Abstract
Cancers arising from the bladder urothelium often exhibit lineage plasticity with regions of urothelial carcinoma adjacent to or admixed with regions of divergent histomorphology, most commonly squamous differentiation. To define the biologic basis for and clinical significance of this morphologic heterogeneity, here we perform integrated genomic analyses of mixed histology bladder cancers with separable regions of urothelial and squamous differentiation. We find that squamous differentiation is a marker of intratumoral genomic and immunologic heterogeneity in patients with bladder cancer and a biomarker of intrinsic immunotherapy resistance. Phylogenetic analysis confirms that in all cases the urothelial and squamous regions are derived from a common shared precursor. Despite the presence of marked genomic heterogeneity between co-existent urothelial and squamous differentiated regions, no recurrent genomic alteration exclusive to the urothelial or squamous morphologies is identified. Rather, lineage plasticity in bladder cancers with squamous differentiation is associated with loss of expression of FOXA1, GATA3, and PPARG, transcription factors critical for maintenance of urothelial cell identity. Of clinical significance, lineage plasticity and PD-L1 expression is coordinately dysregulated via FOXA1, with patients exhibiting morphologic heterogeneity pre-treatment significantly less likely to respond to immune checkpoint inhibitors.
Similar content being viewed by others
Introduction
For decades, platinum-based combination chemotherapy was the primary systemic treatment for patients with metastatic bladder cancer1,2,3. More recently, immune checkpoint inhibitors were shown to induce profound and durable responses in patients with metastatic bladder cancer who progressed after platinum-based chemotherapy1,3,4,5,6. However, a majority of bladder cancers are intrinsically resistant to immune checkpoint inhibitors and even for those patients that respond, acquired resistance is a major clinical challenge.
Cancers arising from the bladder urothelium display a wide spectrum of histomorphologies, with regions of urothelial carcinoma often adjacent to or admixed with regions of squamous, glandular, sarcomatoid, small cell, or other histologic variants7,8,9,10. These morphologic variants are often underreported and unrecognized9,10. While genomic differences have been reported between morphologically distinct regions of some mixed histology tumors11,12, the molecular basis for morphologic heterogeneity in bladder cancer has not been systematically studied.
Transcriptomic-based molecular classification of urothelial carcinomas has revealed that the basal subtype is enriched for tumors with squamous differentiation, the most common bladder cancer variant13,14. As bladder tumors of the basal subtype are less likely to respond to the anti-PD-L1 antibody atezolizumab4, we hypothesized that bladder cancers with mixed histology and regions of squamous differentiation may be intrinsically resistant to immunotherapy due to the presence of a pre-existent treatment-resistant population of cancer cells. To date, biomarker analyses incorporated into immunotherapy clinical trials have been unable to address this question as only a single tumor region is typically analyzed by bulk sequencing. While such analyses can yield important insights, they cannot account for the confounding effects of intra-tumoral and tumor-to-tumor heterogeneity. To determine whether morphologic heterogeneity is a marker for genomic and immunologic heterogeneity in bladder cancers with mixed histology, we performed an integrated genomic analysis of morphologically and spatially distinct regions of urothelial and squamous morphology from tumors with heterogeneous histopathology.
Here, we show that the urothelial and squamous regions are derived from a common shared precursor but there is no recurrent genomic alteration exclusive to the urothelial or squamous morphologies. We also show that lineage plasticity in urothelial carcinoma with squamous differentiation is associated with loss of expression of transcription factors critical for maintaining urothelial cell identity and luminal phenotype such as FOXA1, GATA3, and PPARG. By functional analysis, we show that lineage plasticity and PD-L1 expression is coordinately dysregulated via FOXA1 repression. Morphologic heterogeneity in the form of squamous differentiation is a marker of intratumoral genomic and immunologic heterogeneity in patients with bladder cancer and a biomarker of intrinsic immunotherapy resistance. Of clinical significance, patients whose tumors exhibit morphologic heterogeneity pre-treatment are significantly less likely to respond to immune checkpoint inhibitors.
Results
Regions of urothelial and squamous differentiation have a shared clonal origin
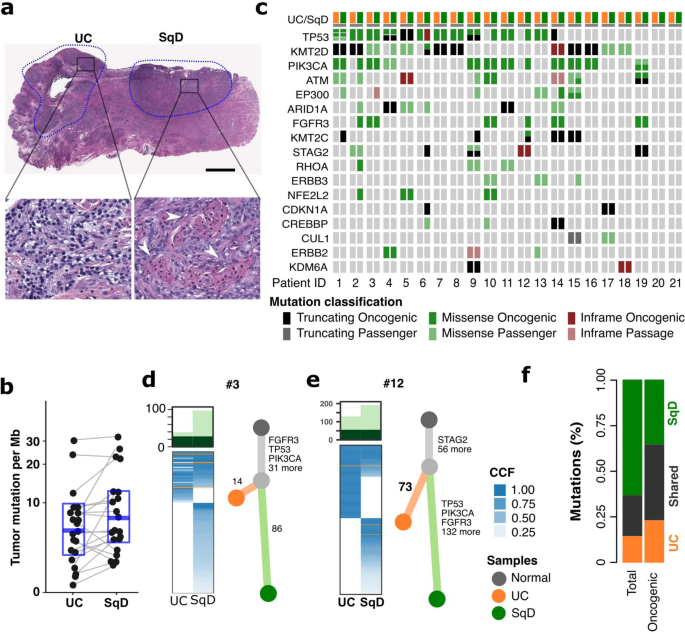
To determine whether there were differences in the genomic landscape of bladder cancers with urothelial and squamous differentiation, we performed a central histologic review of a prospectively sequenced cohort of 848 primary bladder tumors. We found that 69% (587/848) had a pure urothelial carcinoma histology (UC), whereas 82 (10%) had morphologic evidence of squamous differentiation (SqD). Targeted sequencing of 341 or more cancer-associated genes (MSK-IMPACT), revealed higher rates of TP53 and CDKN2A alterations in SqD vs UC but no somatic alteration exclusive to the UC or SqD tumors (Supplementary Fig. 1). We next performed whole exome sequencing (WES) of paired, macrodissected regions of UC and SqD of 21 bladder tumors in which distinct and separable UC and SqD regions were present (total 42 whole exomes) (Fig. 1a). Mean tumor mutational burden (TMB) was higher in the SqD (10.2 mutations/megabase [mut/Mb]) versus the UC regions (7.8 mut/Mb, p = 0.02, Wilcoxon paired test, Fig. 1b). Consistent with the higher TMB, the SqD regions also had a higher neoantigen burden than the patient-matched UC regions (p < 0.001, Wilcoxon paired test, Supplementary Fig. 2a). The SqD regions also exhibited higher karyotypic complexity with a higher median ploidy relative to the paired UC regions (median 2.6 vs 2.3, p = 0.02, Wilcoxon paired test, Supplementary Fig. 2b). Consistent with the genomic landscape of UC as previously defined by the Cancer Genome Atlas (TCGA) and others13,15, known or presumed oncogenic mutations were identified in TP53, chromatin modifying genes (KMT2D, ARID1A, KMT2C, KDM6A), and mitogenic signaling pathways (PIK3CA, FGFR3, ERBB2) in both UC and SqD regions (Fig. 1c).
