology of obesity-associated asthma precludes the development of new therapies that could effectively treat asthma in patients with obesity. Our study uncovers a previously undescribed mechanism for obesity-associated asthma that is based on the largely unexplored ability of CCK hormone and its receptor CCKAR in promoting ASM contraction. Furthermore, our study provides direct pre-clinical evidence for the repurposing of potent CCKAR antagonists as new, potentially effective therapies for asthma patients with obesity.
Although CCK increases airway constriction ex vivo36, neither the identity of the cognate receptor nor the in vivo implication of such airway constriction was known. Our study first identified CCKAR as a highly expressed GPCR in ASM cells from an unbiased RNA sequencing dataset that led us to investigate its role in CCK-induced airway constriction. Using genetic ablation and pharmacologic intervention, we firmly established the role of CCK and CCKAR in contracting ASM cells. We also found that CCK is elevated in the lungs of mouse models of obesity-associated asthma, which implied that CCK/CCKAR mediates AHR in the obese. Indeed, blocking CCKAR using potent CCKAR antagonists attenuates obesity-associated AHR in two different obese mouse models. Our results support a model in which elevated CCK level associated with obesity increases CCKAR signaling in the lung to constrict ASM cells to drive bronchoconstriction and AHR. Antagonizing such elevated CCK/CCKAR signaling in the ASM blocks obesity-induced AHR and thus presents a completely new way to treat asthma in patients with obesity (Fig. 4g).
Our studies clearly established the in vivo role of CCK-induced airway constriction as blocking the heightened CCK/CCKAR signaling using potent antagonists decreased the pulmonary resistance to methacholine challenge in two distinct mouse models of obesity-associated asthma—a genetically obese mouse model and a diet-induced mouse model. CCKAR antagonists are intended for gastric ulcer to inhibit gastrointestinal motility and gastric secretions and are known to possess favorable safety profile55,56. Thus, our data showing that CCKAR antagonists attenuated AHR support the idea that these drugs could potentially be repurposed for the treatment of asthma patients with obesity. We used two antagonists (proglumide and devazepide) that possess different selectivity profiles with respect to CCK receptors. Devazepide, in addition to being more potent in reducing AHR, is also the more selective CCKAR antagonist than the pan-CCK receptor-targeting proglumide. Thus, the use of devazepide or other highly selective CCKAR antagonists would be a priority for asthma drug repurposing to limit the undesirable effects brought by the possibility of blocking the other CCK receptor (CCKBR). CCKAR antagonists are normally administered via oral or intraperitoneal delivery that increases drug bioavailability in the systemic circulation. We showed that intranasal administration of CCKAR antagonists is sufficient to reduce AHR in obese mice. Through this delivery method, the potential unwanted effects of CCKAR inhibition in extra-pulmonary tissues is likely limited. Potent bronchodilators are commonly delivered via nebulization, suggesting that CCKAR antagonists may be developed in similar formulation to effectively abrogate asthma symptoms while avoiding undesirable systemic effects.
Our data suggest that, at least in the mouse with obesity-associated AHR, the heightened CCK/CCKAR signaling in the lung appears to lead to increased ASM contraction that is independent of airway inflammation as CCKAR antagonists did not alter markers of inflammation. This is supported by our in vitro biophysical studies showing that CCK causes direct ASM contraction. While our studies focused largely on inhibiting CCKAR-mediated contraction, the absence of effect of CCKAR antagonists on BAL cell differential counts or inflammatory cytokines nevertheless suggests that blocking CCKAR-mediated ASM contraction is sufficient to suppress obesity-associated AHR.
Our study focused on the expression and function of CCKAR in ASM cells, which are the main effector of bronchoconstriction, but previous studies had reported the expression of CCK receptors in other lung cell types, including bronchial epithelial cells, alveolar epithelial cells, pulmonary macrophages, and pulmonary vascular endothelial cells29,30,32, whose roles in asthma are tightly linked to inflammation. Epithelial cells are the airway’s first line of defense against inhaled allergens and pathogens, and epithelial damage initiates airway inflammation57; pulmonary macrophages are dysfunctional in allergic asthma and are major sources of TNF-α, IL-1β, IL-6 and IL-858; and vascular endothelial cells may contribute to airway inflammation and remodeling through eotaxin, IL-1α, GM-CSF, and VEGF59. In pulmonary macrophages, CCK was shown to exert anti-inflammatory activity in response to endotoxin shock inducer, lipopolysaccharide31,32. As we did not observe significant changes in airway inflammation in the obese mouse models as indicated by the absence of significant differences in the levels of inflammatory molecules/cytokines as well as in percentage of macrophages in BAL, it is unlikely that the reduction in AHR by CCKAR antagonists was linked to the reported function of CCK in lung macrophages. Other studies also reported a role for CCK in promoting cell proliferation of lung cancer cells. However, as our dose regimen is considered acute treatment, it is also unlikely that the action of CCKAR antagonists in reducing AHR in our mouse models was through inhibition of proliferation of asthma-relevant cells such as bronchial epithelial cells. Thus, while CCK receptors are also expressed in non-ASM cells in which they mediate cellular processes in the context of other lung disorders, CCKAR signaling in ASM cells appears to be the major driver of obesity-associated AHR.
