Abstract
The pursuit of di-coordinate boron radical has been continued for more than a half century, and their stabilization and structural characterization remains a challenge. Here we report the isolation and structural characterization of a linear di-coordinate boron radical cation, achieved by stabilizing the two reactive atomic orbitals of the central boron atom by two orthogonal π-donating and π-accepting functionalities. The electron deficient radical cation undergoes facile one-electron reduction to borylene and binds Lewis base to give heteroleptic tri-coordinate boron radical cation. The co-existence of half-filled and empty p orbitals at boron also allows the CO-regulated electron transfer to be explored. As the introduction of CO promotes the electron transfer from a tri-coordinate neutral boron radical to a boron radical cation, the removal of CO under vacuum furnishes the reverse electron transfer from borylene to yield a solution consisting of two boron radicals.
Similar content being viewed by others
Introduction
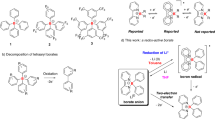
Electron transfer reaction of low-coordinate high spin metal complexes upon substrate binding is vital for the biological activities of a variety of metalloenzymes. For instance, the activity and function of heme proteins associated with the redox reaction of the system can be modulated by the coordination of gaseous substates like O2, NO, and CO to the five-coordinate high spin ferrous center of heme1. While the co-existence of the metal-based half-filled (SOMO) and empty (LUMO) frontier molecular orbitals is rather common in transition metal complexes, electron-deficient low-coordinate open-shell main-group compounds are extremely rare and the ligand-regulated electron transfer has not been explored2,3,4,5. As depicted in Fig. 1a, main-group radicals with no accessible empty valence orbital cannot accept pair of electrons and, thus, frustrate the binding of the substrate at the central atom. Among s- and p-block elements, the synthesis of low-coordinate electron-deficient boron, carbon, nitrogen, and oxygen radicals is exceptionally difficult due to the propensity of these open-shell species to form strong chemical bonds. Considering the overall charge of the molecule and the coordination number at the central atom, neutral di-coordinate boryl radical should be the most probable target in the series (Fig. 1b). However, as tri-coordinate and tetra-coordinate boron radicals6 have been extensively explored for applications in organic synthesis7,8,9,10,11, polymerization12,13,14,15, and optoelectronic materials16,17,18,19, their corresponding five-electron counterparts could only be examined in silico20. All attempts in realizing the electron-deficient di-coordinate boron radical from chemical reduction of boranes21,22,23,24,25,26,27,28,29,30,31,32,33,34 and borinium ion were unsuccessful35,36. Although the heavier Group 13 analogs were readily obtained from reaction of E(I) (E = Ga, In, Tl) precursors with boryllithium or reduction of diborylchloroindium with potassium2, the lack of accessible monovalent B(I) precursors and the relatively strong boron-containing chemical bonds should be responsible for the inability to achieve di-coordinate boron radical.
Looking back on the development of carbon radical, the incorporation of both π-donating and π-accepting substituents at a radical center noticeably boosted the lifetime of the radical due to the favorable synergetic stabilization of the unpaired electron. Inspired by the captodative stabilization effect of carbon radical37,38,39, we hypothesized that a linear di-coordinate boron radical could also be accomplished through attaching a captor and a dative group on boron radical (Fig. 1c). However, unlike the captodative effect in which the singly occupied orbital is overlapping with both the donor and the acceptor orbitals, the push-pull stabilizing groups of a linear di-coordinate boron radical are expected to interact with two orthogonal atomic orbitals of boron. As the π-donating substituent could mitigate the extreme Lewis acidity associated with di-coordinate boron atom, the orthogonal π-accepting group could stabilize the unpaired electron through delocalization. Given the fact that the π-acidity of cyclic(alkyl)(amino)carbene (CAAC) has been proven critical in accomplishing isolable main-group radical species40,41,42, we envision that a linear di-coordinate boron radical cation bearing a π-donating bulky amino substituent and a π-acidic CAAC ligand should be an accessible target (Fig. 1d). In this work, we report the synthesis and characterization of a di-coordinate boron radical cation, and demonstrate that the redox event of boron radical cation can be regulated by CO complexation.
Results and discussion
Synthesis of di-coordinate boron radical cation
The CAAC-stabilized amino-chloroborenium [1][OTf] obtained from the reaction of equimolar amount of CAAC and TMP-BCl2 was reduced in toluene with one equivalent of cobaltocene to give neutral boron radical 2•, which was isolated as red solid in 73% yield (Fig. 2a). Radical 2• displays a strong isotropic EPR absorption centered at giso = 2.0047 with hyperfine coupling to 14N and 11B/10B nuclei of 6.62 G and 0.77/0.26 G, respectively (Fig. 2b). The obtained relatively weak hyperfine coupling to boron is typical for CAAC-stabilized neutral boron radicals43,44,45,46,47. Crystalline 2• obtained from slow evaporation of a pentane solution of 2• in a glovebox was analyzed with single crystal X-ray diffraction. As illustrated in Fig. 2c, the presence of a trigonal planar boron center is confirmed by the sum of the E-B-E angles of 360.0°. Structural parameters of 2• are reminiscent of the reported CAAC-stabilized boron radicals. The B–C [1.526(4) Å] and B–N [1.468(4) Å] bond distances are relatively short compared to typical B–C and B–N single bonds due to the delocalization of three electrons in the angular Cπ-Bπ-Nπ orbital. The population of the pπ orbital of boron weakens the B–Cl π-interaction leading to a markedly elongated B–Cl bond [1.854(3) Å], which could be readily cleaved with chloride abstracting agent (vide infra).