a Hematoxylin and Eosin (H&E) staining of a representative mixed histology bladder cancer with discrete and separable regions of urothelial carcinoma, NOS (UC), and squamous differentiation (SqD). In comparison to the urothelial component, the squamous regions were characterized by the presence of tumor cells with keratin formation (block arrow), a microscopic feature unequivocal for squamous differentiation. Scale bar = 2 mm. b Whole exome sequencing of macrodissected UC and SqD regions of 21 mixed histology bladder cancers revealed a significantly higher tumor mutational burden in the SqD versus UC regions (two-sided Wilcoxon paired test, p 0.02). c Oncoprint showing the mutation status of genes commonly mutated in bladder cancer. For each patient, the mutation status in the UC component is shown on the left, and the SqD component on the right. d, e Phylogenetic analysis of two representative urothelial carcinomas with squamous differentiation highlighting that the UC and SqD components were derived from a shared precursor. The number of non-synonymous mutations in each region is shown on top with shared mutations represented in dark green and mutations private to each component in light green. The cancer cell fraction (CCF) of individual mutations is indicated by the degree of blue shading. The UC (orange), SqD (green), and their hypothetical normal cell of origin (gray) are indicated by the different colors. f Average of concordant and discordant mutations between paired UC and SqD samples revealed higher concordance of oncogenic and likely oncogenic mutations (63.2%) versus all nonsynonymous mutations (33.9%).
Phylogenetic analysis of the WES data confirmed that the morphologically distinct regions of all 21 tumors arose from a shared precursor with a median of 68 (range 16 – 852) non-synonymous mutations shared between the UC and SqD regions (Fig. 1d, e, Supplementary Fig. 3). Clonality analysis further supported that the two components were clonally related and did not arise independently (p < 0.001, Supplementary Table 2)16,17. To better quantitate the relative burden of private versus shared mutations in the SqD and UC regions, we calculated a “phylogenic ratio” for each pair, defined as the number of private mutations divided by the number of shared mutations. The median phylogenic ratio was 1.1 in the SqD and 0.8 in UC regions, indicating that there was a similar number of private mutations in each of the morphologically distinct regions. However, in six tumors (30%), the phylogenic ratio of the SqD region was over 4-fold higher than the patients’ matched UC sample, suggesting that the SqD component in these cases had evolved significantly further than the UC component from the inferred common shared precursor (Cases 2, 3, 9, 10, 11, and 12). In sum, the data were indicative of early-branched evolution.
While there was significant mutational discordance between the UC and SqD regions of individual tumors, mutational concordance was higher for known or presumed oncogenic mutations (63.2% of oncogenic/likely oncogenic mutations were shared versus 33.9% of all nonsynonymous mutations) with some notable discordant mutations (Fig. 1f). For example, FGFR3 S249C, an oncogenic and therapeutically actionable hotspot mutation, was detected in only the SqD regions of two cases (tumors 2 and 12, Fig. 1d, e and Supplementary Fig. 3). WES did not, however, identify a specific gene or pathway that was significantly more frequently mutated in the SqD or UC regions. Additionally, tumors from UC and SqD regions had a high degree of concordance in their mutational signatures, with an APOBEC mutational signature the most common mutational signature in most of the paired regions18 (Supplementary Fig. 4).
Lineage plasticity in bladder cancers with squamous differentiation
As targeted and whole exome DNA sequencing did not identify a shared mutational alteration or genomic signature unique to the SqD or UC components, we next sought to determine whether dysregulation of gene expression was the basis for the lineage plasticity observed in bladder cancers with mixed UC-SqD histology. We, therefore, performed expression profiling of separable SqD and UC regions of 12 bladder tumors from the WES cohort for which RNA quality was sufficient for whole transcriptome RNA-sequencing (RNAseq, total of 24 regions). Based on centroid clustering analysis of expression data, all 12 SqD regions were basal-squamous subtype based on the TCGA molecular classification schema13. Notably, 8 of the UC regions were also basal-squamous subtype, with only four pairs exhibiting discordant transcriptional subtypes (three UC regions were luminal-infiltrated, one luminal-papillary, Fig. 2a).
a RNA sequencing analysis of 12 pairs of macrodissected UC and SqD regions from bladder tumors with mixed histology. Each region was classified using the TCGA molecular signature classification schema. All 12 SqD regions were basal/squamous subtype as were 8 of the 12 UC regions. Four tumors had discordant molecular subtypes. b Expression of BASE47 (left) and UPK/KRT (right) genes were evaluated by single sample GSEA analysis. Analysis was performed on 12 biologically independent UC-SqD pairs, Error bars represent standard error, p = 0.0005, two-sided paired Wilcoxon rank sum test. c Single-cell RNA-Seq analysis of a bladder carcinoma with extensive squamous differentiation revealed five clusters of tumor cells (clusters 1–5). d Trajectory analysis of these cell populations indicated a linear progression from cells resembling basal cells to those more similar to differentiated squamous cells. e Single-cell expression of select genes. Cluster 1 (61 cells) had an expression signature characteristic of the basal phenotype (high KRT5). Cluster 2 (64 cells) and Cluster 3 (152 cells) had expression signatures indicative of early squamous differentiation similar to that of suprabasal (spinous) cells (stratum spinosum), which are normally located immediately superficial to the basal cells of the epidermis (high DSC2, DSG2). Clusters 4 (231 cells) and 5 (220 cells) had a late squamous differentiation signature similar to that of granular squamous cells (stratum granulosum), which are normally located between the stratum spinosum and stratum corneum of the epidermis (high KRT13, KRT16).