Our findings suggest an autocrine stimulatory loop of CCK-activated, CCKAR-mediated ASM contraction where free fatty acids induce the expression and secretion of CCK in ASM cells. However, our studies have some limitations. Our experimental results cannot rule out the possibility that CCK in the GI and in the CNS also contribute to the AHR in the obese mice. Although our data suggests that CCK is produced in the lungs as shown by our qRT-PCR, immunostaining, and ELISA data, GI-produced CCK that has entered the circulation and reached the lungs could potentially activate CCKAR signaling in ASM. Plasma levels of CCK are elevated in obese rodents or after high-fat diet intake60,61. Individuals with high circulating CCK levels may thus be prone to altered lung function and heightened AHR. Similarly, conditions that lead to elevation of plasma CCK may trigger asthma symptoms. It was reported that exercise elevates plasma CCK levels in individuals with exercise-induced asthma62. Similarly, plasma CCK was observed to be increased by high altitude63. It is possible that the worsening of asthma symptoms associated with these clinical scenarios is related to elevated plasma CCK levels. Our results, however, do not identify which CCK source (circulating or ASM-secreted) contributes more greatly to obesity-induced AHR. It is likely that both CCK sources, independently or synergistically, lead to the contraction of ASM once CCKAR is engaged. Similarly, circulating FFAs may initiate ASM contraction when they induce the production and the secretion of CCK. Regardless of the initiating event, the resulting AHR may be alleviated by blocking CCK/CCKAR signaling with the antagonists.
Our experimental results also do not rule out the possibility that CCKAR signaling in the CNS affects obesity-associated AHR. It has been shown that metabolic hormones can regulate AHR in obese mice by acting on cholinergic neurons in the brainstem64,65 though this activity has not been reported for CCK. Although CCK levels are elevated in obese rodents or after high-fat diet intake60,61, CCK itself is not able to cross the blood-brain barrier66,67. Its potential action in the CNS-mediated control of obesity-associated AHR, if at all, would likely have to be through brainstem-derived CCK. However, previously published reports of the level of CCK in the brain of obese mice (ob/ob) showed either no significant difference or lower compared to lean controls68,69,70. Furthermore, another published study showed that the level of binding of CCK to its receptors in the brain of db/db mice is unchanged71, suggesting that there is no increased CCKAR signaling in the brain that could have contributed to the increased AHR of the db/db mice that we observed in our study. Tissue-specific conditional CCK or CCKAR knockout in obese mice may be needed to completely rule out the possible role of CCK/CCKAR in the brainstem in modulating obesity-associated AHR.
In summary, there is an urgent need to develop new asthma therapeutics especially for asthma patients with obesity. Our present study reveals that CCKAR mediates CCK-induced contraction of ASM cells and that CCK/CCKAR signaling is a mechanism underlying obesity-induced AHR in mice. Such findings imply that blocking CCKAR may be developed into a new asthma therapy for asthma patients with obesity, who still rely on ineffective symptomatic treatments. Our study provides critical pre-clinical evidence for repurposing CCK/CCKAR antagonists, many of them already developed for gastrointestinal diseases61, to treat obesity-associated asthma. Future clinical studies built upon our findings here may lead to the development of much-needed effective therapies for asthma patients with obesity.
Methods
Ethics statement
The animal experiments were approved by the Harvard Medical Area Institutional Animal Care and Use Committee (HMA-IACUC) under the protocol #IS506-6. Primary ASM cells were isolated from lung tissues obtained from the National Disease Resource Interchange (NDRI). Their use was approved by the University of Pennsylvania Institutional Review Board. Use of these de-identified cells does not constitute human research.
Cell culture and reagents
Primary cultures of human ASM cells were cultured as described72. ASM cultures were maintained in F12 (HAM) nutrient medium (Gibco) supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 300 μg/ml L-glutamine, 4.8 mM HEPES, 0.34 mM CaCl2, and 2.4 mM NaOH. Passages 3–8 were used in the experiments.
Air-liquid interface (ALI) culture of normal human bronchial epithelial (HBE) cells (Cat. CC-2541, Lonza) was established and maintained until use73. HBE cells were first seeded in collagen-coated transwell inserts (0.4 μm pore size, Corning) and cultured under submerged conditions in a 1:1 mixture of bronchial epithelial basal media (Lonza) and DMEM (Mediatech, Tewksbury, MA) supplemented with bovine pituitary extract (52 mg/mL), hydrocortisone (0.5 mg/mL), human epidermal growth factor (0.5 ng/mL), epinephrine (0.5 mg/mL), insulin (5 mg/mL), triiodothyronine (6.5 ng/mL), transferrin (10 mg/mL), gentamicin (50 mg/mL), amphotericin-B (50 ng/mL), BSA (1.5 mg/mL), and retinoic acid (50 nM). When the cells reached confluence, the medium was removed from the apical surface. Air-liquid interface (ALI) culture was maintained up to at least 14 days.
