Abstract
In endochondral bone development, bone-forming osteoblasts and bone marrow stromal cells have dual origins in the fetal cartilage and its surrounding perichondrium. However, how early perichondrial cells distinctively contribute to developing bones remain unidentified. Here we show using in vivo cell-lineage analyses that Dlx5+ fetal perichondrial cells marked by Dlx5-creER do not generate cartilage but sustainably contribute to cortical bone and marrow stromal compartments in a manner complementary to fetal chondrocyte derivatives under the regulation of Hedgehog signaling. Postnatally, Dlx5+ fetal perichondrial cell derivatives preferentially populate the diaphyseal marrow stroma with a dormant adipocyte-biased state and are refractory to parathyroid hormone-induced bone anabolism. Therefore, early perichondrial cells of the fetal cartilage are destined to become an adipogenic subset of stromal cells in postnatal diaphyseal bone marrow, supporting the theory that the adult bone marrow stromal compartments are developmentally prescribed within the two distinct cells-of-origins of the fetal bone anlage.
Similar content being viewed by others
Introduction
Bones are incredibly malleable organs capable of growing exponentially in early life and maintaining their structures and functions throughout life. The inherent flexibility of bones is endowed at least in part by the unique property of bone-forming osteoblasts that can be generated from multiple cellular origins1,2,3,4,5,6. Most bones in mammals are formed through the endochondral pathway, in which initial cartilage templates established in a vasculature-free environment are gradually replaced by bones associated with invading vasculature7. The two most developmentally important cellular sources of endochondral bones lie in the cartilage template proper and its surrounding perichondrium. It is now commonly accepted that fetal chondrocytes within cartilage templates can transform into osteoblasts and marrow stromal cells to establish the nascent marrow space2,5,8,9,10. Cells in the perichondrium are equally important, as these cells can contribute both to the cortical bone and the marrow space11. The partnership between the cartilage template and the perichondrium provides the anatomical foundation of endochondral bones, ultimately establishing diverse compartments in postnatal long bones.
The perichondrium is composed of multiple layers of elongated fibroblasts in a vasculature-rich environment. Cells constituting the perichondrium of the fetal cartilage are not completely defined. The osteogenic perichondrium is the inner layer of the perichondrium where the first osteoblasts appear in endochondral bones12. The formation of osteoblast precursor cells in the osteogenic perichondrium is executed at least in part due to the actions of Indian Hedgehog (Ihh) released from adjacent pre-hypertrophic chondrocytes13. Osterix (Osx)-expressing cells are located in the innermost layer of the perichondrium and represent the committed precursor cells of the osteoblast lineage. Osx+ cells translocate into the nascent marrow space, and their descendants eventually populate the area as osteoblasts and marrow stromal cells4. Importantly, these Osx+ cell derivatives are transient, and eventually disappear from the perichondrium and the marrow space in subsequent postnatal stages. The identity of earlier perichondrial cells that constantly replace Osx+ cells in the perichondrium remain unidentified.
Previous studies reported several constitutively active cre and inducible creER lines that can target perichondrial cells, including Prrx1-creER14, Hoxa11-creER15,16, Sox9-creER17, Axin2-creER18, Ctsk-cre19, and Fsp1-cre20,21. While these studies substantially contributed to the field, these reported lines may have potential limitations. In particular, these creER lines also mark at least some of chondrocytes within the growth plate. Moreover, Hoxa11-creER is active exclusively in the zeugopod bones (ulna, radius, tibia, and fibula)15, while Sox9-creER is specific to perichondrial cells exclusively in the rib cartilage17. Additionally, the constitutively active cre lines lack temporal control and have activities in other cell types, such as osteoclasts (Ctsk-cre) and other fibroblasts (Fsp1-cre). Therefore, a perichondrial cell-specific inducible mouse genetic tool would facilitate the understanding of the function of early perichondrial cells.
In this study, we aimed to achieve two major goals. First, we sought to define the identity of as-yet unidentified early perichondrial cells that can provide a perpetual source of osteoblasts and marrow stromal cells, using a combination of single-cell RNA-seq analyses and in vivo lineage-tracing approaches. Second, we sought to define how these identified early perichondrial cells contribute postnatally to functionally distinct compartments of developing bones, including the cortical bone and the bone marrow, and to determine whether osteoblasts and bone marrow stromal cells derived from the two cells-of-origins have distinct features and functions, using comparative in vivo lineage-tracing approaches. The cell type-specific creER lines – Dlx5-creER for early fetal perichondrial cells and Fgfr3-creER for fetal chondrocytes – highlight distinctive cell fates and contributions of the two cell types. Our findings support the theory that the adult bone marrow stromal compartments are developmentally prescribed within the two distinct cells-of-origins of the fetal cartilage.
Results
Putative cells-of-origins of the fetal cartilage
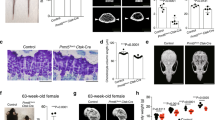
First, to discover the putative cellular origins of the fetal cartilage, we performed single-cell RNA-sequencing (scRNA-seq) analyses of the chondrocyte-perichondrial cell lineage at embryonic day (E) 13.5 in mice. Both perichondrial cells and chondrocytes at the fetal stage can be fate-mapped by a cre recombinase driven by a Col2a1 promoter (Col2a1-cre)22 (Fig. 1a). We dissociated limb mesenchymal cells of Col2a1-cre; R26RtdTomato mice at E13.5, and profiled fluorescence-activated cell sorting (FACS)-isolated Col2a1cre-tdTomato+ cells using the 10X Chromium Single-Cell Gene Expression Solution platform (Fig. 1b, c and Supplementary Fig. 1a). Louvain community detection after cell cycle regression revealed 9 clusters (Fig. 1d), including three clusters of chondrocytes abundant in Sox9 (Cluster 0,1,2; Fig. 1e and Supplementary Fig. 1b, see also Fig. 1a for immunostaining) and two clusters of perichondrial cells abundant in Prrx1 (Cluster 4,5).
a–e Single-cell RNA-seq analysis of Col2a1-cre-marked chondrocytes and perichondrial cells at E13.5. a: Col2a1-cre; R26RtdTomato femur immunostained for SOX9. R round layer, F flat layer, PH prehypertrophic layer, PC perichondrium, Grey DIC. Scale bar: 200 µm. n = 3 mice. b, c FACS (b) and scRNA-seq (c) strategy for Col2a1cre-tdTomato+ cells (red box). Shown are cells isolated from Col2a1-cre; R26RtdTomato limbs (left) or R26RtdTomato control limbs (right). d UMAP plot of major classes of Col2a1cre-tdTomato+ cells (Cluster 0 – 9, 7,889 cells). Pooled from n = 5 mice. Dotted box: Feature plot of tdTomato. e Feature plots of representative genes enriched in each cluster. Cluster 0,1,2: Sox9+ (green dotted contour), Cluster 1: Col2a1+, Acan+, Fgfr3+, Cluster 1,5: Sp7+, Cluster 4,5: Prrx1+, Dlx5+, Cluster 5: Runx2+. Violet: high expression, yellow: low expression. f Fgfr3-GFP; Osx-mCherry femur immunostained for SOX9. Scale bar: 200 µm. n = 4 mice. g RNAScope analyses of Dlx5. Scale bar: 200 µm. n = 4 mice. h RNA velocity analysis. Dynamical model-based RNA velocity vectors superimposed on the UMAP plot. The origins of black arrows represent the inferred initial states, namely Cluster 1 (blue arrowhead) and Cluster 5 (red arrowhead). i Initial state and root cell inference. Top: colored by GPCCA-based, CellRank-computed initial state probability. Violet indicates low probability while yellow indicates high probability. Bottom: Cluster 5 is the inferred root cell population.
The fetal chondrocyte-perichondrial cell lineage was largely contiguous and shared an overlapping set of marker genes. Of the chondrocyte clusters, cells in Cluster 1 were enriched for Col2a1, Fgfr3, Acan, Ihh, and Sp7, representing relatively mature chondrocytes (Fig. 1e). Among the perichondrial cell clusters, cells in Cluster 5 were enriched for Runx2 and Sp7, representing perichondrial cells partially committed to the osteoblast lineage4,23. Both Dlx5 and Prrx1 were enriched in Cluster 4 and 5. Dlx5 is an upstream regulator of Runx224 and a co-factor of Sp7 (also known as osterix, Osx)25. Dlx5 was enriched both in Cluster 4 and 5, identified as the most significantly differentially expressed gene in Cluster 5 and most abundantly expressed at the border of Cluster 4 and 5 (Fig. 1e). In contrast, Prrx1 was diffusely expressed in other clusters including chondrocytes (Fig. 1e), indicating Dlx5 might serve as a reliable cell type-specific marker for early perichondrial cells.
We further examined in vivo expression patterns of potential cell type-specific genes that we identified above using independent approaches, including Fgfr3-GFP; Sp7(Osx)-mCherry double transgenic reporters, RNAscope assays and SOX9 immunostaining. Importantly, SOX9 proteins and Fgfr3-GFP (MMRRC:031901) activities were essentially confined to fetal chondrocytes within cartilage templates, with Fgfr3-GFP+ cells representing a more differentiated subset of SOX9+ cells located toward the center of the cartilage template (Fig. 1f). Osx-mCherry activities were observed not only in the perichondrium but also in the cartilage template representing an even more differentiated subset of SOX9+Fgfr3-GFP+ chondrocytes. Additionally, while Col2a1 mRNA was almost exclusively expressed within the cartilage template, Dlx5 mRNA was predominantly expressed in the perichondrium (Fig. 1g and Supplementary Fig. 1c). Therefore, these in vivo gene expression patterns are concordant with our cluster designation of chondrocytes and perichondrial cells in the scRNA-seq dataset.
To predict the putative cells-of-origins in the fetal cartilage and the perichondrium, we subsequently performed RNA velocity analysis26. Two putative cells-of-origins were computationally predicted; (1) the edge of Cluster 1 corresponding to relatively mature chondrocytes, and (2) the midpoint between Clusters 4 and 5 corresponding to relatively immature perichondrial cells (Fig. 1h). Tracing these velocity vectors backward using CellRank inferred Cluster 5 as a putative origin for the differentiation process (Fig. 1i).
Therefore, these computational analyses revealed that perichondrial cells immediately outside of the osteogenic perichondrium might provide a putative cell-of-origin of the fetal chondrocyte-perichondrial cell lineage. We focused on Dlx5 and Fgfr3 as markers for early perichondrial cells and fetal chondrocytes, respectively, in subsequent in vivo lineage-tracing studies.