To better quantify differences in the transcriptional profiles between the paired UC and SqD regions, we performed single sample gene set enrichment analysis (ssGSEA)19,20. This revealed that the SqD regions expressed higher levels of basal-squamous genes based on the BASE47 gene set21 (p = 0.07, paired Wilcoxon rank sum test) and UPK/KRT gene sets20 (p < 0.001, paired Wilcoxon rank sum test), whereas the matched UC regions expressed higher levels of luminal genes based on the BASE47 (p = 0.06, paired Wilcoxon rank sum test) and UPK/KRT gene sets (p = 0.02, paired Wilcoxon rank sum test) (Fig. 2b). These differences were observed even in cases in which both the UC and SqD regions were basal-squamous subtype (Fig. 2b).
To determine if quantitative differences in the expression of basal-squamous genes were identifiable at the single cell level in tumors with squamous morphology, we performed single cell RNA sequencing (sc-RNAseq) of a bladder urothelial carcinoma with extensive squamous differentiation. After excluding immune and stromal cell populations (Supplementary Fig. 5), we identified five distinct clusters of tumor cells (labeled as 1–5, Fig. 2c, Supplementary Figs. 6a, b). Trajectory inference analysis indicated a linear progression of these populations that phenotypically mirror the differentiation pattern of normal epidermis (Fig. 2d). Cells in cluster 5 were phenotypically similar to basal cells of the urothelium as well as skin epidermis with high expression of Keratin 5 (KRT5) and ribosome protein genes suggesting a high proliferative index (Fig. 2e). In contrast, cells in clusters 3 and 4 were phenotypically similar to early differentiation suprabasal (spinous) squamous cells with high levels of desmocollins and desmogleins (DSC2/DSG3) and transglutaminase (TGM1). Finally, clusters 1 and 2 resembled late differentiation (granular) squamous cells, with overexpression of filaggrin (FLG), as well as activated keratinocytes in an intermediate state of cell differentiation between basal and suprabasal cells, with overexpression of type I cytoskeletal keratins KRT16 and KRT1322,23,24,25 (Fig. 2e, Supplementary Fig. 6a). Notably, many of the genes that were up-regulated by bulk RNA-seq analysis in the SqD regions of tumors with mixed histology were also up-regulated in sub-population 2 of this squamous differentiated tumor (Supplementary Figs. 5c and 6c). Finally, despite the extensive squamous morphology evident in this tumor, some cells within clusters 1–3 had relatively high expression of FOXA1 (Fig. 2e), suggesting that a subset of tumor cells continued to express luminal differentiation markers. In sum, our bulk and sc-RNAseq results suggest that SqD in bladder tumors with mixed histology can be viewed as a continuous/gradual process that recapitulates the differentiation stages of the normal epidermis, with the squamous morphology representing the extreme of the basal-squamous phenotype. The bulk and single-cell transcriptomic analyses also provide molecular evidence that such a transition is well underway in regions of UC prior to discernable morphologic evidence of squamous differentiation at the histologic level, highlighting the limitations of classifying tumors based on morphologic assessments under light microscopy.
Regions of SqD in urothelial carcinomas with mixed histology are characterized by loss of FOXA1, GATA3, and PPARγ expression
To identify individual genes contributing to the squamous phenotype, we further analyzed the bulk RNAseq data to identify genes differentially expressed in the UC or SqD regions of mixed histology tumors. We identified 718 significantly upregulated and 651 downregulated genes in the SqD as compared to the UC regions (Fig. 3a; Fold Change >2 and FDR < 0.05). A subset of the genes upregulated in the SqD regions were genes previously reported to be highly expressed in squamous carcinomas of various lineages, including the peptidase kallikrein genes KLK1326, KLK1027, and KLK1228, the cornification-related genes CNFN and PLA2G4E29, the small proline-rich protein genes SPRR2D and SPRR2E30, the keratin genes KRT1631, KRT3132 and KRTDAP33, the interleukin IL36G34, and the desmogleins DSG1/3/4 and desmocollins DSC2/335,36 (Fig. 3b). Consistent with the sc-RNAseq data above, GSEA also revealed that the top two gene sets upregulated in the SqD regions included genes known to play a role in normal epidermis development and genes associated with the cornified envelope (Fig. 3c, Supplementary Figs. 7a–c). Conversely, genes significantly upregulated in the UC regions compared to SqD regions included FOXA1, GATA3, and PPARG, transcription factors that play a critical role in maintenance of urothelial cell identity20,37,38,39 (Wilcoxon signed-rank test, Supplementary Fig. 7d). Immunohistochemistry further confirmed the significantly decreased expression of FOXA1, GATA3 and PPARγ in the SqD versus UC regions of mixed histology bladder tumors (Fig. 3d, Supplementary Table 1, all p < 0.05, paired Wilcoxon test, H-score)40.
a Differential gene expression analysis of RNAseq data from 12 biologically independent samples following macrodissection of paired UC and SqD regions revealed 718 significantly upregulated and 651 significantly downregulated genes in the SqD versus the UC regions (defined as log2 FoldChange >1 and FDR < 0.05). b Volcano plot of the same 12 UC-SqD pairs showing differentially expressed genes with log FoldChange on the x-axis and log p-value on the y-axis. Highlighted are genes associated with urothelial differentiation (left, blue color) or squamous differentiation during normal skin development (right, red color). c Gene Set Enrichment Analysis (GSEA) identified gene sets associated with epidermis development and the cornified envelope as the top gene sets upregulated in the SqD versus UC regions (two-sided paired Wilcoxon test). d Representative H&E and immunohistochemical stains showing loss of FOXA1, GATA3, and PPARɣ protein expression exclusive to the SqD regions of mixed histology tumors. Scale bar = 500 μm.