The reaction of 2• with K[B(C6F5)4] resulted in the formation of a linear di-coordinate boron radical cation [3][B(C6F5)4]. A DCM solution consisting of an equimolar amount of 2• and K[B(C6F5)4] turned gradually from dark red to yellow-greenish to give [3][B(C6F5)4] in 93% yield (Fig. 2a). The formation of a new radical species was corroborated by EPR spectroscopy. While the giso value of 2.0047 is identical to that of 2•, a drastic change in the splitting pattern of [3]+• is recognized (Fig. 2e). The spectrum can be satisfactorily simulated with one boron nucleus and two inequivalent nitrogen nuclei. Although the obtained hyperfine coupling constants of 3.82 G and 1.27 G to 11B (I = 3/2) and 10B (I = 3) are relatively small in comparison with triarylborane radical anions6, the shift of spin density from CAAC to boron upon chloride abstraction is evident. The unpaired electron of [3]+• is also coupled with two inequivalent 14N (I = 1) nuclei with hyperfine coupling constants of 8.32 G and 2.42 G, which could be respectively assigned to the nitrogen atom of CAAC and TMP. The considerable contribution of one boron and two nitrogen atoms to the splitting of the EPR signal suggests that the CAAC-B-TMP linkage is preserved in [3]+• in the solution. The observed increased delocalization of the unpaired electron to the B-TMP unit is consistent with the enhanced electron deficiency of boron upon chloride abstraction. To verify the existence of a di-coordinate boron radical species, lime green single crystals of [3][B(C6F5)4] suitable for X-ray diffraction analysis were obtained by cooling a DCM/pentane solution of [3][B(C6F5)4] at −30 °C. As shown in Fig. 3f, a linear di-coordinate boron radical with a C10-B–N1 bond angle of 172.56(19)° is corroborated. The dihedral angle of 67.1° between the planes consisting of the ylidene center of CAAC and the nitrogen atom of TMP is consistent with the presence of two perpendicular π-bonding interactions at the central sp hybridized boron atom. The B–C and B–N bond [C10-B1 1.457(3) Å, B1-N1 1.347(3) Å] are shortened by about 6.9 and 12.1 pm with respect to that of 2•, confirming the enhanced π-interactions at the both sides. The solid-state structural features of [3]+• are well reflected on its solution EPR spectrum, in which a stronger coupling of the unpaired electron to the B-TMP moiety is recognized.
DFT calculations of 2• and [3]+•
To have a better insight into the bonding situations of 2• and [3]+•, density functional theory (DFT) calculations were performed at the UCAM-B3LYP/6-31G**/SMD(CH2Cl2) level of theory (see Supplementary Methods 1.7). All bond distances and angles of the optimized 2• and [3]+• agree well with those determined experimentally. The assembly of two perpendicular planar substituents at the central boron atom of [3]+• in an allene-like linear arrangement is also captured by computation. The discernable enlargement of a(11,10B) from 0.77/0.26G in 2• to 3.82/1.27G in [3]+• can also be elucidated with the spin density distribution calculation. As depicted in Fig. 2d, g, the unpaired electron of 2• and [3]+• are mainly localized within the NCAAC-CCAAC-B unit. However, a drastic shift of the spin density from the ylidene center to boron is observed upon chloride abstraction. The enhanced electron deficiency of boron is responsible for the umpolung of the unpaired electron distribution in the B–C π-bond from C/B = 47.6/31.7 (in 2•) to C/B = 18.8/46.4 (in [3]+•), leading to the observed stronger coupling of the electron spin to the boron nucleus in EPR. Mulliken charge distribution analysis reveals that the positive charge is predominantly residing on the central boron atom of [3]+• (+ 0.28, Supplementary Fig. 26), indicating the significant involvement of the borinium ion character to the electronic structure of [3]+•. The distribution of LUMO, manifesting mainly the 2p orbital of boron, is in line with the electrophilic nature of the boron atom (Fig. 3). Two orthogonal π-bonding interactions of the di-substituted boron radical involving TMP and CAAC are also captured in the frontier molecular orbitals of [3]+•. As SOMO-3 represents the B = N π-bonding interaction, the share of an unpaired electron between boron and CAAC is depicted in SOMO (Fig. 3).