Reagents used in the experiments included: A71623 (Cat. 2411), proglumide (Cat. 1478) and devazepide (Cat. 2304) from Tocris (Minneapolis, MN); lorglumide (Cat. L109, Sigma-Aldrich (St. Louis, MO); normal saline (Cat. S5819, Teknova, Hollister, CA); acetylcholine chloride (Cat. A6625), Tween-80 (Cat. P4780), DMSO (Cat D2650), hexanoic acid (Cat. W255912), 9-decenoic acid (Cat. W366005), myristoleic acid (Cat. M3525) and palmitoleic acid (Cat 76169) from Sigma-Aldrich (St. Louis, MO).
Quantitative real-time (qRT)PCR
Total RNA was extracted using the RNEasy kit according to the manufacturer’s instructions (Qiagen, Cat. 74106). RNA was then reverse-transcribed using the Superscript III First-Strand Synthesis System (Life Technologies, Cat. 18080-051). Quantitative PCR was performed using SYBR green master mix (Qiagen, Cat. 214145) with the following gene-specific primers synthesized by Integrated DNA Technologies (Coralville, IA):
Human CCKAR forward: 5′- TGCTCAAGGATTTCATCTTCG
Human CCKAR reverse: 5′- TGGTCACCAGATTAAAGGTAGATACA
Human CCK forward: 5′- CTGGCAAGATACATCCAGCA
Human CCK reverse: 5′- CCATGTAGTCCCGGTCACTT
Human ACTB (β-actin) forward: 5′- CCAACCGCGAGAAGATGA
Human ACTB (β-actin) reverse: 5′- CCAGAGGCGTACAGGGATAG
Human 18 s rRNA forward: 5′- CCGATTGGATGGTTTAGTGAG
Human 18 s rRNA reverse: 5′—AGTTCGACCGTCTTCTCAGC
Mouse CCK forward: 5′- GCTGATTTCCCCATCCAAA
Mouse CCK reverse: 5′- GCTTCTGCAGGGACTACCG
Mouse CCKAR forward: 5′- GATGCCAGCCAGAAGAAATC
Mouse CCKAR reverse: 5′- ACAGCCATCGCTATCCTCAT
Mouse ACTB (β-actin) forward: 5′- CTAAGGCCAACCGTGAAAAG
Mouse ACTB (β-actin) reverse: 5′- ACCAGAGGCATACAGGGACA
Immunofluorescence staining
Immunofluorescence staining of lung tissue sections were performed in paraffin-embedded lung tissue53. The tissue slides were deparaffinized using xylene and rehydrated by washing with 100% ethanol, 95% ethanol, 70% ethanol, and finally distilled water. Antigen retrieval was performed by bringing slides to a boil in 10 mM citrate buffer, pH 6.0, then matinining at a sub-boiling temparture for 10 min. After cooling sildes for 30 min, the slides were washed with PBS. Lung tissue sections were blocked with PBS supplemented with 5% normal donkey serum and 0.2% Triton X-100 for 1 h at room temperature. Primary antibodies used were anti-CCKAR (Pierce, Cat. PA3-116; 1:500 dilution), anti-CCK (Santa Cruz Biotechnology, Inc., Cat. Sc-21617; 1:25 dilution), and anti-α-SMA (Sigma, Cat. C6198; 1:200 dilution). For isotype controls, normal rabbit whole serum (ThermoScientific, Cat. 31883) and normal goat IgG (Sta. Cruz, Cat. Sc-2028) were used. Secondary antibodies used were AlexaFlour 488 anti-rabbit (Life Technologies, Cat. A21206, 1:200 dilution) and anti-goat CFTM488A (Sigma-Aldrich, Cat. SAB4600032, 1:200 dilution). ProLong gold anti-fade mountant with DAPI (Life Technologies, Cat. P36941) was used for mounting samples. Confocal images were taken using Leica SP8X Confocal Microscope and the images were processed using ImageJ.