Intratumoral morphologic heterogeneity is associated with intratumoral immunologic heterogeneity
Intratumoral genetic heterogeneity can result in a large subclonal mutational burden that may facilitate the selection of tumor subclones resistant to immune checkpoint blockade41. We, therefore, hypothesized that morphologic heterogeneity could be a predictive biomarker of poor response to anti-PD-1/PDL-1 therapies in bladder cancer patients. To test this hypothesis, we performed a detailed histologic review of bladder tumors collected from 29 patients with locally advanced or metastatic bladder cancer that were treated with the anti-PD-L1 antibody atezolizumab on a therapeutic protocol (clinicaltrials.gov NCT02108652)6. This analysis was restricted to performing histopathologic review of hematoxylin and eosin-stained slides from the tumors included in this study and correlation with response to atezolizumab therapy and did not include use of any genomic data from that study6. Of the 9 patients who experienced durable clinical benefit, histopathologic evaluation revealed overwhelming tumor morphologic monotony and a lack of morphologic heterogeneity in 7 patients (78%). In contrast, 16 of 20 patients (80%) who derived no clinical benefit from atezolizumab had tumors with mixed histology (p = 0.01, Fisher’s exact test, Fig. 4a).
a Morphologic heterogeneity was associated with a lack of clinical benefit in bladder cancer patients treated with the anti-PD-L1 antibody atezolizumab (n = 29) (p = 0.01, Fisher’s exact test). b RNAseq data was used to classify the UC and SqD components from the 12 UC-SqD pairs based on TCGA immune subtypes. c Immune cell deconvolution analysis was performed using single sample Gene Set Enrichment analysis (ssGSEA, left) or the CIBERSORTX algorithm (right). The size of the circles is reflective of the p-value and the red * indicates significantly enriched immune cells in either the SqD or UC fractions (two-sided Paired sample Wilcoxon test). d Immune cell deconvolution scores for individual UC-SqD paired regions were plotted for significantly enriched immune cell types identified by either ssGSEA or CIBERSORTX. Analyses were performed on 12 biologically independent samples following macrodissection of paired UC and SqD regions. All analyses used paired sample Wilcoxon test. The line in the center of box plot denotes the median. The lower and upper bounds of the box indicate 1st and 3rd quartiles, respectively. The whisker reaches to the maximum and minimum point within the 1.5x interquartile range. Data beyond the end of the whiskers are outliers. e PD-L1 immunohistochemical analysis (clone SP263) of two representative tumors showing significantly higher PD-L1 expression in the SqD region versus the adjacent UC region (p = 0.02, Wilcoxon rank sum test, also Supplementary Table 1) of mixed histology tumors. Scale bar = 1 mm. Additional microscopic images from Case 2 taken at higher magnifications are included in Supplementary Fig. 13.
Given the association between heterogeneous histomorphology and intrinsic resistance to immune checkpoint blockade, we investigated whether variations in tumor histopathology were associated with heterogeneity of the immune tumor microenvironment. More specifically, we performed tumor immune subtype analysis42 and immune cell deconvolution analysis of the RNAseq data of the macrodissected UC and SqD regions analyzed above. A C2-interferon-gamma (IFNγ)-dominant immune signature was observed in all 12 SqD regions and 9 UC regions. This immune signature is characterized by significant enrichment for M1/M2 macrophage and CD8 expressing T-cells, and high T-cell receptor diversity42. Discordant immune signatures were noted in the paired UC-SqD regions of three tumors (two UC regions were classified as C1-wound healing and one as C3-inflammatory subtype) (Fig. 4b). Immune cell deconvolution analysis of the macrodissected UC and SqD regions further revealed that the UC regions were enriched for signatures of M2 macrophages (p = 0.012), CD56 bright (active) NK cells (p < 0.001), and cytotoxic (CD8+) and effector memory T (TEM) cells (p = 0.03) (for all analyses, paired sample Wilcoxon test was used), whereas SqD regions were enriched for M0 macrophages (Fig. 4c, d, Supplementary Fig. 8). In sum, the results suggest that there is a predisposition in SqD tumors for an IFNγ dominant immune signature and that there are spatial differences in the immune cell microenvironment within tumors of mixed histology.
We hypothesized that the immune exclusion signature of SqD as compared to UC regions may partly explain the low response rate to immune checkpoint inhibitors in basal-squamous UC tumors despite the dominant IFNɣ immune signature4,43,44. Factors that can contribute to spatial differences in immune cell populations include (1) regional differences in the expression of cellular proteins that play a mechanical barrier function such as filaggrin (FLG) and desmosomal proteins such as DSC345, and (2) metabolic barriers related to increased hypoxia, decreased fatty acid metabolism and increased glycolysis46,47, all of which were preferentially altered in SqD regions as compared to UC regions in our cohort (Supplementary Fig. 8).
To validate the difference in immune cell infiltrates between UC and SqD regions noted by immune cell deconvolution analysis of the RNAseq data, we performed immunohistochemical staining for PD-L1 and observed significantly higher PD-L1 protein expression on tumor cells in the SqD as compared to UC regions of 14 of 15 tumors (Fig. 4e and Supplementary Table 1, p = 0.02, paired Wilcoxon rank sum test). The tumor-infiltrating immune cells in SqD regions also had greater PD-L1 expression than the tumor-infiltrating immune cells in the UC regions (p = 0.02, paired Wilcoxon rank sum test). As illustrated by the two examples shown in Fig. 4e, the SqD regions had strong and diffuse PD-L1 expression whereas the UC regions were largely PD-L1 negative. Multiplex immunofluorescence staining confirmed the high PD-L1 expression on the tumor cells, particularly those with squamous features (Supplementary Fig. 9). In sum, our analyses indicate that morphologic heterogeneity is a marker for both genomic and immunologic intratumoral heterogeneity and that patients with mixed histology tumors have heterogeneous PD-L1 expression and are less likely to derive clinical benefit from anti-PD-L1 therapy.
FOXA1 is a bladder cancer cell-intrinsic repressor of the IFNγ transcriptional signature and CD274/PD-L1 expression
FOXA1 has previously been shown to regulate PD-L1 (CD274) expression in regulatory T-cells48. As the squamous regions of mixed histology tumors had both upregulation of PD-L1 and downregulation of FOXA1 expression, we hypothesized that urothelial to squamous lineage plasticity and immune heterogeneity were coordinately dysregulated in mixed histology bladder cancers through changes in FOXA1 gene expression. To explore this possibility, we generated a series of FOXA1 knockout (FOXA1-KO) sublines through CRISPR-Cas9 mediated gene editing of UM-UC-1 bladder cancer cells. Loss of FOXA1 expression, as shown by quantitative PCR and immunoblot analysis, was sufficient to induce PD-L1 mRNA and protein expression in multiple UM-UC-1 single-cell clones (Fig. 5a, Supplementary Figs. 10a, b). Conversely, ectopic expression of FOXA1 in UM-UC-3 cells, a bladder cancer cell line with high basal PD-L1 expression, was sufficient to downregulate CD274/PD-L1 expression (Fig. 5b, Supplementary Fig. 10c, d).