Reactions of [3]+•
With [3]+• in hand, we then explored its electrochemical reactions. The cyclic voltammogram of [3]+• in DCM features a reversible reduction wave centered at E1/2 = −1.00 V (supplementary Fig. 19), showing that the corresponding CAAC-amino borylene, CAAC-B-TMP (4), can be obtained from the one-electron reduction of [3]+• (Fig. 4a). As expected, mixing an equimolar amount of tetrakis(dimethylamino)ethylene (TDAE) and [3]+• resulted in the clean formation of 4 with the detection of a 11B NMR signal at 71.8 ppm comparable to the value reported for CAAC-coordinated aminoborylenes43,45. Compared with [1]+, which exhibits a reversible reduction at E1/2 = −1.18 V with a peak potential difference of 0.50 V (supplementary Fig. 17), the formation of a di-coordinated boron radical cation does not result in a drastic shift of the reduction potential. However, a considerably narrower redox peak separation of 0.27 V is observed for [3]+•. The obtained not-so-anodic reduction potential and the narrow peak separation of [3]+• could be attributed to the strengthened B–C and B–N π-bonding interactions in the linear di-coordinate boron radical cation that mitigate the electron deficiency of the central boron atom and rigidify the central C = B = N core. In contrast to the facile reduction, no identifiable product could be obtained from oxidation of [3]+•, potentially due to the instability of the resulting di-substituted boron dication, [5]2+, under the reaction conditions. All attempts in oxidizing [3]+• to [5]2+ with [NO][SbCl6], Ag[Al(OC(CF3)3)4], and [(4-Br-C6H4)3N][SbCl6] were unsuccessful (Fig. 4a).
According to DFT calculations, there is a substantial borinium ion character to the electronic structure of [3]+•. Thus, we have also examined the reaction of [3]+• with 4-N,N-dimethylaminopyridine (DMAP) to afford a tri-coordinate boron radical cation [6]+•. Upon addition of DMAP, the DCM solution of [3]+• turned from lime green to violet instantaneously (Fig. 4b). X-ray diffraction analysis on a single crystal of [6][B(C6F5)4] obtained from a DCM/pentane solution at –30 °C corroborated the formation of a tri-coordinate boron radical cation, a much less explored boron radical class (Fig. 4b)46,47,48,49,50. Compared to the reported homoleptic analogs, the base coordination reaction of [3]+• represents a straightforward synthetic method to boron radical cations with a heterolepic coordination environment51,52. The EPR spectrum of [6]+• was obtained as a broad signal at –196 °C, which can be satisfactorily simulated with hyperfine coupling to one boron atom (a(11,10B) = 5.33/1.79G) and three nitrogen atoms (a(14NCAAC) = 8.29 G, a(14NTMP) = 2.63G, and a(14NDMAP) = 1.96G) (supplementary Fig. 23).
CO-regulated electron transfer
Inspired by the [Mes2B]+ caused C = O scission of CO253, we have also examined the reaction of [3]+• towards CO2 and CO. However, [3]+• displays no reaction towards CO2 and decomposes upon exposure to CO. The inability of [3]+• in CO2 binding could be attributed to the lower Lewis acidity of [3]+• than [Mes2B]+, which was corroborated with hydride ion affinity (HIA) calculations (supplementary Table 7)54. The presence of an α-amino group and an additional electron at boron significantly diminish the acidity of [3]+• to afford a HIA of –29.4 kcal/mol, which is 34.2 kcal/mol lower than [Mes2B]+ (–63.6 kcal/mol). Surprisingly, the corresponding borylene–carbonyl complex (7), which could be independently prepared from 4 and CO43, was obtained when a 1:1 mixture of 2• and [3]+• was allowed to react with CO at room temperature (Fig. 5a). The formation of 7 accompanied with an equimolar amount of chloroborenium ion [1]+ is intriguing, as 2• with a reduction potential comparable to [3]+• cannot reduce the di-coordinate boron radical cation to the corresponding borylene (4). As shown in supplementary Fig. 14, no detectable signal of 4 is identified in the NMR spectra of the radical mixture. Upon exposure to CO, two 11B NMR signals at −5.6 ppm and 33.0 ppm attributed to 7 and [1]+ were detected. One interesting property of 7 is its instability under vacuum (supplementary Fig. 16). Upon evacuation, 7 would lose the CO ligand to afford 4 in nearly quantitative yield, implying that the electron transfer between 2• to [3]+• can be regulated with CO. We hypothesized that observed CO-regulated electron transfer is due to an anodic shift of the radical cation upon CO-binding and the weak boron–CO interaction in 7. As the electron deficient nature of carbonyl-boryl radical cation [8]+• promotes the electron transfer from 2• to [8]+•, the reverse transfer of electron from 4 to [1]+ is observed when 7 decomposes under vacuum to give 4.
a Reaction of 2• and [3]+• with CO. b The proposed CO-coordination-initiated electron transfer and the calculated redox potential of relevant species. c EPR spectrum and molecular structure of [9]+•. Dipp = 2,6-diisopropylphenyl, CAAC = cyclic(alkyl)(amino)carbene, CNXyl = 2,6-dimethylphenylisocyanide.