Generation of CCKAR-knockout cells
Two CCKAR-knockout cell lines (KO1 and KO2) were generated using CRISPR with the following guide RNAs (gRNAs): TAGAAAGCGGCACATATTCG for KO1 and CCGCTTGTTCCGAATCAGCA for KO2. Guides were cloned into lentiCRISPRv2 vector containing hSpCas9 cassette (Addgene)54. Oligonucleotides targeting the site sequence were synthesized with 3-bp NGG PAM sequence flanking the 3′ end, annealed and cloned into the BsmBI-digested lentiCRISPRv2 vector. The resulting plasmids were transformed into Stbl3 bacteria (ThermoFisher Scientific, Cat. C737303) and purified using Mini-prep Kit (Qiagen, Cat. 27104). Lentiviruses were produced by co-transfecting the lentiCRISPRv2 containing gRNAs with the packaging plasmids pVSVg and psPAX2 (Addgene) in HEK293T cells (Cat. CRL-3216, American Type Culture Collection, ATCC). Lentiviral transduction in ASM cells was performed in the presence of polybrene (8 µg/ul) for 24 hours. Stably transduced ASM cells were selected using puromycin (ThermoFisher Scientific, Cat. A1113803). T7E1 assay was performed to determine knockout efficiency. For the T7E1 assay, the genomic region harboring the target of gRNAs was first PCR amplified, subjected to denaturing and reannealing temperatures (95 °C for 2 min, ramp down at −2 °C/s to 85 °C, ramp down at −0.1 °C/s to 25 °C, and stopped at 16 °C. T7E1 (New England Biolabs, Cat. M0302S) cleavage reaction was then performed at 37 °C for 20 min. The PCR products were visualized using 1.5% agarose gel.
Optical magnetic twisting cytometry
Cell stiffness was measured using optical magnetic twisting cytometry41. ASM cells were cultured to confluence in collagen-coated 96-well plate (Stripwell Microplates, Corning, NY). After overnight serum starvation, cells were incubated with ferrimagnetic beads (4.5 μm in diameter, produced in house, and coated with poly-L-lysine) for 20 min to allow beads to attach to cell surfaces74. The beads were then magnetized with a strong magnetic pulse in the horizontal direction and twisted with a much weaker oscillatory magnetic field (0.77 Hz) in the vertical direction. The ratio of magnetic torque to bead motion41,74 was used to determine cell stiffness; values are expressed in arbitrary units (a.u). To determine relative changes in cell stiffness after drug treatment, the average of stiffness normalized to baseline was calculated.
Traction force microscopy
Cell contraction was measured using traction force microscopy43. Glass bottom 96-well plates containing custom elastic polyacrylamide substrate were prepared using a multi-layered approach. The plates were treated with 6 N NaOH for 1 h followed by silane solution for additional 1 h. Fluorescent bead solution (1 μm carboxylate-modified microspheres, Invitrogen) was added to the wells and allowed to air-dry overnight. Glutaraldehyde (0.25% in PBS) was then added for 30 min followed by washing. After drying, acrylamide gels (5.5% acrylamide, 0.076% bisacrylamide, Young’s modulus = 1.8 kPa, thickness = 200 μm) were cast using a custom-made gel caster (Matrigen Life Technologies, CA). Gel surfaces were functionalized using sulfo-SANPAH (sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino]hexanoate, 0.2 mg/ml) and coated with 0.2 μm sulfate microspheres (Invitrogen). Collagen (40 μg/ml) was then added to coat plates prior to seeding of ASM cells. ASM cells were cultured to confluence in the plates using the regular FBS-containing ASM medium followed by switching to serum free medium containing insulin (5.7 μg/ml) and transferrin (5 μg/ml). The cells were maintained for additional 48 h in this medium until the time of experimentation (e.g., drug treatment). For the traction microscopy procedure, a set of flourescence images per well were obtained using an inverted microscope (DMI 6000B, Leica Inc): before plating the cells (reference), prior to drug exposure (baseline), and 1 h after drug exposure (treatment). The cell-exerted displacement field was determined by comparing the baseline or treatment image to the reference image. The contractile force (per unit area) was computed from the obtained displacement field using Fourier-transform traction microscopy. The average contractile force was then determined by computing the root mean squared value from each force map. Finally, the force-response ratio of treatment vs. baseline contraction was calculated.
Mouse models of obesity-associated AHR and drug treatment
Obese mice and their corresponding lean controls were acquired from the Jackson Laboratory and were allowed at least one week for acclimation prior to experimentation. A maximum of 4 mice per cage were permitted and animals were checked at least weekly after arrival. Standard monitoring practices were applied for animals including monitoring for persistent recumbence, intractable pain, severe weight loss, tumor, severe central nervous system signs, dyspnea and cyanosis, prolapse of the penis or rectum, limb or spinal fractures, and dystocia. Water and enrichment (nestlet) were also provided in the cages (static caging system). The temperature in the animal room was maintained at 70 F and relative humidity at 50%. Light and dark cycle was from 7:00 am to 7:00 pm.
High fat diet (HFD)-fed mice were obtained from Jackson Laboratory; these were male C57BL/6 J mice placed on a high fat diet where 60% of calories were derived from fat in the form of lard (D12492, Research Diets) starting at 8 weeks until the time of experiments. Mice placed on a regular chow diet (PicoLab 5058, Lab Diets) served as corresponding lean controls. Mice were 21–24 weeks of age at the time of experiments. Mice fed with HFD gain weight very rapidly and develop AHR compared to their corresponding lean controls, which have normal airway reactivity54. Db/db mice (B6.BKS(D)-Leprdb/J), which lack the long form of the leptin receptor, were used as the mouse model of genetic obesity. Db/db mice are substantially obese compared to the age- and sex-matched WT controls (C57BL/6 J) and exhibit increased airway responsiveness to methacholine even in the absence of any inciting stimuli (e.g., ovalbumin challenge)9. Db/db and WT mice (male or female) were 10–12 weeks of age at the time of experiments.