a CRISPR-Cas9 gene editing was used to generate isogenic UM-UC-1 bladder cancer cells with loss of FOXA1 expression. Immunoblot analysis showing that loss of FOXA1 expression was associated with increased PD-L1 expression. This analysis was performed in triplicate. Source data are provided as a Source Data file. b UM-UC-3 cells were transfected with FOXA1 or with empty vector. Immunoblot analysis was then performed to quantitate expression of FOXA1, PD-L1, and GAPDH as a loading control. This analysis was performed in triplicate. c Differential gene expression (FDR q < 0.05) in parental and FOXA1-KO UM-UC-1 bladder cancer cells. Loss of FOXA1 expression was associated with upregulation of 1358 genes including CD274 (PD-L1) and other interferon response genes, and downregulation of 2187 genes. d Gene set enrichment analysis (GSEA) identified the interferon alpha and gamma pathways as the top two gene sets altered following FOXA1 knockout in UM-UC-1 bladder cancer cells. e FOXA1 mRNA expression was significantly negatively correlated with CD274 (which encodes PD-L1) mRNA expression in the TCGA bladder cancer cohort (two-sided Spearman correlation). f FOXA1 regulon activity profiles for 404 samples in the TCGA BLCA cohort sorted by FOXA1 regulon activity (left), molecular subtypes and CD274 gene expression (right). g Kaplan-Meier plot of the bladder TCGA cohort13 showed that disease-specific survival (DSS) was significantly associated with positive vs. negative FOXA1 regulon activity status (Log-rank p-value = 0.01). Numbers indicate patients in each group and, in curved parentheses, deceased patients. h Correlation between FOXA1 and CD274 mRNA expression across 24 cancer types from the TCGA analysis showed that the most significant inverse association between these genes was observed in bladder cancer.
To characterize the global transcriptional changes resulting from FOXA1 loss-of-function in bladder cancer cells, we performed whole transcriptome RNA sequencing of the parental UM-UC-1 cells and two FOXA1-KO sublines. Differential gene expression analysis identified 1358 significantly upregulated and 2187 significantly downregulated genes in the FOXA1-KO as compared to the parental UM-UC-1 cells, including many interferon target genes such as CD274 (PD-L1), ISG15, and IFI44L (Fig. 5c, FDR < 0.05). Furthermore, analysis of the upregulated genes via GSEA suggested a critical role for FOXA1 in repression of interferon target genes (Fig. 5d, and Supplementary Fig. 10e). We also confirmed that there was a reciprocal relationship between FOXA1 and PD-L1 expression in human bladder cancers using RNA sequencing data from the bladder cancer TCGA cohort (Fig. 5e; Spearman correlation; r = −0.45; p < 0.001). This finding was further supported by analyzing FOXA1 regulon activity in paired UC and SqD regions. Regulon refers to target gene sets controlled (induced and/or repressed) by the same regulator genes13, and in our cohort, FOXA1 regulon activity was decreased in SqD compared to UC regions of individual bladder cancers (paired Wilcoxon rank sum test, Supplementary Fig. 11). Finally, to further validate our findings, we re-analyzed FOXA1 regulon activity across molecular subtypes in the TCGA bladder cancer cohort13 and in another previously published study49. This analysis confirmed the presence of decreased FOXA1 regulon activity in basal/squamous tumors as compared to luminal tumors and identified a significant inverse correlation between FOXA1 regulon activity and PD-L1 expression in both cohorts (Fig. 5f, Supplementary Fig. 11). Decreased FOXA1 regulon activity was also associated with shorter overall and disease-specific survival in the bladder TCGA cohort (Logrank p = 0.01, Fig. 5g, Supplementary Fig. 11). Additionally, of the 24 TCGA cancer types, an inverse association between FOXA1 and CD274 gene expression was most significant in bladder cancer (Fig. 5h).
FOXA1 knockout induces genome-wide epigenetic reprogramming and increased expression of PD-L1 and other interferon-stimulated genes
To identify the genes dysregulated by FOXA1 loss in a bladder cancer context, we next performed ChIP-Seq analysis of the parental UM-UC-1 and FOXA1-KO isogenic cells. This analysis identified 21,766 genomic loci occupied by FOXA1, most of which were annotated as genomic enhancers (n = 13,972, 64%, defined as 50 kb upstream of Transcriptional Start Site (TSS), or 5 kb downstream of Transcriptional End Site [TES]). Intergenic regions were the second most common loci (n = 6074, 28%), followed by promoter sites (n = 1720, 8%, 2 kb up- or down-stream of TSS) (Fig. 6a). Genome-wide changes in histone H3 lysine 27 acetylation (H3K27ac), a marker of active enhancers and promoters, has been reported in several FOXA1 dysregulated cancer types50,51,52,53,54,55,56. We, therefore, hypothesized that increased expression of PD-L1 and other interferon-stimulated genes (ISGs) in bladder cancer cells following FOXA1 loss-of-function is partly a result of changes in H3K27ac modification. To explore this possibility, we performed a comprehensive analysis of FOXA1-occupied sites and investigated changes in H3K27ac modification following FOXA1 knockout, and correlated these changes with changes in gene expression profiles. H3K27ac ChIP-Seq analysis identified 14,977 genomic regions with H3K27ac enrichment in either parental UM-UC-1 or FOXA1-KO cells (FDR < 0.05). The majority of these H3K27ac regions mapped to enhancer (n = 9302, 62%) and intergenic (n = 3064, 21%) regions and less frequently to proximal promoter regions (n = 2605, 17%) (Supplementary Fig. 12a). Together, 16% (4778) of the genomic regions were identified as having both H3K27ac enrichment and an occupied nearby (within 5 kb) FOXA1 binding site. Additionally, 55% and 29% of genomic regions were associated with either an occupied FOXA1 binding site or H3K27ac enrichment, respectively (Fig. 6b). The sites exhibiting both H3K27ac enrichment and an occupied FOXA1 binding site (16%) were proportionally annotated as enhancer (67%), intergenic (23%) and promoter regions (10%) (Fig. 6c).