Dlx5+ ePCs: Dlx5-creER marks early perichondrial cells
We utilized tamoxifen-inducible Dlx5-creER27 and Osx-creER4 lines to mark fetal perichondrial cells and an Fgfr3-creER P1-derived artificial chromosome (PAC) transgenic line28 to mark fetal chondrocytes. These lines were crossed with R26RtdTomato and Col1a1(2.3 kb)-GFP reporters, and to visualize the corresponding cell types, the triple transgenic mice were pulsed at E12.5 and analyzed after 24 h at E13.5.
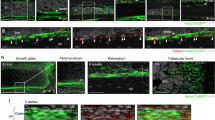
We first defined the characteristics of cells marked by Dlx5-creER, Osx-creER and Fgfr3-creER at this stage (Dlx5CE-E12.5, OsxCE-E12.5 and Fgfr3CE-E12.5 cells, respectively). Dlx5CE-E12.5 cells were located in the outer perichondrium, which was outside of the SOX9+ cartilage template and Col1a1(2.3 kb)-GFP+ osteogenic perichondrium; in fact, Dlx5CE-E12.5 cells only minimally overlapped with SOX9+ or Col1a1(2.3 kb)-GFP+ cells (Fig. 2a). Importantly, Dlx5CE-E12.5 cells did not overlap with MYH3+ skeletal muscle cells or EMCN+ endothelial cells, demonstrating their perichondrial identities (Fig. 2b). In contrast, OsxCE-E12.5 cells occupied the inner perichondrium, and adjoined or overlapped with Col1a1(2.3 kb)-GFP+ cells (Fig. 2a, c). Additionally, many OsxCE-E12.5 cells were located within the pre-hypertrophic layer of cartilage templates as reported previously4. Fgfr3CE-E12.5 cells were located within cartilage templates but not in the outer perichondrium (Fig. 2a).
a–d Localization of Dlx5-creER+, Osx-creER+, or Fgfr3-creER+ cells at E13.5 (pulsed at E12.5), visualized by cre-inducible R26RtdTomato reporter, with Col1a1(2.3 kb)-GFP reporters to mark osteoblasts. a E13.5 cartilage template immunostained for SOX9. Left panels: Scale bar: 200 µm. Right panels: magnified view of the boxed areas (1–3). n = 4 mice per each group. b Col1a1(2.3 kb)-GFP; Dlx5-creER; R26RtdTomato perichondria at E13.5, immunostained for MYH3 (skeletal muscles, left) or EMCN (endothelial cells, right). Grey: DAPI. Scale bar: 20 µm. n = 4 mice per each group. c Quantification of Dlx5-creER+, Osx-creER+, or Fgfr3-creER+tdTomato+ cells. Left: percentage of tdTomato+ cells among Col1a1-GFPneg perichondrial cells. Center: percentage of Col1a1-GFP+tdTomato+ cells among Col1a1-GFP+ osteogenic perichondrial cells. Right: percentage of SOX9+tdTomato+ cells among SOX9+ chondrocytes. n = 4 mice per each group. Two-tailed, one-way ANOVA followed by Tukey’s post-hoc test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. d Dlx5-creER and Fgfr3-creER can mark mutually exclusive cell populations in the fetal perichondrium and cartilage template, respectively, whereas Osx-creER marks cells that overlap with those two cell types. Source data are provided as a Source Data file.
We further quantified Dlx5CE-E12.5, OsxCE-E12.5 and Fgfr3CE-E12.5 tdTomato+ cells in the perichondrium and the cartilage template. First, we quantified Col1a1(2.3 kb)-GFPnegtdTomato+ perichondrial cells in the outer perichondrium. Dlx5CE-E12.5 cells accounted for approximately half of Col1a1-GFPneg perichondrial cells (55.6 ± 4.0%), while OsxCE-E12.5 or Fgfr3CE-E12.5 cells overlapped only minimally with these cells (OsxCE-E12.5: 6.8 ± 1.0%, Fgfr3CE-E12.5: 2.1 ± 0.7%, Fig. 2c, left panel). Second, we quantified Col1a1(2.3 kb)-GFP+tdTomato+ osteogenic perichondrial cells in the inner perichondrium. A majority of osteogenic perichondrial cells was composed of OsxCE-E12.5 cells (73.1 ± 9.5%), while Dlx5CE-E12.5 or Fgfr3CE-E12.5 cells were minimally present in this area (Dlx5CE-E12.5: 9.9 ± 0.7%, Fgfr3CE-E12.5: 6.2 ± 2.6%, Fig. 2c, center panel). Third, we quantified SOX9+tdTomato+ chondrocytes in the cartilage template proper. As expected, Fgfr3CE-E12.5 cells constituted a substantial fraction of SOX9+tdTomato+ chondrocytes, and OsxCE-E12.5 cells composed a smaller but a sizable fraction of these cells. In contrast, virtually no Dlx5CE-E12.5 cells constituted these chondrocytes (Dlx5CE-E12.5: 2.2 ± 0.4%, OsxCE-E12.5: 18.0 ± 3.9%, Fgfr3CE-E12.5: 36.6 ± 1.8%, Fig. 2c, right panel).
Therefore, Dlx5-creER can preferentially mark Col1a1-GFP negative cells in the outer perichondrium, which are distinct from osteogenic perichondrial cells or chondrocytes marked by Osx-creER or Fgfr3-creER, respectively. In other words, Dlx5-creER and Fgfr3-creER can mark mutually exclusive cell populations in the fetal perichondrium and cartilage template, respectively, whereas Osx-creER marks cells that overlap with those two cell types (Fig. 2d).
Dlx5+ ePCs contribute to cortical bone and marrow stroma
Subsequently, we traced the fate of Dlx5-creER+ early perichondrial cells during the formation of the primary ossification center. After 3 days of chase at E15.5, Dlx5CE-E12.5 cells expanded and contributed to the perichondrium and the newly formed bone collar (periosteum), while only minimally contributing to the marrow space (Fig. 3a-1). In contrast, OsxCE-E12.5 and Fgfr3CE-E12.5 cells populated the nascent marrow space in large numbers (Fig. 3a–2,3), indicating that the nascent marrow space may be initially occupied by the cells derived from the cartilage template. The apparently higher contribution of OsxCE-E12.5 cells to the marrow space than that of Fgfr3CE-E12.5 cells at this stage likely reflects the fact that Osx-creER marks pre-hypertrophic and hypertrophic chondrocytes that can promptly move into the marrow space, while Fgfr3-creER marks proliferating chondrocytes that take some time to move into the marrow space. Importantly, OsxCE-E12.5 cells disappeared from the perichondrium and the pre-hypertrophic layer after translocation (Fig. 3a-2), while Fgfr3CE-E12.5 cells expanded within the cartilage template (Fig. 3a-3), highlighting that Fgfr3-creER can mark chondroprogenitor cells whereas Osx-creER marks pre-hypertrophic chondrocytes with transient nature.
a–c Cell-fate analysis of Dlx5-creER+, Osx-creER+, or Fgfr3-creER+ cells at E15.5 (pulsed at E12.5), visualized by cre-inducible R26R-tdTomato reporter, with Col1a1(2.3 kb)-GFP reporters for osteoblasts. a E15.5 whole femurs. Scale bar: 200 µm. Right panels: magnified view of the boxed areas. n = 4 mice per each group. b E18.5 whole femurs. Right panels: magnified view of the boxed areas showing perichondrium, growth plate and bone collar. Arrowhead: groove of Ranvier and periosteum. Scale bar: 500 µm. n = 4 mice per each group. c Quantification of lineage-marked tdTomato+ cells. Upper left: percentage of tdTomato+ cells among total periosteal cells. Upper center: percentage of Col1a1-GFP+tdTomato+ cells among Col1a1-GFP+ cortical osteoblasts. Upper right: percentage of Col1a1-GFP+tdTomato+ cells among Col1a1-GFP+ trabecular osteoblasts. Lower left: number of tdTomato+ BMSCs. Lower right: percentage of SOX9+tdTomato+ cells among SOX9+ growth plate chondrocytes. n = 4 mice per each group. Two-tailed, one-way ANOVA followed by Tukey’s post-hoc test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. d Diagram depicting lineage contribution of Dlx5-creER+, Osx-creER+, and Fgfr3-creER+ cells in fetal endochondral bone development, at E15.5 and E18.5. Source data are provided as a Source Data file.
After 6 days of chase at E18.5, when the marrow space expanded further and hematopoiesis was established, Dlx5CE-E12.5 cells contributed to trabecular bone osteoblasts and marrow stromal cells (Fig. 3b-2). Dlx5CE-E12.5 cells also expanded in the periosteum and the perichondrium, contributing to the groove of Ranvier (Fig. 3b-1, arrowheads) and the cortical bone. In contrast, Fgfr3CE-E12.5 cells continued to contribute to the marrow space, the growth plate cartilage and part of the perichondrium (Fig. 3b-5,6). Importantly, OsxCE-E12.5 cells continued to contribute to the marrow space but not to the perichondrium (Fig. 3b-3,4), underscoring their transient nature in the perichondrium as reported previously29.
Additionally, we defined the presumed migratory path of Dlx5CE-E12.5 cells from the perichondrium to the marrow space by analyzing serial time points from E13.5 to E18.5. Consistent with our findings described above, Dlx5CE-E12.5 cells expanded within the Col1a1-GFPneg outer domain of the perichondrium for two days (Supplementary Fig. 2a, b), and subsequently started to move laterally to the Col1a1-GFP+ osteogeonic perichondrium after 3 days of chase at E15.5 (Supplementary Fig. 2c). After 4 days of chase at E16.5, Dlx5CE-E12.5 cells moved further laterally toward the nascent marrow space and massively expanded therein, and progressively differentiated into Col1a1(2.3 kb)-GFP+ osteoblasts of the trabecular and cortical compartments (Supplementary Fig. 2d–f). These findings demonstrate that Dlx5+ perichondrial cells can travel laterally to enter the marrow space.