To verify the proposed binding initiated electron transfer, we conducted DFT calculations on the reduction potential of [1]+, [3]+• and [8]+• 55. As summarized in Fig. 5b, the calculated reduction potentials of [1]+ and [3]+• estimated respectively at –0.98 V and –1.18 V are comparable with that determined experimentally. The obtained reversed order is potentially a result of cation-anion interaction in the CV experimental setup. The calculated reduction potential of [8]+• is ca. 0.57 V shifted anodic from [3]+• at –0.61 V, providing the theoretical support for the enhanced electron deficiency of boron radical cation upon CO coordination. With the calculated reduction potentials, we propose a coordination-initiated electron transfer mechanism for the formation of carbonyl–borylene complex. As depicted in Fig. 5b, the coordination of a π-acidic ligand, CO, to [3]+• lowers the reduction potential of the resulting boryl radical cation [8]+•, which then undergoes subsequent one-electron reduction with 2• to afford 7 and [1]+.
As all attempts to isolate [8]+• were unsuccessful due to the rapid decomposition of the carbonyl-boryl radical cation in the absence of reductant, we decided to mimic the carbonyl binding event with a bulkier isoelectronic analog, 2,6-dimethylphenylisocyanide (xylyl-NC). The addition of xylyl-NC to a DCM solution of [3]+• resulted in an instant formation of a dark blue solution, from which dark blue crystals were obtained by adding a few drops of pentane and storing at –30 °C. As demonstrated in Fig. 5c, the binding of isocyanide to the di-coordinate boron radical cation resulted in bending the CCAAC–B–NTMP angle from 172.5(2)o in [3]+• to 127.1(3)° in [9]+•. Compared to [3]+•, the CCAAC–B bond is elongated from 1.457(3) to 1.536(5) Å due to the competing π-accepting property of the isocyanide group. However, the electron back donation from boron radical to the π* orbital of isocyanide does not lead to appreciable elongation of the C≡N bond, potentially as a consequence of steric repulsion between CAAC and xylyl group. As the consequence of the weak B–CCN interaction, the coordination of xylyl-NC does not lead to a significant shift in redox potential of boron radical cation. Thus, [9]+• with a reversible reduction wave at –1.0 V (vs. Fc/Fc+ in DCM) cannot be reduced by 2• to afford the corresponding isocyanide-borylene adduct (10).
In conclusion, a di-coordinate boron radical cation, [3]+• stabilized by adjacent orthogonal π-donating and π-accepting groups has been isolated and fully characterized experimentally and computationally. As the crystal structure of [3]+• confirms the linear geometry at boron, DFT calculations identify a significant contribution of borinium ion character to the push-pull stabilized boron radical cation. While the transformation of [3]+• to borylene 4 is readily achieved with a mild reductant, further oxidation to the elusive di-coordinate boron dication [5]2+ could not be accomplished. The coordination of [3]+• with DMAP represents a facile synthetic method to heteroleptic tri-coordinate boron radical cation [6]+•. Interestingly, the coordination with π-acidic ligand, like CO, triggers subsequent electron transfer from 2• to [8]+• to yield the corresponding carbonyl–borylene complex 7. The removal of CO under vacuum transforms 7 to 4, which then gives an electron to [1]+ to afford a solution consisting of two radicals, 2• and [3]+•. Such CO-regulated electron transfer could be viewed as a boron-radical based CO-transportation or CO-controlled release of low-coordinate boron radical. Currently, the reaction of [3]+• towards other Lewis bases is under investigation. Fine-tuning the stereoelectronic properties of di-coordinate boron radical cation by replacing CAAC and TMP is also undergoing in this laboratory.
Methods
General methods
All the manipulations were conducted using standard Schlenk-line techniques or in a glovebox under nitrogen atmosphere. All solvents were degassed and dried by either molecular sieves (DCM, toluene, hexane), or Na/K (pentane and ether). Cobaltocene (CoCp2), lithium diisopropylamide (LDA), tetrakis(dimethylamino)ethylene (TDAE), 4-dimethylaminopyridine (DMAP) and 2,6-dimethylphenylisocyanide (CNXyl) were purchased and used without further purification. Cyclic (alkyl)(amino)carbene (CAAC), 2,2,6,6-tetramethylpiperidinyl boron dichloride (TMP-BCl2), potassium tetrakis(pentafluorophenyl) borate (K[B(C6F5)4]) were synthesized according to literature procedures (see Supplementary Methods 1.2). SC-XRD were carried out on Bruker D8 VENTURE at 100 K. NMR spectra were recorded using Bruker AvanceIII-400 (1H: 400.2 MHz, 11B: 128.4 MHz, 13C: 100.6 MHz, 19F: 376 MHz). EPR spectra were carried out on either Bruker EMXnano (0.3162 mW) or EMXmicro (21.10 mW). CV spectra were collected on CH Instruments (Model 660D) at a scan rate of 100 mV/s. DFT calculations were carried out with Gaussian 16 program package at the theory level of UCAM-B3LYP/6-31 G(d,p) with SMD(DCM) as the solvation model.