a ChIP-Seq was performed in duplicates using chromatin extracted from UM-UC-1 parental and UM-UC-1 FOXA-1-KO cells. 21,766 FOXA1 binding sites were identified in the ChIP-Seq data by MACS2 (FDR < 0.001). Genomic regions where FOXA-1 binding sites were located were then annotated as promoter (2 kb+/− transcriptional start site—TSS), enhancer (between −50 kb from TSS and 5 kb after transcriptional end site—TES), or intergenic (other sites). b Integrated ChIP-Seq analysis of FOXA1 binding sites and H3K27ac revealed 14971 differentially modified H3K27ac loci enriched in either parental UM-UC-1 cells or those with FOXA1 KO (55% were unique FOXA1 binding, 29% were histone H3K27ac enriched sites, and 16% overlapping sites, defined as within 5 kb), c The majority of the overlapping sites were in enhancer regions with fewer sites in the intergenic or promoter regions. d Cluster analysis of the H3K27ac-modified enhancer regions enriched in either the parental UM-UC-1 cells or the isogenic FOXA1-KO cells (left), FOXA1 binding coverage around the same region (middle), and the expression of genes associated with these sites (right). The number of clusters was defined by kmean (k = 2) in H3K27ac enriched peaks. All analyses were performed in duplicate. e Known motif scanning of the sites enriched with H3K27ac modification in the gene promoter regions identified the top 10 significantly enriched motifs (blue dots) and motifs of interferon-regulatory factors (IRF) or interferon-sensitive response element (ISRE) (red dots). f Increased acetylation of CD274 gene regulatory elements including an upstream enhancer (red line region) and the proximal promoter region (blue line) in FOXA1-KO versus parental UM-UC-1 cells (top). For reference, unchanged peaks are shown (grey line). FOXA1 binding sites were also identified in the promoter region of CD274 gene by ChIP-Seq (bottom).
Among the H3K27ac peaks enriched within enhancer and intergenic regions, we identified 5,104 sites located within 10 kb from the gene transcriptional start site. By clustering analysis, there were 2083 (cluster 1) and 1755 (cluster 2) regions demonstrating significant increases or decreases in H3K27ac following FOXA1-KO, respectively (Fig. 6d, left). Enrichment in H3K27ac was also positively associated with increased expression of nearby genes (Fig. 6d, right). Both cluster 1 and cluster 2 exhibited evidence of FOXA1 binding (Fig. 6d, middle), suggesting that changes in FOXA1 binding at genomic enhancer regions was associated with both increased and decreased H3K27ac and parallel changes in target gene expression. Interestingly, a similar analysis of promoter regions showed that H3K27ac modification was enriched at promoter regions in FOXA1-KO cells and associated with increased gene expression (Supplementary Fig. 12b).
As H3K27ac can be a marker for chromatin regions with active regulatory elements57,58, we next performed motif analysis to identify transcription factor binding sites associated with these enriched H3K27ac modifications. In support of an epigenetic role for FOXA1 in accessibility of ISGs, interferon-sensitive response elements (ISRE) were identified in promoter regions with H3K27ac modifications (q < 0.05), as well as motifs for interferon response factors (IRF1, IRF2, and IRF3; all q < 0.05) (Fig. 6e). Notably, three ISRE motifs were identified in the proximal promoter region of the CD274 gene (Supplementary Fig. 12c) and these three ISRE motifs were associated with both a FOXA1-occupied region in parental UM-UC-1 and an enriched H3K27ac modification in FOXA1-KO cells (Fig. 6f). Similarly, cis-regulatory regions with increased H3K27ac following FOXA1 KO also overlapped with FOXA1 bound sites in parental UM-UC-1 for several other ISGs, including IFIT2, IFIT3, IFI35 and STAT2 (Supplementary Fig. 12d). In sum, the results suggest that increased H3K27ac enhances accessibility of interferon response factors following genetic ablation of FOXA1, resulting in increased ISG expression.
FOXA1 knockout increases IRF1 expression and its binding to the CD274 promoter, and upregulates PD-L1 expression
Our results show that FOXA1 KO results in increased PD-L1 expression, as well as increased H3K27ac at the CD274 promoter. We also show that at the CD274 promoter, and throughout the genome, areas of increased H3K27ac were significantly enriched for ISRE motifs following FOXA1 KO. Focusing on CD274 as a model ISG, we hypothesized that FOXA1 serves as a repressor of CD274 in the absence of IFNɣ exposure. Since IRF1 is a key transcriptional activator of CD274 following IFNɣ stimulation, we additionally hypothesized that IRF1 competes with and displaces FOXA1 after treatment with type II interferon. To explore these hypotheses, we performed DNA-affinity purification of nuclear proteins from UM-UC-1 cells treated in the absence or presence of IFNɣ (Fig. 7a, b). As a DNA probe, we used a 30 bp 5′ biotinylated fragment of the human CD274 promoter, including two functional ISRE motifs, overlapping with the identified FOXA1 binding motif. Importantly, this FOXA1 bound region shares sequence homology with a FOXA1 binding region of mouse Cd27448. In addition to this wild-type probe, a “scrambled” probe and a probe with mutations in the nucleotides hypothesized to be important for FOXA1 binding within the ISRE element were used as controls (see materials and methods and Supplementary Fig. 14). DNA affinity purification experiments followed by western blotting show that in the absence of IFNɣ, FOXA1 exhibits significantly greater binding to the wild-type CD274 probe relative to scrambled (Student’s t-test; p = 0.0319) or FOXA1 mutant (Student’s t-test; p = 0.0256) CD274 probes (Fig. 7b). In the presence of IFNɣ, FOXA1 still showed significantly greater binding to wild-type CD274 promoter relative to scrambled (Student’s t-test; p = 0.0208) and FOXA1 mutant (Student’s t-test; p = 0.0123) probes (Fig. 7b). However, there were no significant differences in FOXA1 binding relative to IFNɣ treatment. While FOXA1 was not regulated via IFNɣ treatment, IRF1 was significantly upregulated following IFNɣ stimulation (Fig. 7a), resulting in increased IRF1 binding to wild-type CD274 relative to scrambled and mutant probes (Fig. 7b). These results indicate IRF1 does not compete with FOXA1 binding to the CD274 promoter following IFNɣ stimulation.