We further quantified Dlx5CE-E12.5, OsxCE-E12.5 and Fgfr3CE-E12.5 tdTomato+ cells at E18.5 in various bone compartments established by this stage. First, we quantified tdTomato+ cells in the periosteum. Dlx5CE-E12.5 cells occupied essentially the entire periosteum (89.7 ± 2.9%), while OsxCE-E12.5 or Fgfr3CE-E12.5 cells were only minimally present in this area (OsxCE-E12.5: 4.1 ± 1.3%, Fgfr3CE-E12.5: 11.7 ± 3.1%, Fig. 3c, upper left). Second, we quantified Col1a1(2.3 kb)-GFP+tdTomato+ osteoblasts in the cortical bone. Dlx5CE-E12.5 cells contributed to a large majority of cortical bone osteoblasts (periosteal and endosteal osteoblasts, 71.4 ± 2.5%), while OsxCE-E12.5 or Fgfr3CE-E12.5 cells contributed to a much smaller fraction of these cells (OsxCE-E12.5: 19.6 ± 3.8%, Fgfr3CE-E12.5: 21.0 ± 3.5%, Fig. 3c, upper center), indicating that a majority of cortical bone osteoblasts are derived from Dlx5+ perichondrial cells. Third, we quantified Col1a1(2.3 kb)-GFP+tdTomato+ osteoblasts in the trabecular bone. Importantly, Dlx5CE-E12.5 perichondrial cells and Fgfr3CE-E12.5 chondrocytes contributed to an equivalent fraction of trabecular bone osteoblasts (Dlx5CE-E12.5: 51.3 ± 5.4%, Fgfr3CE-E12.5: 49.2 ± 4.2%, Fig. 3c, upper right), indicating that trabecular bone osteoblasts at this stage might be equally derived from the two cells-of-origins of Dlx5+ perichondrial cells and Fgfr3+ chondrocytes. We also found that OsxCE-E12.5 cells contributed to a large fraction of these cells at this stage (67.9 ± 4.8%), indicating that OsxCE-E12.5 cells overlap extensively both with Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells. Fourth, we quantified tdTomato+ bone marrow stromal cells (BMSCs) within the marrow space. Dlx5CE-E12.5 cells contributed to a higher number of BMSCs in the marrow space than the other cell types do (Dlx5CE-E12.5: 842.7 ± 91.9 cells, OsxCE-E12.5: 511.0 ± 84.8 cells, Fgfr3CE-E12.5: 500.0 ± 94.0 cells, Fig. 3c, lower left), indicating that Dlx5+ early perichondrial cells may preferentially contribute to marrow stromal cells. Fifth, we quantified SOX9+tdTomato+ chondrocytes with the growth plate. As expected, only Fgfr3CE-E12.5 cells, but not Dlx5CE-E12.5 or OsxCE-E12.5 cells, contributed to growth plate chondrocytes at this stage (Fgfr3CE-E12.5: 70.0 ± 5.1%, Dlx5CE-E12.5: 1.0 ± 0.7%, OsxCE-E12.5: 0%, Fig. 3c, lower right), demonstrating that Dlx5+ early perichondrial cells do not become growth plate chondrocytes.
Therefore, trabecular bone osteoblasts and BMSCs within the marrow space have dual origins in the fetal perichondrium and the cartilage template among Dlx5-creER+ cells and Fgfr3-creER+ cells, respectively, while Osx-creER+ cells overlap with these cell types. In contrast, periosteal cells are almost entirely formed from the outer perichondrium, and growth plate chondrocytes are entirely formed from the cartilage template (Fig. 3d).
Dlx5+ ePCs regulate marrow formation via Hedgehog pathway
We next investigated molecular mechanisms that coordinate the differentiation of Dlx5-creER+ perichondrial cells and Fgfr3-creER+ fetal chondrocytes to achieve the formation of the bone marrow space. To this end, we performed additional computational analyses by CellChat30 on our scRNA-seq dataset (Figs. 1 and 4 red dotted area) to unravel intercellular interactions. This analysis can identify the signaling pathways regulating the two cells-of-origins present in the fetal cartilage, namely, Fgfr3+ Cluster 1 and Dlx5+ Cluster 5.
a–f CellChat intercellular communication analysis of the E13.5 Col2a1-cre-marked chondrocyte and perichondrial cell scRNA-seq dataset. a Outgoing and incoming interaction strength of signaling pathways. Each circle represents the number of interactions a cluster participates in. (Left): Cluster 1 has a low tendency to send signals but a high tendency to receive signals in overall pathways. (Right): Cluster 1 exhibits a high propensity to both send and receive signals via the Hh pathways. Dotted box: UMAP-based plot and cluster labels of the E13.5 scRNA-seq data. b Feature plots of Ihh and Ptch1. Violet: high expression, yellow: low expression. c Violin plots displaying the expression distribution of Ihh and Ptch1 in each cluster. d Chord diagram shows the signals linking Cluster 1 and other clusters via the Hh pathway. e Heatmap represents the relative importance of each cluster. Dark green: higher importance, white: lower importance. Cluster 1 is scored as the dominant sender, receiver, and influencer of the Ihh-Ptch1 signaling pathway. Cluster 5 is measured as a dominant receiver. f Hierarchical plot showing the intercellular interactions of the chondrocyte-perichondrial cell lineage via the Ihh-Ptch1 signaling pathway. g Functional analysis of Hedgehog signaling in Dlx5-creER+ early perichondrial cells and Fgfr3-creER+ fetal chondrocytes in bone marrow development. Left panels: Histological images of Dlx5-creER; Ptch1fl/+; R26RtdTomato (Dlx5-Control), Dlx5-creER; Ptch1fl/fl; R26RtdTomato (Dlx5-Ptch1 cKO), Fgfr3-creER; Ptch1fl/+; R26RtdTomato (Fgfr3-Control), Fgfr3-creER; Ptch1fl/fl; R26RtdTomato (Fgfr3-Ptch1 cKO), femur at E18.5 (pulsed at E12.5). Scale bar: 500 μm. n = 8 (Dlx5-Control), n = 11 (Dlx5-Ptch1 cKO), n = 10 (Fgfr3-Control), n = 8 (Dlx5-Ptch1 cKO) mice per each group. Right panels: quantification of whole femur length (top left), bone marrow length per total bone length (middle left), distal cartilage length per total bone length (bottom left), Col1a1-GFP+ trabecular area per total bone marrow area (top right), number of chondrocytes per column (middle right) and total number of columns in growth plate (bottom right). n = 8 (Dlx5-Control), n = 11 (Dlx5-Ptch1 cKO), n = 10 (Fgfr3-Control), n = 8 (Dlx5-Ptch1 cKO) mice per group. Two-tailed, Mann–Whitney’s U test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. Source data are provided as a Source Data file.
The overall level of signaling among clusters varied by cell type, with a variety of strengths of outgoing and incoming interactions (Fig. 4a, left panel). Hedgehog (Hh) Pathways showed strong outgoing and incoming interaction strengths in Fgfr3+ Cluster 1, and incoming interaction strength in Dlx5+ Cluster 5 (Fig. 4a, right panel), indicating potential functional significance of Hh pathways in regulating the two cells-of-origins. This could be explained by the fact that Ihh was most strongly expressed in Cluster 1, whereas its cognate receptor Ptch1 was expressed both in Cluster 1 and 5 (Fig. 4b, c). The CellChat analysis further revealed that Cluster 1 was classified as the most important sender, receiver and influencer of Hh signaling, while Cluster 5 was listed as the second most important receiver (Fig. 4d. e). Among genes in the Hh signaling pathway, Indian hedgehog (Ihh) was identified as an autocrine signaling factor, expressed by and targeting Ptch1+ cells in Cluster 1 (Fig. 4f). The Ihh-Ptch1 paracrine signaling originating from Cluster 1 targeted the other clusters (Fig. 4e, f); among these interactions, the paracrine signaling association between Cluster 1 and Cluster 5 was the strongest, wherein Cluster 5 received the strongest signals from Cluster 1 (Fig. 4f). Therefore, Ihh-Ptch1 signaling was predicted as an important pathway mediating intercellular interactions between the fetal perichondrium and cartilage template via paracrine regulations.
We further performed functional analyses of the Hedgehog signaling pathway regulating the differentiation of the two cells-of-origins types i.e. Dlx5+ early perichondrial cells and Fgfr3+ fetal chondrocytes. To this end, we conditionally deleted Ptch1 in these cells using Ptch1-floxed alleles. Dlx5-creER; Ptch1fl/+; R26RtdTomato (Dlx5-Control) and Dlx5-creER; Ptch1fl/fl; R26RtdTomato (Dlx5-Ptch1 cKO) mice, as well as Fgfr3-creER; Ptch1fl/+; R26RtdTomato (Fgfr3-Control) and Fgfr3-creER; Ptch1fl/fl; R26RtdTomato (Fgfr3-Ptch1 cKO) mice were pulsed at E12.5, and analyzed at E18.5. Deletion of Ptch1 leads to constitutive activation of Hedgehog signaling in these cells31,32,33.
Interestingly, the whole femur length was not altered in the both mutant (Dlx5-Ptch1 cKO and Fgfr3-Ptch1 cKO) mice. However, the bone marrow length relative to the total length was significantly reduced in both Dlx5-Ptch1 cKO and Fgfr3-Ptch1 cKO mice compared to Control (Fig. 4g). This reduction was more pronounced in Fgfr3-Ptch1 cKO mice, associated with an increase of the distal cartilage length relative to the total length, suggesting a delay in the cartilage-to-marrow stroma transition. In contrast in Dlx5-Ptch1 cKO mice, only the bone marrow length relative to the total length was reduced without increasing the cartilage length (Fig. 4g, second column from the right), supporting the theory that Dlx5+ perichondrial cells regulate the marrow formation without involving chondrocytes within cartilage templates. Further, the Col1a1(2.3 kb)-GFP+ trabecular area per the marrow area was significantly reduced in Fgfr3-Ptch1 cKO, associated with an increase in the number of chondrocytes in each column while the total number of chondrocyte columns was unchanged (Fig. 4g, first column from the right), further suggesting a delay in the chondrocyte-to-osteoblast transition.
Therefore, Dlx5+ early perichondrial cells directly regulate the formation of the marrow space, whereas Fgfr3+ fetal chondrocytes regulate bone formation within the marrow space through chondrocyte-to-osteoblast transition, at least in part mediated through Hedgehog signaling.
Dlx5+ ePCs contribute to postnatal diaphyseal marrow stroma
We further set out to define how Dlx5-creER+ early perichondrial cells contribute postnatally to the bone and marrow stromal compartment. For this purpose, we analyzed the cell fates of Dlx5CE-E12.5, OsxCE-E12.5 and Fgfr3CE-E12.5 cells after 4 weeks of chase at postnatal day (P) 21.