Synthesis of [1][OTf]
A TMP-BCl2 (1.85 g, 8.38 mmol) hexane solution was slowly added into a mixture of CAAC (4.00 g, 8.38 mmol) and LiOTf at −78 °C. The resulting solution was then slowly warmed to room temperature and stirred for another 16 h. After the reaction, all volatiles were removed under vacuum and the solid residue was washed by 10 mL of pentane for two times before the addition of 20 mL of dichloromethane. The DCM solution was then filtered and the filtrate was dried to afford [1][OTf] as white powder (4.66 g, 84%). X-ray quality crystals were obtained by layering a DCM solution of [1][OTf] with pentane. 1H NMR (400.2 MHz, CD2Cl2, ppm): δ 7.53 (t, 1H, para-Dipp), 7.39 (d, 2H, meta-Dipp), 2.78 (sept, 2H, CH(CH3)2), 2.46 (s, 2H, CH2), 2.20-1.5 (m, 16H, H2CCy,TMP), 1.63 (s, 6H, C(CH3)2), 1.54 (br. s, 6H, NC(CH3)2),1.40 (d, 6H, CH(CH3)2), 1.29 (d, 6H, CH(CH3)2), 1.12 (br. s, 6H, NC(CH3)2). 11B NMR (128.4 MHz, CD2Cl2, ppm): δ 33.1. 13C{1H} NMR (100.6 MHz, CD2Cl2, ppm): δ 145.7, 132.3, 131.9, 127.1, 85.3, 62.0, 59.6, 44.0, 40.0, 39.1, 33.3, 33.1, 32.0, 29.4, 28.9, 27.1, 25.8, 24.5, 22.6, 14.9. Anal. Calcd. for C33H53BClF3N2O3S: C, 59.95; H, 8.08; N, 4.24. Found: C, 59.98; H, 8.02; N, 4.09. E1/2 = –1.18 V, peak different 502 mV.
Synthesis of 2•
In a solution CoCp2 (1.00 g, 5.29 mmol) in toluene (20 mL), [1][OTf] (3.61 g, 5.29 mmol) were added in one portion. After addition, the reaction mixture was stirred over-night, and the solvent was removed in vacuo. The product was extracted by 10 mL of pentane to give 2• as reddish powder (1.98 g, 73%). Single crystals were obtained by slow evaporation of a n-pentane solution of 2• at room temperature. Anal. Calcd for C32H53BClN2: C, 75.06; H, 10.43; N, 5.47. Found: C 75.47; H 10.04; N 5.45. E1/2 = −1.15 V, peak different 430 mV.
Synthesis of [3][B(C6F5)4]
An equimolar amount of 2• (1.00 g, 1.95 mmol) and K[B(C6F5)4] was allowed to react in 30 mL of DCM at room temperature for 3 h. Afterwards, all insoluble solids were removed by filtration with celite and the filtrate was dried in vacuo. The solid residue was then washed with 10 mL of pentane to afford [3][B(C6F5)4] as yellow-greenish powder (2.09 g, 93%). X-ray quality crystals were obtained from layering a DCM solution of [3][B(C6F5)4] with pentane at –40 oC. Anal. Calcd for C56H53B2F20N2: C, 58.20; H, 4.62; N, 2.42. Found: C,58.49; H, 4.51; N, 2.31. E1/2 = −1.00 V, peak different 270 mV.
Synthesis of 4
An equimolar amount of [3][B(C6F5)4] (50.0 mg, 0.043 mmol) and TDAE was mixed in 2 mL of o-difluorobenzene. The reaction mixture was stirred for 1 h before the removal of solvent in vacuo. Compound 4 was extracted from the solid residue with 3 mL of hexane and obtained as yellow powder (18.4 mg, 89%). 1H NMR (400.2 MHz, C6D6, ppm): δ 7.15-7.10 (m, 3H, Dipp), 3.71 (sept, 2H, CH(CH3)2), 2.33 (m, 2H, H2CCy) 1.94 (s, 2H, CH2), 1.80-1.40 (m, 10H, H2CCy,TMP), 1.38 (s, 6H, C(CH3)2), 1.37 (d, 6H, CH(CH3)2), 1.31 (d, 6H, CH(CH3)2), 1.24 (br. s, 6H, NC(CH3)2), 1.15 (br. s, 6H, NC(CH3)2). 11B NMR (128.4 MHz, C6D6, ppm): δ 71.8. 13C{1H} NMR (100.6 MHz, C6D6, ppm): δ 151.7, 142.5, 126.5, 123.9, 63.8, 55.7, 53.2, 47.5, 44.4, 38.3, 35.3, 31.5, 27.8, 27.5, 26.9, 26.3, 25.9, 25.5, 17.2.