Western blotting (a) and quantification (b) following DNA affinity purification for FOXA1 (n = 3) and IRF1 (n = 2) in parental UM-UC-1 treated in the presence and absence of IFNɣ. In the absence of IFNɣ, FOXA1 exhibits significantly greater binding to the wild-type CD274 probe relative to scrambled negative control DNA probe (Student’s t-test; p = 0.0319) or mutant (Student’s t-test; p = 0.0256) CD274 probes. In the presence of IFNɣ, FOXA1 still showed significantly greater binding to wild-type CD274 promoter relative to scrambled DNA probe (Student’s t-test; p = 0.0319) and FOXA1-binding mutant (Student’s t-test; p = 0.0256) CD274 probes. There were no significant differences in FOXA1 binding relative to IFNɣ treatment. Following IFNɣ treatment, IRF1 shows increases in binding to wild-type CD274 relative to scrambled and mutant probes. Q-RT-PCR data are expressed as the mean ± S.D. from independent experiments of FOXA1 (n = 3), IRF1 (n = 2), respectively. Source data are provided as a Source Data file (c) and western blotting (d) shows that FOXA1 KO in UM-UC-1 results in significant increases in IRF1 expression at the mRNA and protein levels, respectively. Data of Q-RT-PCR are expressed as the mean ± S.D. from independent experiments (n = 3). Source data are provided as a Source Data file. Western blotting (e) and quantification (f) following DNA affinity purification for FOXA1 and IRF1 in parental UM-UC-1 (Ctrl) and FOXA1 KO UM-UC-1 (FOXA1 KO) cell lines. IRF1 purified from FOXA1 KO UM-UC-1 exhibited a 26-fold increase in binding to the CD274 promoter fragment relative to parental UM-U-C1. IRF1 was unable to be purified from parental and FOXA1 KO UM-UC-1 cells with scrambled negative control oligo (n = 2). Ectopic expression of IRF1 in parental UM-UC-1 cells followed by Q-RT-PCR (n = 4). Data are expressed as the mean ± S.D. from independent experiments (g) and western blotting (h). Source data are provided as a Source Data file. Overexpression of IRF1 significantly increased expression of i CD274 (n = 4, Student’s t-test; p = 0.0344), j STAT2 (n = 4, Student’s t-test p = 0.0321), k ISG15 (n = 4, Student’s t-test p = 0.0008), l IFIT2 (n = 3 Student’s t-test; p = 0.0338), m IFIT3 (n = 4, Student’s t-test; p = 0.0009) and n IFI35 (n = 4, Student’s t-test; p = 0.0406). Data are expressed as the mean ± S.D. from independent experiments. *p < 0.05 was considered as a statistically significant. ns not significant. All tests are unpaired two-sided Student’s t-test.
Interestingly, we noted that FOXA1 KO resulted in significant increases in IRF1 transcript (Fig. 7c; Student’s t-test; p < 0.0001) and protein levels (Fig. 7d). Based on this, we tested the hypothesis that increased IRF1 expression following FOXA1 KO results in increased binding of IRF1 to the CD274 promoter, and subsequently increased CD274 expression. Indeed, DNA affinity purification from FOXA1 KO UM-UC-1 cells identified an ~26-fold increase in IRF1 binding to the wild-type CD274 promoter relative to parental UM-UC-1 (Fig. 7e, f). To determine if increased IRF1 expression was sufficient to drive expression of CD274 and other ISGs even in the absence of IFNɣ stimulation, we next transiently overexpressed IRF1 in parental UM-UC-1 cells (Fig. 7g, h). Ectopic overexpression of IRF1 resulted in a significant increase in the expression of CD274 (Fig. 7i; Student’s t-test; p = 0.0344), STAT2 (Fig. 7j; Student’s t-test p = 0.0321), ISG15 (Fig. 7k; Student’s t-test p = 0.0008), IFIT2 (Fig. 7l; Student’s t-test; p = 0.0338), IFIT3 (Fig. 7m; Student’s t-test; p = 0.0009), and IFI35 (Fig. 7n; Student’s t-test; p = 0.0406). In sum, these results show that increased IRF1 expression is sufficient to activate the expression of ISGs, including CD274 in an IFNɣ-independent manner.
Discussion
Elucidating the relationship between genomic and immune heterogeneity is crucial to understanding mechanisms of intrinsic and acquired resistance to immune checkpoint blockade in patients with bladder cancer and other cancer types. Here, we performed comprehensive multiplatform analyses of spatially distinct regions of urothelial and squamous morphology from mixed histology bladder cancers. While WES confirmed that both the UC and SqD regions of individual tumors were derived from a shared precursor, we observed significant mutational discordance between the two morphologically distinct regions consistent with early branched evolution. Of clinical significance, heterogeneous histomorphology was a marker for immune heterogeneity within the tumor microenvironment and differential PD-L1 expression on tumor cells, and patients whose bladder tumors had marked intratumoral morphologic heterogeneity were less likely to benefit from treatment with the anti-PD-L1 inhibitor atezolizumab. Functional studies indicated that lineage plasticity and immune cell heterogeneity were coordinately dysregulated through changes in the expression of the FOXA1 transcription factor, the loss of which was sufficient to induce PD-L1 expression on tumor cells via increased IRF1 expression and its binding to the CD274 promoter.
While we did not identify a recurrent genetic alteration unique to the squamous regions of mixed histology tumors, the squamous components generally exhibited greater divergence from their nearest shared evolutionary ancestor and greater tumor mutational burden, neoantigen load and ploidy than the matched UC regions. The molecular basis for the higher mutational burden of the SqD regions was not clear as in most cases, the paired UC and SqD regions demonstrated similar mutational processes with almost all cases exhibiting APOBEC-associated mutational signatures13,18. Our analysis also identified discordant mutations in genes long thought to represent early clonal events during bladder carcinogenesis such as TP53, RB1, and FGFR3, the latter being unique to the SqD regions of two cases.