As expected, Dlx5CE-E12.5 cells substantially contributed to the postnatal cortical bone compartment. These cells became undifferentiated periosteal cells and Col1a1(2.3 kb)-GFP+ cortical bone osteoblasts (Fig. 5a, b and Supplementary Fig. 3a). However, in bone marrow, Dlx5CE-E12.5 cells contributed only to the diaphyseal marrow stromal compartment. These cells became Col1a1(2.3 kb)-GFP+ trabecular bone osteoblasts and Cxcl12-GFPhigh reticular stromal cells in the diaphysis, but did not generate the same cell types in the metaphysis (Fig. 5a, b and Supplementary Fig. 3a). In contrast, OsxCE-E12.5 cell-derived osteoblasts and marrow stromal cells largely disappeared by this stage due to their transient nature6,22. Fgfr3CE-E12.5 cells became cortical and trabecular osteoblasts as well as Cxcl12-GFPhigh stromal cells predominantly in the metaphyseal marrow space, seemingly in a mutually exclusive manner to Dlx5CE-E12.5 cell derivatives (Fig. 5a, b and Supplementary Fig. 3a). Interestingly, both Dlx5CE-E12.5 cells and Fgfr3CE-E12.5 cells had disappeared from the perichondrium (groove of Ranvier) by this stage, and appeared to make only minimal contribution to the endosteum.
a–d Contribution of fetal (E12.5) Dlx5-creER+, Osx-creER+ or Fgfr3-creER+ cells to postnatal (P21) skeletal compartments. Cxcl12GFP/+; Dlx5-creER; R26RtdTomato ((a): left, (b): upper left), Cxcl12GFP/+; Osx-creER; R26RtdTomato ((a): center, (b): upper center), Cxcl12GFP/+; Fgfr3-creER; R26RtdTomato ((a): right, (b): upper right), Col1a1(2.3 kb)-GFP; Dlx5-creER; R26RtdTomato ((b): middle left, lower left), Osteocalcin (Ocn) -GFP; Osx-creER; R26RtdTomato ((b): middle center, lower center) or Col1a1(2.3 kb)-GFP; Fgfr3-creER; R26RtdTomato femurs ((b):middle right, lower right). n = 4 mice per group. (a): Whole femurs with growth plates on top. Scale bar: 500 µm. n = 4 mice per group. (b): (Upper panels): metaphyseal bone marrow (top), diaphyseal bone marrow (bottom). Scale bar: 20 µm. (Middle panels): metaphyseal cortical bone (left), diaphyseal cortical bone (right). Scale bar: 20 µm. Lower panels: groove of Ranvier. Scale bar: 50 µm. (c): Quantification of tdTomato+ bone marrow stromal cells (left) and osteoblasts/cytes (right), based on distance from growth plate. Red: Dlx5-creER+, Green: Osx-creER+, Blue: Fgfr3-creER+ cell-derivatives. n = 4 mice per each group. (d): Flow cytometry analysis of CD45/Ter119/ CD31neg cells at P21. Bone marrow cells isolated from Cxcl12GFP/+; Dlx5-creER; R26RtdTomato femurs (pulsed at E12.5), Cxcl12GFP/+; Osx-creER; R26RtdTomato femurs (pulsed at E12.5) and Cxcl12GFP/+; Fgfr3-creER; R26RtdTomato femurs (pulsed at E12.5). n = 5 (Dlx5-E12.5), n = 5 (Osx-E12.5), n = 10 (Fgfr3-E12.5) mice per group. Two-tailed, One-way ANOVA followed by Tukey’s post-hoc test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. e Contribution of fetal (E12.5) Dlx5-creER+, Osx-creER+ or Fgfr3-creER+ cells to adult (3 M) skeletal compartments. Upper panels: metaphysis. Lower panels: diaphysis. Scale bar: 500 µm. n = 4 mice per each group. Source data are provided as a Source Data file.
We further defined how Dlx5CE-E12.5 cells contributed to the developing bone marrow space and cortical bone using quantitative histological approaches. Dlx5CE-E12.5 perichondrial cells predominantly contributed to BMSCs and osteoblasts and osteocytes in the distal area (3–7 mm from the growth plate), whereas Fgfr3CE-E12.5 cells contributed to these cells in the proximal area (0–3 mm from the growth plate, Fig. 5c). OsxCE-E12.5 cells showed a similar pattern to Dlx5CE-E12.5 cells, but contributed to BMSCs and osteoblasts to a much lesser extent.
Additionally, we performed flow cytometry analysis of cells isolated from femurs of triple transgenic mice carrying a Cxcl12-GFP reporter (Fig. 5d and Supplementary Fig. 4) for further quantification. Importantly, Dlx5CE-E12.5 contributed to a substantial fraction of Cxcl12-GFPhigh reticular stromal cells, at a level fewer than Fgfr3CE-E12.5 cells (Dlx5CE-E12.5: 19.5 ± 3.1%, Fgfr3CE-E12.5: 26.4 ± 3.7%). The remaining fraction of Cxcl12-GFPhigh stromal cells might be derived from other perichondrial cells or chondrocytes at E12.5 that were not marked either by Dlx5-creER or Fgfr3-creER. In contrast, OsxCE-E12.5 cells made essentially no contribution to Cxcl12-GFPhigh reticular stromal cells at this stage (OsxCE-E12.5: 1.0 ± 0.4%). Similarly, not all the osteocytes were derived from either Dlx5CE-E12.5 or Fgfr3CE-E12.5 cells, suggesting that other perichondrial cells or chondrocytes may contribute to the remaining fraction.
The differential contribution of Dlx5-creER+ perichondrial cells and Fgfr3-creER+ chondrocytes continued further onto the adult stage. At three months of age, Dlx5CE-E12.5 cells were present in the diaphyseal marrow stromal compartment and contributed to Perilipin+ marrow adipocytes, while Fgfr3CE-E12.5 cells were present in the metaphyseal marrow stromal compartment and contributed to alkaline phosphatase (ALP)+ osteoblast-like cells on the bone surface (Supplementary Fig. 3b). Only a small number of OsxCE-E12.5 cells persisted in the diaphyseal marrow space at this stage (Fig. 5e).
These findings demonstrate that Dlx5-creER+ early perichondrial cells make sustained contribution to diaphyseal bone and marrow stromal compartment in a manner distinct from Fgfr3-creER+ fetal chondrocytes (Supplementary Fig. 3c).
We also defined how the two cell types (Dlx5-creER+ perichondrial cells and Fgfr3-creER+ chondrocytes) are related to previously identified chondrogenic cell types marked by Col2a1-creER22,34,35. Analysis of Col2a1-creER; R26RtdTomato mice at E13.5 after 24 h of pulse at E12.5 revealed that Col2a1CE-E12.5 cells were present not only in the cartilage template but also in the perichondrium (Supplementary Fig. 5a). After 2 weeks of chase at P7, Col2a1CE-E12.5 cells contributed to essentially all perichondrial cells and growth plate chondrocytes, as well as to cells of the metaphyseal periosteum and cortical and marrow stromal components; this contribution was far broader than that of Fgfr3CE-E12.5 or Dlx5CE-E12.5 cells (Supplementary Fig. 5b). The same trend continued after 1 month of chase at P21 (Supplementary Fig. 5c). Therefore, Col2a1-creER marks both early perichondrial cells and chondrocytes at the fetal cartilage, demonstrating the utility of Dlx5-creER and Fgfr3-creER to deconvolute the contribution of the chondrocyte-perichondrial cell lineage.
Adipocyte-biased state of Dlx5+ ePC-derived BMSCs
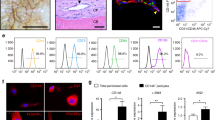
Our findings that Dlx5-creER+ early perichondrial cells and Fgfr3-creER+ chondrocytes contributed to the different bone marrow stromal compartments (diaphyseal and metaphyseal, respectively) prompted us to investigate molecular differences of the two types of (perichondrium-derived and cartilage-derived) BMSCs. To reveal the unique molecular signature of Dlx5-creER+ cell-derived BMSCs, we performed a comparative bulk RNA-seq analysis of Dlx5CE-E12.5 and Fgfr3CE-E12.5 Cxcl12-GFPhigh cells at P21. Cxcl12-GFPhightdTomato+ cells were isolated by FACS from Cxcl12GFP/+; Dlx5-creER; R26RtdTomato and Cxcl12GFP/+; Fgfr3-creER; R26RtdTomato bones at P21 (pulsed at E12.5; Fig. 6a).
a Diagram of comparative bulk RNA-seq analysis of Cxcl12-GFPhigh stromal cells at P21 that are derived from fetal Dlx5+ perichondrial cells and Fgfr3 + chondrocytes. GFPhightdTomato+ (Dlx5CE-E12.5 and Fgfr3CE-E12.5) cells were isolated from P21 Cxcl12GFP/+; Dlx5-creER; R26RtdTomato and Cxcl12GFP/+; Fgfr3-creER; R26RtdTomato femurs (pulsed at E12.5) by FACS. n = 3 (Dlx5CE-E12.5: pooled from n = 4 mice), n = 2 (Fgfr3CE-E12.5: pooled from n = 4 mice) biologically independent samples. b Principal component analysis (PCA) plot of five biologically independent samples. Circles: Cxcl12-GFPhighDlx5-E12.5 cells, Triangles: Cxcl12-GFPhighFgfr3-E12.5 cells. x-axis: PC1, 96.4% variance, y-axis: PC2, 2.1% variance. c Volcano plot for differentially expressed genes (DEGs). x-axis: log2fold-change, y-axis: -log10adjusted p-value. Total of 10,524 genes (excluding known pseudogenes). 1379 and 1854 genes upregulated in Dlx5CE-E12.5 (blue) and Fgfr3CE-E12.5 (red) cells, respectively. Two-sided exact test for difference in means of the negative binomial distribution, followed by Benjamini-Hochberg (B-H) method adjusted for multiple comparisons. d Heatmap of representative DEGs associated with chondrocyte, osteoblast or adipocyte differentiation. Star: statistically significant DEGs between Dlx5CE-E12.5 and Fgfr3CE-E12.5. e Gene Ontology enrichment analysis based on 1379 and 1854 genes upregulated in Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells, respectively. Top 20 GO terms ranked by B-H adjusted p-value. x-axis: number of genes enriched in the corresponding GO term. Color indicates adjusted p-value. One-sided Fisher’s exact test, followed by B-H adjustment for multiple testing. f Colony-forming unit fibroblast (CFU-F) assay of E12.5-pulsed Dlx5-creER; R26RtdTomato (upper left panels) and Fgfr3-creER; R26RtdTomato (lower left panels) bone marrow cells. MB: Methylene blue staining. Scale bar: 5 mm. Right panel: Percentage of tdTomato+ colonies among total CFU-Fs. Dlx5CE-E12.5: n = 4, Fgfr3CE-E12.5: n = 7 mice. Two-tailed, Mann–Whitney’s U-test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. g Dlx5+ fetal outer perichondrial cells contribute to diaphyseal adipogenic marrow stromal cells, while Fgfr3+ fetal chondrocytes contribute to osteogenic metaphyseal stromal cells. Source data are provided as a Source Data file.