Mice were treated via intranasal administration of CCKAR antagonists (proglumide or devazepide). Proglumide was administered at 50 mg/kg (3x) and devazepide at 25 μg/kg (2x) for a total volume of 25 μl (12.5 μl in each nostril). The vehicles used to deliver the antagonists are normal saline for proglumide and 0.05% DMSO/ 0.05% Tween-80/normal saline for devazepide. After experiments, mice were euthanized with an overdose of sodium pentobarbital (200 mg/kg i.p.).
Measurement of Airway Hyperresponsiveness (AHR)
This study was approved by the Harvard Medical Area Standing Committee on Animals. The forced-oscillation technique was used to assess AHR using the Flexivent machine (flexiWare7, Scireq, Montreal, QC, Canada) which was conducted at least two hours after the last administration of CCKAR antagonists or vehicle. Mice were first anesthetized with xylazine (7 mg/kg) and sodium pentobarbital (50 mg/kg) and an incision along the tracheal wall was made to expose the trachea. The trachea was then cannulated with a tubing adaptor and then connected to the machine. The mice were ventilated and instrumented for the measurement of pulmonary mechanics and airway responsiveness in response to increasing doses of methacholine75. The chest wall was opened to expose the lungs to atmosphere pressure and a positive end expiratory pressure of 3 cm H20 was applied. Obese mice were ventilated at a respiratory rate of 180 breaths/minute while lean mice were ventilated at 150 breaths/min. Changes in pulmonary resistance (RL) were assessed at the baseline and after delivery of aerosolized PBS and aerosolized methacholine in increasing doses.
CCK enzyme immunoassay
CCK Enzyme Immunoassay Kit (Cat. RAB0039 St. Louis, MO) was used to detect CCK in samples. This assay is based on the principle of competitive enzyme immunoassay. Lung tissue lysates were prepared using RIPA buffer (Thermo Scientific, Waltham, MA) supplemented with protease and phosphatase inhibitor cocktails (Roche, Mannheim Germany) and protein concentration was measured using BCA assay (Thermo Scientific, Waltham, MA). Standards and equal amounts of samples were mixed with biotinylated CCK (final concentration was 20 pg/mL in every sample). A 96-well plate was coated with anti-CCK antibody and incubated overnight at 4 °C with gentle shaking (1–2 cycles/sec). Following incubation, the wells were washed 5 times with wash buffer. Samples and standards were added into appropriate wells. A blank well-containing assay diluent was also included. The plate was incubated overnight at 4 °C with gentle shaking (1–2 cycles/sec). Following incubation, the wells were washed 5 times with wash buffer. A solution of HRP-streptavidin was added to each well and the plate was incubated for 45 min with gentle shaking at room temperature. The solution was discarded and the plate washed 5 times with wash buffer. TMB One-Step Substrate Reagent was then added to each well and the plate was incubated for 30 min at room temperature in the dark with gentle shaking. Following incubation, a stop solution was added to each well and absorbance was read at 450 nm (SpectraMax 190 Microplate Reader, Molecular Devices). A four-parameter logistic regression model was then used to plot the standard curve and to determine the amount of CCK in the samples.
Free Fatty Acid Quantification
For the detection of FFA in samples, the Free Fatty Acid Assay Kit was used (Cat. ab65341, Abcam, Waltham, MA). For lung samples, FFAs were extracted using Lipid Extraction Kit (Cat. Ab211044, Abcam, Waltham, MA) from equal weight of lung tissue samples (40 mg). Serum samples were directly assayed. The FFA Assay Kit converts fatty acids into their CoA-derivatives which are then oxidized with the concomitant generation of color or fluoresence. Fifty (50) μl of samples (dissolved in supplied assay buffer if needed) were added to each well in a 96-well plate. The acyl-CoA synthesis was then performed by adding 2 μl of ACS reagent into the wells, mixed, and incubated at 37 °C for 30 min. A reaction mix (44 μl assay buffer, 2 μl fatty acid probe, 2 μl enzyme mix, and 2 μl enhancer was added to each well and incubated at 37 °C for 30 min in the dark. After incubation the absorbance was measured at 570 nm (SpectraMax 190 Microplate Reader, Molecular Devices). A standard curve was generated using palmitic acid and the concentration of fatty acid was determined using formula, [FFA] = Fa/Sv (nmol/μl or mM) where Fa is the fatty acid amount in the well obtained from the standard curve and Sv is the sample volume (μl) added to the sample well. For lung samples, the amount of FFA was expressed per weight (g) of lung.