Synthesis of [6][B(C6F5)4]
DCM (10 mL) was added to the mixture consisting of [3][B(C6F5)4] (50 mg, 0.043 mmol) and DMAP (5.5 mg, 0.043 mmol) at ambient temperature. After stirring for 1 h, solvent was removed with vacuum and the solid residue was washed by 10 mL of pentane to afford [6][B(C6F5)4] as violet powder, which was further purified with re-crystallization from DCM/pentane solution (37.7 mg, 68%). Single crystals suitable for X-ray diffraction analysis were obtained from layering a DCM solution of [6][B(C6F5)4] with pentane at –40 oC. Anal. Calcd for C63H63B2F20N4: C, 59.22; H, 4.97; N, 4.38. Found: C, 58.91; H, 4.95; N, 4.05.
Synthesis of 7
DCM (0.5 mL) was added to the J-Young’s NMR tube consisting of 2• (17.7 mg, 0.035 mmol) and [3][B(C6F5)4] (40.0 mg, 0.035 mmol) at ambient temperature. The solution was degassed with three freeze-pump-thaw cycles. Then, the NMR tube was then connected though a tygon tubing to a CO-filled 100 mL Schlenk flask at ambient pressure. After the exposure to CO atmosphere to for 18 h, all volatiles were removed in vacuo. 7 was obtained from the reaction mixture by hexane extraction and isolated as reddish powder. 1H NMR (400.2 MHz, CD2Cl2, ppm): δ 7.36 (t, 1H, para-Dipp), 7.22 (d, 2H, meta-Dipp), 2.96 (sept, 2H, CH(CH3)2), 2.71 (m, 2H, H2CCy), 2.10 (s, 2H, CH2), 1.90-1.60 (m, 8H, H2CCy), 1.50-1.40 (m, 6H, H2CTMP), 1.31 (s, 6H, C(CH3)2), 1.30 (d, 6H, CH(CH3)2), 1.25 (d, 6H, CH(CH3)2), 1.21 (br. s, 6H, NC(CH3)2), 1.00 (br. s, 6H, NC(CH3)2). 11B NMR (128.4 MHz, CD2Cl2, ppm): δ −5.6. 13C{1H} NMR (100.6 MHz, C6D6, ppm): δ 229.4 (m, BCO), 202.5 (m, CCAACB),149.8, 134.3, 130.2, 126.0, 67.6, 55.4, 54.0, 48.2, 42.7, 37.7, 36.2, 32.21, 30.76, 28.2, 28.1, 27.2, 26.3, 25.2, 23.6, 19.0.
Synthesis of [9][B(C6F5)4]
DCM (10 mL) was added to the mixture consisting of [3][B(C6F5)4] (100 mg, 0.086 mmol) and CNXyl (12.5 mg, 0.095 mmol) at ambient temperature. After stirring for 1 h, the reaction solution was concentrated to about 2 mL before the addition of 20 mL of pentane. The precipitates were collected and dried to afford [9][B(C6F5)4] as violet powder (91 mg, 81%). X-ray quality crystals were obtained from layering a DCM solution of [9][B(C6F5)4] with pentane at –40 oC. Anal. Calcd for C65H62B2F20N3•CH2Cl2 + 0.5C5H12: C, 58.44; H, 5.01; N, 2.98. Found: C, 59.18; H, 4.80; N, 3.07. E1/2 = –1.02 V, peak different 103 mV.
Data availability
Experimental details on the synthesis and characterization of all reported compounds can be found in the supporting information. Data supporting the formation of all isolated compounds include NMR spectra, EPR spectra, cyclic voltammogram, and DFT calculations. Crystallographic data of 2•, [3][B(C6F5)4], [6][B(C6F5)4], and [9][B(C6F5)4] have been deposited to CCDC database with the deposition number of CCDC 2167148, 2167149, 2167150, and 2167151.
References
Shimizu, T., Lengalova, A., Martínek, V. & Martínková, M. Heme: emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 48, 5624–5657 (2019).
Protchenko, A. V. et al. Stable GaX2, InX2 and TlX2 radicals. Nat. Chem. 6, 315–319 (2014).
Wang, G. et al. A stable, crystalline beryllium radical cation. J. Am. Chem. Soc. 142, 4560–4564 (2020).
Czernetzki, C. et al. A neutral beryllium(I) radical. Angew. Chem. Int. Ed. 60, 20776–20780 (2021).
Protchenko, A. V. et al. Oxidative bond formation and reductive bond cleavage at main group metal centers: reactivity of five-valence-electron MX2 radicals. J. Am. Chem. Soc. 136, 10902–10905 (2014).
Su, Y. & Kinjo, R. Boron-containing radical species. Coord. Chem. Rev. 352, 346–378 (2017).
Duret, G., Quinlan, R., Bisseret, P. & Blanchard, N. Boron chemistry in a new light. Chem. Sci. 6, 5366–5382 (2015).
Friese, F. W. & Studer, A. New avenues for C–B bond formation via radical intermediates. Chem. Sci. 10, 8503–8518 (2019).