Principal component analysis (PCA) revealed that biological replicates of Dlx5CE-E12.5 and Fgfr3CE-E12.5 Cxcl12-GFPhigh cells grouped by lineage (Fig. 6b, Dlx5CE-E12.5: circles, 1–3, Fgfr3CE-E12.5: triangles, 1–2), demonstrating the unique molecular characteristics of these two cell types. Accordingly, a large number of genes were differentially expressed between the two cell types, with ~1400 and ~1800 genes upregulated in Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells, respectively (Fig. 6c). Particularly, genes associated with chondrocytes and osteoblasts were upregulated in Fgfr3CE-E12.5 Cxcl12-GFPhigh cells, including Acan, Col2a1, Fgfr3, Sp7, Col1a1, and Dmp1 (Fig. 6d), indicating that these fetal cartilage-derived BMSCs retain a chondrocyte-osteoblast-biased state. In contrast, adipocyte-related genes markers, including Adipoq, Cxcl12, Kitl, Lepr, Lpl, and Ebf3 were upregulated in Dlx5CE-E12.5 Cxcl12-GFPhigh cells, indicating that fetal perichondrium-derived BMSCs possess an adipocyte-biased state (Fig. 6d).
Gene Ontology (GO) enrichment analysis of differentially expressed genes revealed that significant enrichment of biologically relevant GO terms in Dlx5CE-E12.5 cells, including negative regulation of cell migration (GO:0030336) and fat cell differentiation (GO:0045444) and ossification (GO:0001503) (Fig. 6e), revealing the quiescent adipocyte-biased identity of Dlx5+ perichondrium-derived BMSCs. Conversely, many cell division-related GO terms [mitotic cell cycle phase transition (GO:0044772), mitotic nuclear division (GO:0140014), and ossification (GO:0001503)] were enriched in Fgfr3CE-E12.5 cells (Fig. 6e), revealing the actively cycling nature of Fgfr3+ fetal cartilage-derived BMSCs.
Subsequently, we performed colony forming unit-fibroblast (CFU-F) assays of Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells, by plating bone marrow cells isolated from Dlx5-creER; R26RtdTomato or Fgfr3-creER; R26RtdTomato bone marrow at P21 (pulsed at E12.5) at a clonal density. Dlx5CE-E12.5 cells contributed to only a small fraction of CFU-Fs, whereas Fgfr3CE-E12.5 cells contributed to a large majority of CFU-Fs (Dlx5CE-E12.5: 13.2 ± 3.1%, Fgfr3CE-E12.5: 57.1 ± 11.3%, Fig. 6f). Flow cytometry analysis revealed that Cxc12-GFPhigh cells represented 66.2 ± 12.6% and 17.3 ± 4.1% of Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells, respectively, at P21 (Fig. 5d), which does not appear to correlate with their clonogenic activities. These results are consistent with our recent finding that the adipogenic subset of CXCL12high stromal cells (Cxcl12-creER+ stromal cells36, akin to Adipo-CAR cells37) have little colony-forming activities.
Therefore, Dlx5-creER+ perichondrial cell-derived marrow stromal cells possess an adipocyte-biased state, supporting the theory that Dlx5+ early perichondrial cells may preferentially contribute to a pre-adipocyte-like subset of CXCL12+ BMSCs (Adipo-CAR cells) in developing bone marrow (Fig. 6g).
Anabolic and injury responses of Dlx5+ ePC-derived BMSCs
Lastly, we set out to determine the functional significance of Dlx5-creER+ cell-derived marrow stromal cells in the context of elevated osteogenesis. Because Pth1r (encoding PTH/PTHrP receptor) was upregulated in Fgfr3CE-E12.5 CXCL12+ BMSCs, we first tested the response of these cells to bone anabolic agents i.e. intermittent PTH administration (iPTH) by giving once daily injections of PTH (1–34) (100 µg/kg b.w.). Col1a1(2.3 kb)-GFP; Dlx5-creER or Fgfr3-creER; R26RtdTomato mice (pulsed at E12.5) were treated by iPTH for 3 weeks from P21 to P42 (Fig. 7a).
a–c Differential responses of marrow stromal cells derived from Dlx5+ perichondrial cells and Fgfr3+ chondrocytes to intermittent administration of parathyroid hormone (PTH) (iPTH). (b): Col1a1(2.3 kb)-GFP; Dlx5-creER; R26RtdTomato (Dlx5CE-E12.5, left), Col1a1(2.3 kb)-GFP; Fgfr3-creER; R26RtdTomato (Fgfr3CE-E12.5, center), and Col1a1(2.3 kb)-GFP; R26RtdTomato (Control, right) distal femurs, with iPTH or Vehicle for 3 weeks from P21 to P42. Scale bar: 500 µm. (c): Quantification of Col1a1(2.3 kb)-GFP+ cells (left) and Col1a1(2.3 kb)-GFP+tdTomato+ cells (right) in the metaphysis. n = 6 (Dlx5CE-E12.5), n = 5 (Fgfr3CE-E12.5), n = 4 (Control) mice. Two-tailed, One-way ANOVA followed by Tukey’s post-hoc test (left). Two-tailed, Mann-Whitney’s U-test (right). Data are presented as mean ± s.d. Exact P value is indicated in the figures. d–f Injury-responsive osteogenesis of marrow stromal cells derived from fetal perichondrial cells (Dlx5CE-E12.5) and chondrocytes (Fgfr3CE-E12.5) cells. (e): Reactive osteogenesis induced by bone marrow ablation in Col1a1(2.3 kb)-GFP; Dlx5-creER; R26RtdTomato (left panels) and Fgfr3-creER; R26RtdTomato (right panels) femurs at P28 (pulsed at E12.5). These mice underwent surgery at P21. Metaphyseal (upper) and diaphyseal (lower) marrow space after one week of surgery. Scale bar: 500 µm. (f): Quantification of Col1a1-GFP+tdTomato+ cells in the metaphyseal and diaphyseal bone marrow. Percentage of Col1a1-GFP+tdTomato+ osteoblasts within Col1a1-GFP+ osteoblasts in the metaphysis (left) and diaphysis (right). n = 5 (Dlx5CE-E12.5), n = 4 (Fgfr3CE-E12.5) mice. Two-tailed, Mann-Whitney’s U-test. Data are presented as mean ± s.d. Exact P value is indicated in the figures. Source data are provided as a Source Data file.
As expected, this iPTH anabolic regimen substantially increased the trabecular bone mass particularly in the metaphysis associated with a substantial increase in osteoblasts (Fig. 7b, c, left panel) (Col1-GFP+ cells: 324.8 ± 40.7 and 362.0 ± 65.0 cells/area for Dlx5CE-E12.5 and Fgfr3CE-E12.5 femurs, respectively, compared to 114.8 ± 11.6 cells/area in Vehicle-treated mice). Importantly, Fgfr3CE-E12.5 cells contributed to a great majority of newly formed osteoblasts, while virtually no Dlx5CE-E12.5 cells contributed to these cells in the metaphysis (Fig. 7c, right panel; GFP+tdTomato+ cells; 2.4 ± 0.9% and 55.1 ± 5.0% of total Col1-GFP+ cells for Dlx5CE-E12.5 and Fgfr3CE-E12.5 femurs, respectively), demonstrating that Dlx5-creER+ cell-derived BMSCs barely account for iPTH-induced anabolic responses.
Second, we investigated the capability of Dlx5CE-E12.5 and Fgfr3CE-E12.5 cells for injury-responsive osteogenesis using a femoral bone marrow ablation model, which induced direct differentiation of BMSCs within the marrow cavity (Fig. 7d)38. After 7 days of marrow ablation, Dlx5CE-E12.5 cells contributed to de novo bone formation exclusively in the diaphysis, while Fgfr3CE-E12.5 cells contributed predominantly in the metaphysis (Fig. 7e, f; GFP+tdTomato+ cells; 2.2 ± 1.5% and 76.0 ± 7.9% of total Col1-GFP+ cells in metaphyseal bone marrow, 57.4 ± 6.9% and 9.0 ± 3.0% of total Col1-GFP+ cells in diaphyseal bone marrow for Dlx5CE-E12.5 and Fgfr3CE-E12.5 femurs, respectively).
Therefore, Dlx5-creER+ perichondrial cell-derived BMSCs are refractory to bone anabolism by iPTH, and can contribute locally to reactive osteogenesis in the diaphyseal marrow space. In contrast, Fgfr3-creER+ chondrocyte-derived BMSCs have both active anabolic and regenerative osteogenic responses in the metaphyseal marrow space.
Discussion
Here, we discovered a population of early perichondrial cells in the outer layer of the perichondrium defined by Dlx5 expression. We demonstrate that the Dlx5+ early perichondrial cells provide an important cell-of-origin for the diaphyseal bone marrow stroma of postnatal bones (Fig. 8). These Dlx5+ early perichondrial cells represent the second wave of cells populating the developing marrow space, which enter the marrow space following the initial wave of fetal chondrocytes that transform into marrow stroma-constituting cells. These perichondrium-derived cells subsequently occupy the diaphyseal bone marrow as stromal cells with an adipocyte-biased state, some of which also become trabecular bone osteoblasts. Our findings are based on the two cell type-specific inducible genetic tools that we identified in this study – Dlx5-creER for early perichondrial cells and Fgfr3-creER for fetal chondrocytes – which enable the deconvolution of the fetal chondrocyte-perichondrial cell lineage in bone marrow formation.
Dlx5+ fetal outer perichondrial cells translocate into the nascent marrow space and directly differentiate into marrow stromal cells by bypassing a Sox9+ state (chondrocyte-independent pathway). These early perichondrial skeletal cells continue to provide osteoblasts and bone marrow stromal cells well into the postnatal stage, unlike Osx+ perichondrial cells that have a limited life span. As a result, postnatal bone narrow is characterized by transitional mosaicism composed of both chondrocyte-derived and perichondrium-derived stromal cells.