Assessment of airway inflammation
Airway inflammation was assessed and compared between experimental groups using differential cell analysis and measurement of BAL cytokine levels. Lavage was performed in situ with a 20-gauge catheter inserted into the proximal trachea, flushing the lungs 4 times with 0.8 ml PBS. The recovered fluid of the 1st lavage was transferred into a 1.5 ml Eppendorf tube on ice, while the 3 subsequent lavages were pooled into a 5 ml Eppendorf tube on ice. BAL cells were separated from BAL fluid by centrifugation at 500 x g for 10 min at 4 °C. The BAL fluid of the 1st lavage was centrifuged again at 3000 x g for 10 min at 4 °C to spin down cell debris, the supernatant was frozen at −80 °C for further analysis of cytokines in the BAL. For total cell count, BAL cells retrieved from all 4 lavages were pooled and resuspended in 400 μl PBS, then 10 μl of the cell suspension was diluted 4-fold with trypan blue stain and manually counted twice using a hemocytometer. For differential count, a fraction of BAL cell suspension was cytospun onto microscopic slides, air-dried and stained with Diff-Quick. Four hundred (400) cells per sample were counted for the percentages of macrophages, eosinophils, neutrophils, and lymphocytes. For cytokine level analysis, a multiplex assay was performed on BAL samples using Mouse Cytokine 32-Plex Discovery Assay (Eve Technologies, Calgary, AB). Cytokines whose levels were below the detection limit were not included in the supplementary figures.
Statistical analysis
Differences between groups were analyzed using Prism v8; GraphPad Software Inc. No statistical method was used to predetermine sample size and the investigators were not blinded to allocation during experiments and outcome assessment. For both cell culture and animal experiments, random assignment was used to assign samples and subjects to different treatment groups. Both F and P-values were reported for individual variables and their interactions, when appropriate. A P < 0.05 was considered statistically significant. Statistical tests used were noted in figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data in Fig. 1a and Supplementary Data were generated from a previously published RNA-seq data available at the Gene Expression Omnibus Web site (http://www.ncbi.nlm.nih.gov/geo/) under accession GSE52778. All data supporting the findings described in this manuscript are available in the article and in the Supplementary Information and from the corresponding author upon request. Source data are provided with this paper.
Code availability
The analysis codes for this study are available from the corresponding author.
References
2020 GINA Main Report. Global Initiative for Asthma—GINA https://ginasthma.org/gina-reports/.
Doeing, D. C. & Solway, J. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl Physiol. (1985) 114, 834–843 (2013).
Johnson, M. The β -Adrenoceptor. Am. J. Respir. Crit. Care Med 158, S146–S153 (1998).
McGraw, D. W. & Liggett, S. B. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc. Am. Thorac. Soc. 2, 292–296 (2005).
Cockcroft, D. W. & Sears, M. R. Are inhaled longacting β2 agonists detrimental to asthma? Lancet Respir. Med 1, 339–346 (2013).
Mansfield, L. E. The future of the long-acting beta-adrenergic bronchodilators in the treatment of asthma. Allergy Asthma Proc. 29, 103–108 (2008).
Peters, U., Dixon, A. & Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 141, 1169–1179 (2018).
Beuther, D. A. & Sutherland, E. R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med 175, 661–666 (2007).
Shore, S. A. Obesity, airway hyperresponsiveness, and inflammation. J. Appl Physiol. (1985) 108, 735–743 (2010).
Peters-Golden, M. et al. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 27, 495–503 (2006).
Boulet, L.-P. & Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med 101, 2240–2247 (2007).
Camargo, C. A. et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J. Asthma 47, 76–82 (2010).
Sutherland, E. R., Goleva, E., Strand, M., Beuther, D. A. & Leung, D. Y. M. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med 178, 682–687 (2008).
Lavoie, K. L., Bacon, S. L., Labrecque, M., Cartier, A. & Ditto, B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir. Med 100, 648–657 (2006).
Dixon, A. E. et al. Effect of obesity on clinical presentation and response to treatment in asthma. J. Asthma 43, 553–558 (2006).
Dias-Júnior, S. A. et al. Effects of weight loss on asthma control in obese patients with severe asthma. Eur. Respir. J. 43, 1368–1377 (2014).
van Leeuwen, J. C., Hoogstrate, M., Duiverman, E. J. & Thio, B. J. Effects of dietary induced weight loss on exercise-induced bronchoconstriction in overweight and obese children. Pediatr. Pulmonol. 49, 1155–1161 (2014).
McGarry, M. E. et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest 147, 1591–1598 (2015).
Chandra, R. & Liddle, R. A. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 14, 63–67 (2007).
Chaudhri, O., Small, C. & Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1187–1209 (2006).
Liddle, R. A. Cholecystokinin cells. Annu Rev. Physiol. 59, 221–242 (1997).
Beinfeld, M. C., Meyer, D. K. & Brownstein, M. J. Cholecystokinin in the central nervous system. Peptides 2, 77–79 (1981).
Rehfeld, J. F. Cholecystokinin—From Local Gut Hormone to Ubiquitous Messenger. Front. Endocrinol. (Lausanne) 8, 47 (2017).