Lovinger, G. J. & Morken, J. P. Recent advances in radical addition to alkenylboron compounds. Eur. J. Org. Chem. 2020, 2362–2368 (2020).
Qi, J. et al. New radical borylation pathways for organoboron synthesis enabled by photoredox catalysis. Angew. Chem. Int. Ed. 59, 12876–12884 (2020).
Taniguchi, T. Advances in chemistry of N-heterocyclic carbene boryl radicals. Chem. Soc. Rev. 50, 8995–9021 (2021).
Ollivier, C. & Renaud, P. Organoboranes as a source of radicals. Chem. Rev. 101, 3415–3434 (2001).
Ueng, S.-H. et al. N-heterocyclic carbene boryl radicals: a new class of boron-centered radical. J. Am. Chem. Soc. 131, 11256–11262 (2009).
Tehfe, M.-A. et al. N-Heterocyclic carbene-borane radicals as efficient initiating species of photopolymerization reactions under air. Polym. Chem. 2, 625–631 (2011).
Aubry, B. et al. A second-generation chameleon N-heterocyclic carbene–borane coinitiator for the visible-light oxygen-resistant photopolymerization of both organic and water-compatible resins. Macromolecules 51, 9730–9739 (2018).
Min, Y., Dou, C., Liu, D., Dong, H. & Liu, J. Quadruply B←N-fused dibenzo-azaacene with high electron affinity and high electron mobility. J. Am. Chem. Soc. 141, 17015–17021 (2019).
Feng, Z. et al. Stable boron-containing blue-photoluminescent radicals. Chin. J. Chem. 39, 1297–1302 (2021).
Ito, M. et al. Fluorescent organic π-radicals stabilized with boron: featuring a SOMO–LUMO electronic transition. Angew. Chem. Int. Ed. 61, e202201965 (2022).
Loh, Y. K. et al. A crystalline radical cation derived from Thiele’s hydrocarbon with redox range beyond 1 V. Nat. Commun. 12, 7052 (2021).
Zeng, J., Dong, S., Dai, C. & Zhu, J. Predicting dinitrogen activation by five-electron boron-centered radicals. Inorg. Chem. 61, 2234–2241 (2022).
Brotherton, R. J. & McCloskey, A. L. Tetra-(amino)-diborons1,2. J. Am. Chem. Soc. 82, 6242–6245 (1960).
Kuchen, W. & Brinkmann, R.-D. Zur Kenntnis des (C6H5B)n. Z. anorg. allg. Chem. 325, 225–236 (1963).
Wehrmann, R., Meyer, H. & Berndt, A. Diboriranides and a 1,3-diboraallyl system with B-H-B bridge. Angew. Chem. Int. Ed. 24, 788–790 (1985).
Hunold, R., Allwohn, J., Baum, G., Massa, W. & Berndt, A. Compounds with partial boron-carbon triple bonds. Angew. Chem. Int. Ed. 27, 961–963 (1988).
Mennekes, T., Paetzold, P., Boese, R. & Bläser, D. Tetra-tert-butyltetraboratetrahedrane. Angew. Chem. Int. Ed. 30, 173–175 (1991).
Grigsby, W. J. & Power, P. P. Isolation and reduction of sterically encumbered arylboron dihalides: novel boranediyl insertion into C−C σ-bonds. J. Am. Chem. Soc. 118, 7981–7988 (1996).
Anastasi, N. R., Waltz, K. M., Weerakoon, W. L. & Hartwig, J. F. A short synthesis of tetraalkoxydiborane(4) reagents. Organometallics 22, 365–369 (2003).
Wang, Y. et al. A stable neutral diborene containing a BB double bond. J. Am. Chem. Soc. 129, 12412–12413 (2007).
Yamashita, M., Aramaki, Y. & Nozaki, K. Reduction of base-stabilized difluoroboranes to induce rearrangement reactions. N. J. Chem. 34, 1774–1782 (2010).
Braunschweig, H., Ghosh, S., Kupfer, T., Radacki, K. & Wahler, J. High-yield synthesis of a hybrid 2,3,4,5-tetracarba-1,6-nido-hexaborane(6) cluster with an exo-polyhedral boracycle. Chem. Eur. J. 17, 4081–4084 (2011).
Shoji, Y. et al. Boron–boron σ-bond formation by two-electron reduction of a H-bridged dimer of monoborane. J. Am. Chem. Soc. 133, 11058–11061 (2011).
Hübner, A., Bolte, M., Lerner, H.-W. & Wagner, M. Extensive structural rearrangements upon reduction of 9h-9-borafluorene. Angew. Chem. Int. Ed. 53, 10408–10411 (2014).
Kaese, T., Hübner, A., Bolte, M., Lerner, H.-W. & Wagner, M. Forming B–B bonds by the controlled reduction of a tetraaryl-diborane(6). J. Am. Chem. Soc. 138, 6224–6233 (2016).
Budy, H., Kaese, T., Bolte, M., Lerner, H.-W. & Wagner, M. A chemiluminescent tetraaryl diborane(4) tetraanion. Angew. Chem. Int. Ed. 60, 19397–19405 (2021).