Interestingly, Dlx5+ perichondrial cell-derived marrow stromal cells are characterized by pre-adipocyte-like characteristics and do not respond to bone anabolism by intermittent administration of parathyroid hormone (PTH). The emerging concept is that a subset of bone marrow stromal cells termed CXCL12-abundant reticular stromal cells (CAR cells) are composed of two distinct cellular subsets of pre-osteoblast-like (Osteo-CAR) cells and pre-adipocyte-like (Adipo-CAR) cells37. Our recent study demonstrates that Osteo-CAR and Adipo-CAR cells are likely to represent independent cell lineages that do not contribute one another39. Our findings provide preliminary evidence that quiescent pre-adipocyte-like marrow stromal (Adipo-CAR) cells in the diaphyseal marrow space might preferentially originate from the fetal perichondrium, while more active pre-osteoblast-like marrow stromal (Osteo-CAR) cells in the metaphyseal marrow space might predominantly originate from the fetal cartilage during bone marrow development.
One of the central questions regarding the two CAR cell types is whether these cells represent the recent bifurcation of a single progenitor present in adults that gives rise to both cell types or whether these are two wholly distinct cell lineages with distinct origins that happen to share some markers in common. The data presented here argues for the latter possibility, although further studies are needed to delineate such differential lineage contribution. Our findings support the theory that the adult bone marrow stromal compartments are developmentally prescribed within the two cells-of-origins of the fetal cartilage.
Recent investigation demonstrates that leptin receptor (LepR)-expressing marrow stromal cells are composed of two distinct peri-arteriolar and peri-sinusoidal subsets with different functionality. The peri-arteriolar LepR+ cells express osteolectin (encoded by Clec11a), which are rapidly-dividing and short-lived and poised to osteogenesis in a mechanosensitive manner40. This osteolection+ subset of LepR+ cells may coincide with Osteo-CAR cells that are localized to peri-arteriolar surfaces37. Importantly, these LepR+osteolectin+ cells are not fated to adipocytes40 and Osteo-CAR cells are not computationally predicted to give rise to Adipo-CAR cells37. Therefore, these studies also indicate that bone marrow stromal cells poised for osteogenesis and adipogenesis represent two distinct cell lineages. It remains to be determined whether Fgfr3+ fetal chondrocyte-derived CAR cells are similarly rapidly-dividing, short-lived and preferentially localized to peri-arteriolar surfaces.
Of note, Fgfr3+ fetal chondrocytes of the cartilage template appear to generate osteoblasts and stromal cells much more efficiently than PTHrP+ resting chondrocytes of the postnatal growth plate41. We postulate that substantial biological differences lie between stem cells in the cartilage template and those in the postnatal growth plate, which make their eventual cell fates fundamentally different. The mechanism conferring Fgfr3+ fetal chondrocytes the far superior capability to transform into osteoprogenitor cells is unclear at this stage, representing an important agenda for future investigation.
Dlx5+ perichondrial cells are distinct from previously described Osx-expressing osteogenic perichondrial cells that are located in the inner layer of the fetal perichondrium4. Dlx5+ cells represent an earlier cell population of Osx+ cells in the fetal perichondrium, as Dlx5+ cell derivatives sustainably contribute to cortical bone and marrow stromal compartments in postnatal bones, unlike Osx+ cell derivatives that contribute only transiently to these fractions. A potential limitation of our tamoxifen-inducible creER-based lineage-tracing approaches is that additional new recombination may occur for several days after tamoxifen injection, while cells are actively translocating to the nascent marrow space. It is therefore technically challenging to formally distinguish the cell fates of a cell population and its immediate descendants in a fast-evolving process of early bone development. Despite these limitations, we believe that our findings indicate that there may be a long-living cell population within the fetal perichondrium.
Interestingly, the contribution of Dlx5+ cell derivatives to cortical bone and marrow stromal compartments of the metaphyseal marrow space appears to be exhausted at later postnatal stages. We assume that this is due to the cell fate of Dlx5+ perichondrial cells that are primarily destined to become long-lived adipocyte-biased marrow stromal cells, which do not readily convert into short-lived osteoblast-biased marrow stromal cells. We therefore postulate that the Dlx5+ cell-derived osteoprogenitor pool in the marrow space is gradually replaced by those derived from other cellular sources such as Fgfr3+ cells in the metaphyseal marrow space in postnatal stages. Additionally, Osx is also expressed by an osteoblast-biased subset of CAR cells within the postnatal marrow space; however, these Osx+CXCL12+ marrow stromal cells are also transient and gradually disappear from this fraction22, presumably replaced by other cellular sources such as Fgfr3+ cells.
Whether these Dlx5+ early perichondrial cells possess characteristics as skeletal stem cells, such as those identified in the adult periosteum42, needs to be determined by further studies. The partnership between the perichondrium and the cartilage template may be one of the major driving forces for mammalian endochondral bone development.
Methods
Mouse strains
Osx-creER4 and Cxcl12GFP/+ 43 mice have been described previously. Col2a1-cre (JAX003554), Col2a1-creER (JAX006774), Fgfr3-creER (JAX025809), Dlx5-creER (JAX010705), Rosa26-CAG-loxP-stop-loxP-tdTomato (Ai14: R26R-tdTomato, JAX007914), Col1a1(2.3 kb)-GFP (JAX013134), Osteocalcin-GFP (JAX017469), Osterix-mCherry (JAX024850), Ptch1-floxed (JAX030494), FVB/NJ (JAX001800), and C57BL/6 J (JAX000664) mice were acquired from the Jackson laboratory. Fgfr3-GFP (MMRRC:031901-UCD) mice were acquired from the Mutant Mouse Resource and Research Centers. All procedures were conducted in compliance with the Guidelines for the Care and Use of Laboratory Animals approved by the University of Texas Health Science Center at Houston’s Animal Welfare Committee (AWC), protocol AWC-21-0070, and the University of Michigan’s Institutional Animal Care and Use Committee (IACUC), protocol 9496. All mice were housed in a specific pathogen-free condition, and analyzed in a mixed background. Mice were housed in static microisolator cages (Allentown Caging, Allentown, NJ). Access to water and food (irradiated LabDiet 5008, Richmond, IN) was ad libitum. Animal rooms were climate controlled to provide temperatures of 22–23 °C, 40–65% of humidity on a 12 h light/dark cycle (lights on at 0600). For all breeding experiments, creER transgenes were maintained in male breeders to avoid spontaneous germline recombination. Mice were identified by micro-tattooing or ear tags. Tail biopsies of mice were lysed by a HotShot protocol (incubating the tail sample at 95 °C for 30 min in an alkaline lysis reagent followed by neutralization) and used for PCR-based genotyping (GoTaq Green Master Mix, Promega, and Nexus X2, Eppendorf). Perinatal mice were also genotyped fluorescently (BLS miner’s lamp) whenever possible. Mice were euthanized by over-dosage of carbon dioxide or decapitation under inhalation anesthesia in a drop jar (Fluriso, Isoflurane USP, VetOne).
Tamoxifen and induction of cre-loxP recombination
Tamoxifen (Sigma T5648) was mixed with 100% ethanol until completely dissolved. Subsequently, a proper volume of sunflower seed oil (Sigma S5007) was added to the tamoxifen-ethanol mixture and rigorously mixed. The tamoxifen-ethanol-oil mixture was incubated at 60 °C in a chemical hood until the ethanol evaporated completely. The tamoxifen-oil mixture was stored at room temperature until use. Tamoxifen was injected at a dose of 3 mg to pregnant mice intraperitoneally using a 26-1/2-gauge needle (BD309597).
Intermittent administration of parathyroid hormone
Mice were given once daily subcutaneous injections of PTH (1–34) (100 µg/kg b.w., 4095855, Bachem) for 3 weeks from P21 to P42.
Bone marrow ablation surgery
Mice were anesthetized through a nose cone, in which 1.5–2% isoflurane was constantly provided with oxygen through a vaporizer. For bone marrow ablation surgery, right femurs were operated, while left femurs were untreated and used as an internal control. After skin incision, cruciate ligaments of the knee were carefully separated using a dental excavator, and a hole was made in the intercondylar region of femurs using a 26-1/2-gauge needle (BD309597). The endodontic instruments (K-file, #35, 40, 45, 50; GC, Tokyo, Japan) were used in a stepwise manner to remove a cylindrical area of the marrow space. The surgical field was irrigated with saline, and the incision line was sutured.
Histology and immunohistochemistry
Samples were dissected under a stereomicroscope (Nikon SMZ-800), and fixed in 4% paraformaldehyde for a proper period, typically ranging from 3 h to overnight at 4 °C, then decalcified in 15% EDTA for a proper period, typically ranging from 3 h to 14 days. Embryonic samples were not decalcified. Subsequently, samples were cryoprotected in 30% sucrose/PBS solutions and then in 30% sucrose/PBS:OCT (1:1) solutions, each at least overnight at 4 °C. Samples were embedded in an OCT compound (Tissue-Tek, Sakura) under a stereomicroscope and transferred on a sheet of dry ice to solidify the compound. Embedded samples were cryosectioned at 14 µm using a cryostat (Leica CM1850) and adhered to positively charged glass slides (Fisherbrand ColorFrost Plus). Sections were postfixed in 4% paraformaldehyde for 15 min at room temperature. For immunostaining, sections were permeabilized with 0.25% TritonX/TBS for 30 min, blocked with 3% BSA/TBST for 30 min and incubated with rabbit anti-SOX9 polyclonal antibody (1:500, EMD-Millipore, AB5535), rat anti-endomucin (EMCN) monoclonal antibody (1:100, Santa Cruz Biotechnology, sc65495), rabbit anti-MYH3 polyclonal antibody (1:500, Abcam, ab124205), rabbit anti-perilipin A/B polyclonal antibody (1:500, Sigma, P1873) or goat anti-ALPL polyclonal antibody (1:100, R&D, AF2910) overnight at 4 °C, and subsequently with Alexa Fluor 647-conjugated donkey anti-rabbit IgG (A31573) or Alexa Fluor 633-conjugated goat anti-rat IgG (A21049) (1:400, Invitrogen) for 3 hours at room temperature. Sections were further incubated with DAPI (4’,6-diamidino-2-phenylindole, 5 µg/ml, Invitrogen D1306) to stain nuclei prior to imaging.