Wank, S. A. Cholecystokinin receptors. Am. J. Physiol. 269, G628–G646 (1995).
Yu, P. et al. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am. J. Physiol.-Gastrointest. Liver Physiol. 275, G203–G211 (1998).
Kopin, A. S. et al. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest 103, 383–391 (1999).
Sethi, T., Herget, T., Wu, S. V., Walsh, J. H. & Rozengurt, E. CCKA and CCKB receptors are expressed in small cell lung cancer lines and mediate Ca2+ mobilization and clonal growth. Cancer Res 53, 5208–5213 (1993).
Matsumori, Y. et al. Cholecystokinin-B/gastrin receptor: a novel molecular probe for human small cell lung cancer. Cancer Res. 55, 276–279 (1995).
Cong, B. et al. Expression and cell-specific localization of cholecystokinin receptors in rat lung. World J. Gastroenterol. 9, 1273–1277 (2003).
Xu, S.-J., Gao, W.-J., Cong, B., Yao, Y.-X. & Gu, Z.-Y. Effect of lipopolysaccharide on expression and characterization of cholecystokinin receptors in rat pulmonary interstitial macrophages. Acta Pharm. Sin. 25, 1347–1353 (2004).
Cong, B., Li, S.-J., Yao, Y.-X., Zhu, G.-J. & Ling, Y.-L. Effect of cholecystokinin octapeptide on tumor necrosis factor α transcription and nuclear factor-κB activity induced by lipopolysaccharide in rat pulmonary interstitial macrophages. World J. Gastroenterol. 8, 718–723 (2002).
Li, S. et al. CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock 27, 678–686 (2007).
Staley, J., Jensen, R. T. & Moody, T. W. CCK antagonists interact with CCK-B receptors on human small cell lung cancer cells. Peptides 11, 1033–1036 (1990).
Yoder, D. G. & Moody, T. W. High affinity binding of cholecystokinin to small cell lung cancer cells. Peptides 8, 103–107 (1987).
Staley, J., Fiskum, G. & Moody, T. W. Cholecystokinin elevates cytosolic calcium in small cell lung cancer cells. Biochem Biophys. Res. Commun. 163, 605–610 (1989).
Stretton, C. D. & Barnes, P. J. Cholecystokinin-octapeptide constricts guinea-pig and human airways. Br. J. Pharm. 97, 675–682 (1989).
Sriram, K. & Insel, P. A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharm. 93, 251–258 (2018).
Himes, B. E. et al. RNA-Seq Transcriptome Profiling Identifies CRISPLD2 as a Glucocorticoid Responsive Gene that Modulates Cytokine Function in Airway Smooth Muscle Cells. PLOS ONE 9, e99625 (2014).
Behar, J. & Biancani, P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J. Clin. Invest 66, 1231–1239 (1980).
Krishnamurthy, G. T. & Krishnamurthy, S. Gallbladder, Sphincter of Oddi, Cholecystokinin, and Opioid Inter-Relationship. in Nuclear Hepatology: A Textbook of Hepatobiliary Diseases (eds. Krishnamurthy, G. T. & Krishnamurthy, S.) 129–146 (Springer, 2000). https://doi.org/10.1007/978-3-662-22654-4_6.
Park, C. Y. et al. Mapping the cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 298, C1245–C1252 (2010).
Krishnan, R. et al. Reinforcement versus Fluidization in Cytoskeletal Mechanoresponsiveness. PLOS ONE 4, e5486 (2009).
Park, C. Y. et al. High-throughput screening for modulators of cellular contractile force. Integr. Biol. (Camb.) 7, 1318–1324 (2015).
Herness, S. & Zhao, F.-L. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol. Behav. 97, 581–591 (2009).
Oikonomou, E., Buchfelder, M. & Adams, E. F. Cholecystokinin (CCK) and CCK receptor expression by human gliomas: Evidence for an autocrine/paracrine stimulatory loop. Neuropeptides 42, 255–265 (2008).
Silvente-Poirot, S., Escrieut, C. & Wank, S. A. Role of the extracellular domains of the cholecystokinin receptor in agonist binding. Mol. Pharm. 54, 364–371 (1998).
McLaughlin, J. T. et al. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J. Physiol. 513, 11–18 (1998).
McLaughlin, J. et al. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology 116, 46–53 (1999).
Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. North Am. 37, 635–646 viii–ix (2008).
Arner, P. & Rydén, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 8, 147–155 (2015).
Johnston, R. A. et al. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J. Appl Physiol. (1985) 104, 1727–1735 (2008).
Hussain, A. A., Dakkuri, A., Lai, Y.-L., Traboulsi, A. & Hussain, M. A. Nasal administration of albuterol: an alternative route of delivery. J. Pharm. Pharm. 56, 1211–1215 (2004).
Ortiz, M. et al. Intranasal administration of budesonide-loaded nanocapsule microagglomerates as an innovative strategy for asthma treatment. Drug Deliv. Transl. Res. 10, 1700–1715 (2020).