Wegner, B. et al. An organic borate salt with superior p-doping capability for organic semiconductors. Adv. Sci. 7, 2001322 (2020).
Shoji, Y. et al. Tetraaryldiborane(4) can emit dual fluorescence responding to the structural change around the B–B bond. Angew. Chem. Int. Ed. 61, e202113549 (2022).
Viehe, H. G., Merényi, R., Stella, L. & Janousek, Z. Capto-dative substituent effects in syntheses with radicals and radicophiles [New synthetic methods (32)]. Angew. Chem. Int. Ed. 18, 917–932 (1979).
Viehe, H. G., Janousek, Z., Merenyi, R. & Stella, L. The captodative effect. Acc. Chem. Res. 18, 148–154 (1985).
Peterson, J. P. & Winter, A. H. Solvent effects on the stability and delocalization of aryl dicyanomethyl radicals: the captodative effect revisited. J. Am. Chem. Soc. 141, 12901–12906 (2019).
Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): stable carbenes on the rise. Acc. Chem. Res. 48, 256–266 (2015).
Melaimi, M., Jazzar, R., Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): recent developments. Angew. Chem. Int. Ed. 56, 10046–10068 (2017).
Kundu, S., Sinhababu, S., Chandrasekhar, V. & Roesky, H. W. Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents. Chem. Sci. 10, 4727–4741 (2019).
Dahcheh, F., Martin, D., Stephan, D. W. & Bertrand, G. Synthesis and reactivity of a CAAC–aminoborylene adduct: a hetero-allene or an organoboron isoelectronic with singlet carbenes. Angew. Chem. Int. Ed. 53, 13159–13163 (2014).
Bissinger, P. et al. Isolation of a neutral boron-containing radical stabilized by a cyclic (alkyl)(amino)carbene. Angew. Chem. Int. Ed. 53, 7360–7363 (2014).
Ledet, A. D. & Hudnall, T. W. Reduction of a diamidocarbene-supported borenium cation: isolation of a neutral boryl-substituted radical and a carbene-stabilized aminoborylene. Dalton Trans. 45, 9820–9826 (2016).
Yang, W. et al. Persistent borafluorene radicals. Angew. Chem. Int. Ed. 59, 3850–3854 (2020).
Hollister, K. K. et al. Isolation of stable borepin radicals and anions. Angew. Chem. Int. Ed. 61, e202202516 (2022).
Kinjo, R., Donnadieu, B., Celik Mehmet, A., Frenking, G. & Bertrand, G. Synthesis and characterization of a neutral tricoordinate organoboron isoelectronic with amines. Science 333, 610–613 (2011).
Bissinger, P. et al. Boron as a powerful reductant: synthesis of a stable boron-centered radical-anion radical-cation pair. Angew. Chem. Int. Ed. 54, 359–362 (2015).
Huang, J.-S. et al. Cp*-substituted boron cations: the effect of NHC, NHO, and CAAC ligands. Inorg. Chem. 55, 12427–12434 (2016).
Ruiz, D. A., Melaimi, M. & Bertrand, G. An efficient synthetic route to stable bis(carbene)borylenes [(L1)(L2)BH]. Chem. Commun. 50, 7837–7839 (2014).
Wang, H., Zhang, J., Lin, Z. & Xie, Z. Synthesis and structural characterization of carbene-stabilized carborane-fused azaborolyl radical cation and dicarbollyl-fused azaborole. Organometallics 35, 2579–2582 (2016).
Shoji, Y., Tanaka, N., Mikami, K., Uchiyama, M. & Fukushima, T. A two-coordinate boron cation featuring C–B+–C bonding. Nat. Chem. 6, 498–503 (2014).
Clark, E. R., Del Grosso, A. & Ingleson, M. J. The hydride-ion affinity of borenium cations and their propensity to activate H2 in frustrated Lewis pairs. Chem. Eur. J. 19, 2462–2466 (2013).
Yan, L., Lu, Y. & Li, X. A density functional theory protocol for the calculation of redox potentials of copper complexes. Phys. Chem. Chem. Phys. 18, 5529–5536 (2016).
Acknowledgements
This work is supported by the Ministry of Science and Technology of Taiwan (MOST 110-2113-M-002-014-MY3, MOST 107-2113-M-002-007-MY3) and National Taiwan University (110L880503). We would like to thank Prof. Huadong Wang at Fudan University for the insightful discussions.
Author information
Authors and Affiliations
Contributions
Y.-J.L. and W.-C.L. performed the synthetic work, theoretical calculations, and spectroscopic characterizations. Y.-H.L., G.-H.L., and S.-Y.C. determined the crystal structure of the compounds. C.-W.C. conceived the research project and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks David Wilson, Kam-Hung Low and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, YJ., Liu, WC., Liu, YH. et al. A linear Di-coordinate boron radical cation. Nat Commun 13, 7051 (2022). https://doi.org/10.1038/s41467-022-34900-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34900-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.