RNAscope in situ hybridization
Samples were fixed in 4% paraformaldehyde overnight at 4 °C, and then cryoprotected. Frozen sections at 14 µm were prepared on positively charged glass slides. In situ hybridization was performed with RNAscope Multiplex Fluorescent Detection Kit v2 (Advanced Cell Diagnostics 323100) using Dlx5 (478151) and Col2a1 (407221) probes according to the manufacturer’s protocol.
Imaging and cell quantification
Images were captured by an automated inverted fluorescence microscope with a structured illumination system (Zeiss Axio Observer Z1 with ApoTome.2 system) and Zen 2 (blue edition) software. The filter settings used were: FL Filter Set 34 (Ex. 390/22, Em. 460/50 nm), Set 38 HE (Ex. 470/40, Em. 525/50 nm), Set 43 HE (Ex. 550/25, Em. 605/70 nm), Set 50 (Ex. 640/30, Em. 690/50 nm) and Set 63 HE (Ex. 572/25, Em. 629/62 nm). The objectives used were: Fluar 2.5x/0.12, EC Plan-Neofluar 5x/0.16, Plan-Apochromat 10x/0.45, EC Plan-Neofluar 20x/0.50, EC Plan-Neofluar 40x/0.75, Plan-Apochromat 63x/1.40. Images were typically tile-scanned with a motorized stage, Z-stacked and reconstructed by a maximum intensity projection (MIP) function. Differential interference contrast (DIC) was used for objectives higher than 10x. Representative images of at least three independent biological samples are shown in the figures. Quantification of cells on sections was performed using NIH Image J software.
Cell preparation
For embryonic samples, hind limbs were harvested and incubated with 2 Wunsch units of Liberase TM (Sigma/Roche 5401127001) in 2 ml Ca2+, Mg2+-free Hank’s Balanced Salt Solution (HBSS, Sigma H6648) at 37 °C for 15 min on a shaking incubator (ThermomixerR, Eppendorf). For postnatal samples, soft tissues and epiphyses were carefully removed from dissected femurs. After removing distal epiphyseal growth plates and cutting off proximal ends, femurs were cut roughly and incubated with 2 Wunsch units of Liberase TM and 1 mg of Pronase (Sigma/Roche 10165921001) in 2 ml Ca2+, Mg2+-free HBSS at 37 °C for 60 min on a shaking incubator. After cell dissociation, cells were mechanically triturated using an 18-gauge needle with a 1 ml Luer-Lok syringe (BD) and a pestle with a mortar (Coors Tek), and subsequently filtered through a 70 µm cell strainer (BD) into a 50 ml tube on ice to prepare single-cell suspension. These steps were repeated for 5 times, and dissociated cells were collected in the same tube. Cells were pelleted and resuspended in an appropriate medium for subsequent purposes. For cell culture experiments, cells were resuspended in 10 ml culture medium and counted on a hemocytometer.
Flow cytometry
Dissociated cells were stained by standard protocols with the following antibodies (1:500, eBioscience). eFluor450-conjugated CD31 (390, endothelial/platelet), CD45 (30F-11, hematopoietic), Ter119 (TER-119, erythrocytes), allophycocyanin (APC)-conjugated CD31 (390, endothelial/platelet), CD45 (30F-11, hematopoietic), and Ter119 (TER-119, erythrocytes). Flow cytometry analysis was performed using a four-laser BD LSR Fortessa (Ex. 405/488/561/640 nm) and FACSDiva software. Acquired raw data were further analyzed on FlowJo software (TreeStar). Representative plots of at least four independent biological samples are shown in the figures.
Single-cell RNA-seq analysis of fluorescence-activated cell sorting-isolated cells
Cell sorting was performed using a four-laser BD FACS Aria III (Ex.407/488/561/640 nm) high-speed cell sorter with a 100 µm nozzle. tdTomato+ cells were directly sorted into ice-cold DPBS/1% BSA, pelleted by centrifugation and resuspended in appropriate amount of DPBS/1% BSA (1000 cells/μl). Cell numbers were quantified by Countess II automated Cell Counter (ThermoFisher) before loading onto the Chromium Single Cell 3′ v2 microfluidics chip (10x Genomics Inc., Pleasanton, CA). cDNA libraries were sequenced by Illumina HiSeq 4000 using two lanes and 50 base single-end read, generating a total of ~ 770 million reads, or by NovaSeq 6000 using 150 base pair-end read. The sequencing data was first pre-processed using the 10X Genomics software Cell Ranger. For alignment purposes, we generated and used a custom genome fasta and index file by including the sequences of tdTomato-WPRE to the mouse genome (mm10).
Quality Control and Exploration of scRNA Sequencing Data (Seurat)
We carried out quality control (QC) and cell identification steps, by utilizing Seurat 3.044. We created a Seurat object from the raw gene count matrix, by using the Read10X() and CreateSeuratObject() functions. Only features detected in at least 10 cells were retained and only cells with at least 500 features were kept for subsequent analysis. In the QC steps, we filtered out low-quality cells. We excluded cells that had: (1) <2000 or >9000 unique feature counts, or (2) >15% mitochondrial content.
To project high-dimensional data into low-dimensional space for visualizing the cluster structure, we applied the functions of RunUMAP() with dims = 1:30, FindNeighbors() with dims = 1:30, and FindClusters() with resolution = 0.5. We removed a total of two clusters, consisting of cells showing little to no tdTomato expression, using subset(). Of those, 7889 cells met the QC criteria and were used for downstream analysis.
We explored differentially expressed genes (DEGs) across cell clusters by using FindMarkers(). With this approach, we ranked the DEGs with p-values from smallest to largest (statistical significance defined by adjusted p-values <0.05). We used canonical gene markers and the top 100 DEGs of the 10 clusters to assign their cell identities. The UMAP-based cluster visualization and the marker gene expression plots are shown in Fig. 1d, e, respectively. For further analyses of intercellular communication and RNA velocity, we exported the UMAP coordinates of 7889 cells and the clustering results of 10 cell populations (refer to CellChat and scVelo sections).
Quantitative analysis of cell-cell communication networks of scRNA data (CellChat)
To study how intercellular signaling networks among various cell types shape their differentiation response, we employed CellChat30. First, we extracted the normalized count data matrix and cell cluster information from the Seurat object, by applying the function GetAssayData() with the settings of assay = “RNA” and slot = “data”. Subsequently, we generated a CellChat object with createCellChat() and added the corresponding cell identities with setIdent(). We applied subsetData() to subset the expression data of signaling genes, as recommended by the CellChat protocol.
We used CellChatDB.mouse, a database of 2021 validated molecular interactions containing the ligands, receptors, and their cofactors, as the basis for the ligand-receptor analysis. We subsequently applied identifyOverExpressedGenes() and identifyOverExpressedInteractions() to discover the over-expressed genes as well as the over-expressed ligand-receptor pairs (statistical significance level defined by the Bonferroni corrected p-value < 0.05). We utilized projectData() to project the gene expression values of ligand-receptor pairs onto protein-protein interaction (PPI) network.
To infer the communication probability of each signaling pathway, we applied computeCommunProb(), followed by computeCommunProbPathway() and aggregateNet() for computing and aggregating communication networks between cells. We used netAnalysis_computeCentrality() to compute intercellular communication networks of signaling pathways, thus inferring the role(s) of the 10 cell clusters as the sender, receiver, mediator, and/or influencer.
RNA velocity analysis (velocyto and scVelo)
We used the sample-specific aligned bam file from Col2a1cre-E13.5 mouse (GEO Accession Number: GSM3619209), as the input for velocyto to quantify the unspliced and spliced abundances, generating a loom file26. To calculate the RNA velocities of the single cells, we utilized scVelo (version 0.2.2)45. We normalized and log-transformed gene expression using the function scvelo.pp.filter_and_normalize(),with settings of min_shared_counts = 20, n_top_genes = 5500, flavor = “seurat”. We computed the first and second order moments for each cell across its nearest neighbors by using scvelo.pp.moments() with settings of method = ‘umap’, n_neighbors = 30, n_pcs = 30, knn = True. We estimated the velocities by running the likelihood-based dynamical model with scvelo.tl.velocity(mode = “dynamical”), and constructed the velocity graph with scvelo.tl.velocity_graph().
To test for the presence of differential kinetics across cell types that could not be well explained by a single model of the overall dynamics, we applied scvelo.tl.differential_kinetic_test(). We then recomputed the RNA velocities, by using scvelo.pp.neighbor() with similar settings mentioned before, scvelo.tl.velocity(diff_kinetics = True), and scvelo.tl.velocity_graph(). To visualize the RNA velocities, we projected velocities onto the UMAP coordinates, using the function scvelo.tl.velocity_embedding_stream() with the setting of basis = ‘umap’. The UMAP plot is shown in Fig. 1f.
Single-cell fate mapping (CellRank)
To compute a global map of cellular fate potential, we performed cell fate mapping inference by carrying out the protocol from CellRank46. We used cr.tl.initial_states() with default settings. We determined the initial cell population to be cluster 5, based on the generalized Perron Cluster Cluster Analysis (G-PCCA) method, implemented in CellRank46,47. A representative graph of global probabilistic fates at the single-cell level and the inferred root cell population, is presented in Fig. 1g.