Kim, H. Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med 20, 54–61 (2014).
Tucker, R. D. et al. A Cholecystokinin Receptor Antagonist Halts Nonalcoholic Steatohepatitis and Prevents Hepatocellular Carcinoma. Dig. Dis. Sci. 65, 189–203 (2020).
Nadella, S. et al. Cholecystokinin Receptor Antagonist Therapy Decreases Inflammation and Fibrosis in Chronic Pancreatitis. Dig. Dis. Sci. 65, 1376–1384 (2020).
Erle, D. J. & Sheppard, D. The cell biology of asthma. J. Cell Biol. 205, 621–631 (2014).
Fricker, M. & Gibson, P. G. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 50, 1700196 (2017).
Reichard, A. & Asosingh, K. Endothelial Cells in Asthma. Asthma—Biological Evidences (IntechOpen, 2019). https://doi.org/10.5772/intechopen.85110.
Li, J., Ma, W. & Wang, S. Slower gastric emptying in high-fat diet induced obese rats is associated with attenuated plasma ghrelin and elevated plasma leptin and cholecystokinin concentrations. Regul. Pept. 171, 53–57 (2011).
Degen, L., Matzinger, D., Drewe, J. & Beglinger, C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides 22, 1265–1269 (2001).
Hvidsten, D., Jenssen, T. G., Bolle, R. & Burhol, P. G. Plasma gastrointestinal regulatory peptides in exercise-induced asthma. Eur. J. Respir. Dis. 68, 326–331 (1986).
Bailey, D. M. et al. Elevated plasma cholecystokinin at high altitude: metabolic implications for the anorexia of acute mountain sickness. High. Alt. Med Biol. 1, 9–23 (2000).
Leiria, L. O. S. et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Rep. 11, 934–943 (2015).
Arteaga-Solis, E. et al. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 17, 35–48 (2013).
Baldwin, B. A., Parrott, R. F. & Ebenezer, I. S. Food for thought: a critique on the hypothesis that endogenous cholecystokinin acts as a physiological satiety factor. Prog. Neurobiol. 55, 477–507 (1998).
May, A. A., Liu, M., Woods, S. C. & Begg, D. P. CCK increases the transport of insulin into the brain. Physiol. Behav. 165, 392–397 (2016).
Hansky, J. & Ho, P. Cholecystokinin-like peptides in brain and intestine of obese-hyperglycaemic mice. Aust. J. Exp. Biol. Med Sci. 57, 575–579 (1979).
Straus, E. & Yalow, R. S. Cholecystokinin in the brains of obese and nonobese mice. Science 203, 68–69 (1979).
Lavine, J. A. et al. Cholecystokinin Is Up-Regulated in Obese Mouse Islets and Expands β-Cell Mass by Increasing β-Cell Survival. Endocrinology 151, 3577–3588 (2010).
Saito, A., Williams, J. A. & Goldfine, I. D. Alterations of brain cerebral cortex CCK receptors in the ob/ob mouse. Endocrinology 109, 984–986 (1981).
Hu, R. et al. MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB J. 28, 2347–2357 (2014).
Panganiban, R. A. et al. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J. Allergy Clin. Immunol. 142, 1469–1478.e2 (2018).
Zhou, E. H. et al. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl Acad. Sci. USA 106, 10632–10637 (2009).
Johnston, R. A., Zhu, M., Hernandez, C. B., Williams, E. S. & Shore, S. A. Onset of obesity in carboxypeptidase E-deficient mice and effect on airway responsiveness and pulmonary responses to ozone. J. Appl Physiol. (1985) 108, 1812–1819 (2010).
Acknowledgements
This work was supported mainly by a scholar award from the American Asthma Foundation and in part by a grant from the National Institutes of Health (R01HL139496) to Q.L. We thank Dr. Reynold Panettieri for providing human ASM cells.
Author information
Authors and Affiliations
Contributions
R.A.P. and Q.L. conceived the project and designed the experiments. R.A.P. performed most of the experiments. Q.L. supervised the project. C.Y.P, N.S., R.K., and J.F. performed the biomechanical assays and analyses. Z.Y., M.S., D.K., and S.A.S. contributed to AHR assessment in mice. E.I. and M.B.H contributed human samples for immunostaining. B.H., A.K., S.T.W., and K.G.T. supervised the RNA-Seq analyses. R.A.P. and Q.L. wrote the paper with input from other co-authors.
Corresponding author
Ethics declarations
Competing interests
Q.L. is a co-founder, scientific advisor and shareholder of Vesigen Therapeutics. All other co-authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Linhong Deng, Giuseppe D’Agostino and the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panganiban, R.A.M., Yang, Z., Sun, M. et al. Antagonizing cholecystokinin A receptor in the lung attenuates obesity-induced airway hyperresponsiveness. Nat Commun 14, 47 (2023). https://doi.org/10.1038/s41467-022-35739-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35739-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.