Bulk RNA-seq analysis of FACS-isolated cells
Dissociated bone marrow cells harvested from P21 littermate mice were pooled based on the genotype [Cxcl12GFP/+; Dlx5-creER; R26RtdTomato (pulsed at E12.5, Dlx5-E12.5 mice) or Cxcl12GFP/+; Fgfr3-creER; R26RtdTomato (pulsed at E12.5, Fgfr3-E12.5 mice)]. Cell sorting was performed using a four-laser BD FACS Aria III (Ex.407/488/561/640 nm) high-speed cell sorter with a 100 µm nozzle. Cxcl12-GFPhightdTomato+ cells isolated from Dlx5-E12.5 or Fgfr3-E12.5 mice were directly sorted into ice-cold DPBS/10% FBS and pelleted by centrifugation. Total RNA was isolated using PicoPure RNA Isolation Kit (KIT0204, ThermoFisher), followed by DNA-free DNA removal kit (AM1906, ThermoFisher) to remove contaminating genomic DNA. RNA Integrity Number (RIN) was assessed by Agilent 2100 Bioanalyzer RNA 6000 Pico Kit. Samples with RIN > 8.0 were used for subsequent analyses. Complementary DNAs were prepared by SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing (Clontech 634888). Post-amplification quality control was performed by Agilent TapeStation DNA High Sensitivity D1000 Screen Tape system. DNA libraries were prepared by Nextera XT DNA Library Preparation Kit (Illumina) and submitted for NextGen sequencing (Illumina NovaSeq 6000 150 base for pair-end reads for Dlx5E12.5 samples [n = 3 biological replicates], HiSeq 4000, 50 base for single-end reads for Fgfr3E12.5 samples [n = 2 biological replicates]). Reads files were downloaded and concatenated into a single.fastq file for each sample. The quality of the raw reads data for each sample was checked using FastQC to identify quality problems. Tuxedo Suite software package was subsequently used for alignment (using TopHat and Bowtie2). FastQC was used for a second round of quality control (post-alignment). We employed biomaRt48 to convert Ensembl gene identifiers into MGI (Mouse Genome Informatics) mm10 gene symbols. To omit known pseudogenes, we only included protein coding genes, defined by their gene biotypes, in addition to reporter genes, such as cre, eGFP, and tdTomato. We utilized edgeR49 to perform library size normalization and differential expression analysis. For normalization of sequencing depth of each replicate, read counts were scaled to CPM (counts per million). Only genes having CPM greater or equal to 10 in at least two out of the five samples were included. The resulting 10,542 genes were used for subsequent downstream differential expression analysis. We discovered upregulated genes in Dlx5E12.5 and Fgfr3E12.5 samples, which were defined by having an absolute number of log2FC (log2 Fold Change) greater than 1 and B-H (Benjamini-Hochberg) adjusted p-value <0.05. For an unbiased view of the top differentially expressed genes, we used EnhancedVolcanoPlot50 to construct a volcano plot, as shown in Fig. 6c, highlighting the names of the 100 most differentially expressed genes. The gene ranking was based on descending order by log2FC. Next, considering all upregulated genes in Dlx5E12.5 (n = 1379) and in Fgfr3E12.5 (n = 1854) cells, we conducted GO (Gene Ontology) enrichment analysis using ClusterProfiler4.051. The top 20 GO enriched biological processes for each condition were ranked by B-H adjusted p-value and were illustrated in Fig. 6e.
Colony-forming assay and subcloning
Nucleated bone marrow cells were plated into tissue culture 6 well plates (BD Falcon) at a density of <105 cells/cm2, and cultured in low-glucose DMEM with GlutaMAX supplement (Gibco 10567022) and 10% mesenchymal stem cell-qualified FBS (Gibco 12662029) containing penicillin-streptomycin (Sigma P0781) for 10~14 days. Cell cultures were maintained at 37 °C in a 5% CO2 incubator. Representative images of at least three independent biological samples are shown in the figures. For CFU-Fs, cells were fixed with 70% Ethanol for 5 min and stained for 2% methylene blue.
Statistics and reproducibility
Results are presented as mean values ± S.D. Statistical evaluation was conducted using the Mann-Whitney’s U-test or one-way ANOVA. A P value of <0.05 was considered significant. No statistical method was used to predetermine sample size. Some of the data were excluded from the study because of the pre-established criteria such as problems or failures in identifying correct genotypes or birth dates, and issues unrelated to the intervention of the study such as spontaneous malnutrition. Sample size was determined on the basis of previous literature and our previous experience to give sufficient standard deviations of the mean so as not to miss a biologically important difference between groups. The experiments were not randomized. All of the available mice of the desired genotypes were used for experiments. The investigators were not blinded during experiments and outcome assessment. One femur from each mouse was arbitrarily chosen for histological analysis. Genotypes were not particularly highlighted during quantification.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Source Data file. The raw data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The single cell and bulk RNA-seq data presented herein have been deposited in the National Center for Biotechnology Information (NCBI)’s Gene Expression Omnibus (GEO), and are accessible through GEO Series accession numbers GSE126966, GSE197933. Source data are provided with this paper. Reference genome mm10 (Ensembl release 93) for transcript annotation is publicly available on Ensembl, including a gtf file [http://ftp.ensembl.org/pub/release-93/gtf/mus_musculus/Mus_musculus.GRCm38.93.gtf.gz] and a fasta file [http://ftp.ensembl.org/pub/release-93/fasta/mus_musculus/dna/Mus_musculus.GRCm38.dna_sm.primary_assembly.fa.gz]. The scRNA-seq data presented in this study is accessible from the following link through the cellxgene platform: https://welchlab.dcmb.med.umich.edu/view/Dlx5/col2e.h5ad/. Source data are provided with this paper.
References
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Yang, L., Tsang, K. Y., Tang, H. C., Chan, D. & Cheah, K. S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl Acad. Sci. USA 111, 12097–12102 (2014).
Shi, Y. et al. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 8, 2043 (2017).
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010).
Zhou, X. et al. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10, e1004820 (2014).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Yang, G. et al. Osteogenic fate of hypertrophic chondrocytes. Cell Res 24, 1266–1269 (2014).
Aghajanian, P. & Mohan, S. The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 6, 19 (2018).
Wolff, L. I. & Hartmann, C. A second career for chondrocytes-transformation into osteoblasts. Curr. Osteoporos. Rep. 17, 129–137 (2019).
Kronenberg, H. M. The role of the perichondrium in fetal bone development. Ann. N. Y Acad. Sci. 1116, 59–64 (2007).
Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 (2011).
Long, F. et al. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 131, 1309–1318 (2004).
Kawanami, A., Matsushita, T., Chan, Y. Y. & Murakami, S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem. Biophys. Res. Commun. 386, 477–482 (2009).
Pineault, K. M., Song, J. Y., Kozloff, K. M., Lucas, D. & Wellik, D. M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat. Commun. 10, 3168 (2019).
Song, J. Y., Pineault, K. M., Dones, J. M., Raines, R. T. & Wellik, D. M. Hox genes maintain critical roles in the adult skeleton. Proc. Natl Acad. Sci. USA 117, 7296–7304 (2020).
Kuwahara, S. T. et al. Sox9+ messenger cells orchestrate large-scale skeletal regeneration in the mammalian rib. Elife https://doi.org/10.7554/eLife.40715 (2019).
Usami, Y. et al. Possible contribution of Wnt-responsive chondroprogenitors to the postnatal murine growth plate. J. Bone Miner. Res. 34, 964–974 (2019).
Yang, W. et al. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 499, 491–495 (2013).
Bowen, M. E., Ayturk, U. M., Kurek, K. C., Yang, W. & Warman, M. L. SHP2 regulates chondrocyte terminal differentiation, growth plate architecture and skeletal cell fates. PLoS Genet. 10, e1004364 (2014).
Inubushi, T., Nozawa, S., Matsumoto, K., Irie, F. & Yamaguchi, Y. Aberrant perichondrial BMP signaling mediates multiple osteochondromagenesis in mice. JCI Insight https://doi.org/10.1172/jci.insight.90049 (2017).
Ono, N., Ono, W., Nagasawa, T. & Kronenberg, H. M. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat. Cell Biol. 16, 1157–1167 (2014).
Hinoi, E. et al. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 20, 2937–2942 (2006).
Lee, M. H. et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J. Biol. Chem. 280, 35579–35587 (2005).
Hojo, H., Ohba, S., He, X., Lai, L. P. & McMahon, A. P. Sp7/osterix is restricted to bone-forming vertebrates where it acts as a Dlx co-factor in osteoblast specification. Dev. Cell 37, 238–253 (2016).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Rivers, L. E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Ono, N. et al. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev. Cell 29, 330–339 (2014).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Hahn, H. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Johnson, R. L. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671 (1996).
Lum, L. & Beachy, P. A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004).
Nakamura, E., Nguyen, M. T. & Mackem, S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn. 235, 2603–2612 (2006).
Hilton, M. J., Tu, X., Cook, J., Hu, H. & Long, F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132, 4339–4351 (2005).
Matsushita, Y. et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 11, 332 (2020).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Ono, N. et al. Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. J. Cell Physiol. 227, 408–415 (2012).
Matsushita, Y., Chu, A. K. Y., Ono, W., Welch, J. D. & Ono, N. Intercellular interactions of an adipogenic CXCL12-expressing stromal cell subset in murine bone marrow. J. Bone Miner. Res. 36, 1145–1158 (2021).
Shen, B. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021).
Mizuhashi, K. et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 563, 254–258 (2018).
Debnath, S. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 (2018).
Ara, T. et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 (2003).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Lange, M. et al. CellRank for directed single-cell fate mapping. Nat. Methods 19, 159–170 (2022).
Reuter, B., Weber, M., Fackeldey, K., Röblitz, S. & Garcia, M. E. Generalized markov state modeling method for nonequilibrium biomolecular dynamics: exemplified on amyloid β conformational dynamics driven by an oscillating electric field. J. Chem. Theory Comput. 14, 3579–3594 (2018).
Durinck, S., Spellman, P., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Blighe, K., Rana, S., & Lewis, M. EnhancedVolcano: Publication-ready Volcano Plots With Enhanced Colouring And Labeling. R package version 1.14.0 (2022). Available at: https://github.com/kevinblighe/EnhancedVolcano Accessed July 20, 2022.
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Acknowledgements
We thank H.M. Kronenberg (Massachusetts General Hospital) for Osx-creER mice and T. Nagasawa (Osaka University) for Cxcl12GFP/+ mice. This research was supported by National Institute of Health grants R01DE030630, R01DE026666 (to N.O.), R01DE029181 (to W.O.), R01AR065409, R01AR080654 (to S.W.), R01HG010883 (to J.D.W.) and University of Michigan MCubed 2.0 Grant (to N.O., W.O., and S.W.), MCubed 3.0 Grant (to N.O., W.O., and J.D.W.), JSPS grant JP20KK0356 and JST FOREST Program JPMJFR2111 Japan to Y.M. We thank M. Pihalja and K. Saiya-Cork of the University of Michigan Flow Cytometry Core and T. Lau for supporting this study.
Author information
Authors and Affiliations
Contributions
Y.M. and N.O. conceived the project and wrote the manuscript. Y.M., C.T.A., S.O., M.N., W.O., and N.O. performed the experiment. A.K.Y.C. and J.D.W. performed the bioinformatic analysis. S.Y.W. provided the mice and critiqued the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Geert Carmeliet, Matthew Greenblatt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsushita, Y., Chu, A.K.Y., Tsutsumi-Arai, C. et al. The fate of early perichondrial cells in developing bones. Nat Commun 13, 7319 (2022). https://doi.org/10.1038/s41467-022-34804-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34804-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.