Despite the presence of distinct morphologic differences between SqD and UC regions of individual tumors, all twelve SqD regions and nine of twelve UC regions analyzed by RNAseq were basal-squamous subtype based on the TCGA molecular classification schema, with the remaining three UC tumors being luminal or luminal-papillary subtype. Our bulk multi-region and single cell RNA sequencing suggested that in urothelial carcinomas with SqD, lineage plasticity with acquisition of squamous features is a continuous and gradual process in which the squamous morphology represents the extreme of the basal-squamous phenotype. More specifically, subpopulations of cancer cells from the SqD regions recapitulated the spectrum of differentiation stages present in normal epidermis. This was best shown using single cell analysis which was able to identify distinct cell populations with expression profiles characteristic of basal, suprabasal, spinous, granular and cornified squamous cells. Given that the expression profiles of the UC regions of most mixed histology tumors were basal-squamous, our data indicate that the process of SqD is well underway at the molecular level even in regions lacking characteristic squamous histomorphologic features. Overall, these results highlight a limitation of current tumor classification schema which are largely reliant on histologic features identifiable under light microscopy. It is widely acknowledged that the UC regions of bladder tumors with SqD are typically morphologically indistinguishable under light microscopy from non-keratinizing squamous cell carcinomas arising in organs that have a squamous lining, such as squamous tumors of the head and neck and lung. However, in contrast to tumors arising at these other anatomic sites, the squamous designation is only applied to bladder cancers when there is definitive evidence of keratinization or intercellular bridges (representing desmosomes)59. This deliberatively restrictive tumor classification approach was adopted to minimize overcalling of squamous differentiation in tumors that resemble the UC component of mixed histology bladder cancers with UC and SqD features. Our data suggest thus that molecular analysis, in particular gene expression profiling, is better suited than histologic examination under light microscopy for classifying bladder cancers with potential implications for treatment selection and patient outcomes.
A notable finding of our gene expression analyses was that UC regions of mixed histology tumors with SqD consistently expressed higher levels of FOXA1, GATA3, and PPARG, transcription factors previously shown to regulate luminal gene expression20. In addition to its putative role in lineage plasticity, we identified FOXA1 as a tumor cell-intrinsic repressor of ISG expression, exemplified by PD-L1. Using isogenic bladder cancer cells in which FOXA1 was genetically ablated using CRISPR/Cas9, we were able to show that loss of FOXA1 expression was sufficient to induce PD-L1 expression in bladder cancer cells.
Our results support a role for FOXA1 as a pioneer factor that regulates chromatin structure60,61,62, and our findings suggest that global epigenetic reprogramming following FOXA1 loss-of-function contributes to the IFNγ-dominant signature found in the squamous regions of mixed histology bladder cancers. In our integrated analysis of RNAseq and ChIP-seq data, we also observed that genome-wide epigenetic reprogramming following loss of FOXA1 expression was associated with increased H3K27ac at regulatory elements important for the control of CD274 and other interferon sensitive genes. These areas of increased H3K27ac after FOXA1 knockout were significantly enriched for ISRE motifs. Our studies also provide evidence that increased IRF1 expression following FOXA1 KO increases expression of CD274 and other ISGs in an interferon-independent manner, providing one mechanistic explanation of how FOXA1 loss increases expression of CD274 and other ISGs. Previous work published during revision of this manuscript reported that FOXA1 physically interacts with STAT1 and STAT1/STAT2 following treatment with IFNɣ and IFNα, respectively63. Furthermore, this work showed that FOXA1 interaction with STAT proteins serves to repress IFNα-induced gene expression. Our work has identified an additional and likely complementary interferon-independent role for FOXA1 and IRF1 in the activation of CD274 and other ISGs in epithelium. The data also provide a direct link between the molecular mechanisms mediating lineage plasticity in bladder cancer and changes in the composition of immune cells within the tumor microenvironment. While FOXA1 has previously been implicated in the positive regulation of PD-L1 expression in a subset of immune cells48, here we show that FOXA1 also functions as a tumor cell-intrinsic regulator and repressor of PD-L1 expression in a solid tumor (namely urothelial carcinoma). Moreover, our analysis of TCGA data suggests that FOXA1 may also function as a regulator of PD-L1 expression in additional cancer subtypes including squamous cancers of the lung and head and neck.
One limitation of the current study was the sample size. This was in part due to our decision to restrict the analysis to tumors in which urothelial and squamous regions could be separated by macrodissection and analyzed individually using bulk sequencing methods. Much more commonly, urothelial and squamous regions are intermixed in a manner that precludes a paired analysis using bulk RNA and DNA sequencing methods. The recent development of robust methods for global gene expression profiling at the single cell level has opened the possibility of analyzing a broader range of mixed histology tumors. As these methods typically require fresh and not frozen tissue, we have initiated efforts to prospectively perform integrated single cell analysis of mixed histology bladder tumors. While we focused only on tumors with SqD in this study, there are other histologic variants of UC that warrant further investigation, in particular those that are associated with an aggressive clinical course, such as micropapillary, plasmacytoid, small cell and sarcomatoid carcinomas11,12,64,65. While patients with such tumors are often excluded from clinical trials of novel agents such as immune checkpoint inhibitors, the recent FDA-approvals of anti-PD-L1 and anti-PD1 antibodies for patients with bladder cancer should facilitate future biomarker studies to determine how the lineage plasticity observed in other histologic variants impacts the tumor microenvironment.
In conclusion, we performed a multiplatform, integrated analysis of macrodissected UC and SqD regions from mixed histology bladder cancers with SqD. We found that lineage plasticity in bladder cancers with SqD was associated with loss of expression of the FOXA1, GATA3, and PPARG transcription factors. Furthermore, we identified a mechanistic link between lineage plasticity in these tumors and changes in the immune tumor microenvironment. PD-L1 expression on tumor cells was highly discordant in the urothelial and squamous regions. As anti-PD1/PL-1 antibodies are being adopted as standard therapy for patients with locally advanced and metastatic bladder cancer, and as PD-L1 expression is associated with response to these therapies, the work presented here has immediate translational implications for the identification of bladder cancer patients most likely to respond to immune checkpoint blockade. Our data also suggest that morphologic heterogeneity, as a biomarker of genomic and immune heterogeneity, should be assessed for in ongoing and future clinical trials of novel immunotherapy combinations and validated prospectively as a biomarker of intrinsic resistance to immunotherapy across cancer types.
Methods
Patient samples and immunohistochemistry (additional details provided in Supplementary Table 1)
All specimens were obtained from patients following written informed consent and in accordance with institutional review board (IRB) approval at Memorial Sloan Kettering Cancer Center (IRB# 06-107 and IRB# 89-076), Pennsylvania State University College of Medicine (IRB# STUDY00000620) and Vanderbilt University Medical Center (IRB#140888). Tumor samples were from surgical specimens (cystectomy or transurethral resection specimens) and were classified as urothelial carcinoma, NOS (UC), or Squamous Differentiation (SqD) by consensus of three board certified genitourinary pathologists (H.A., J.I.W., and L.L.G.). Tumors with regions of variant histology other than SqD were excluded from this study. Macrodissection was performed with the assistance of hematoxylin and eosin staining to isolate areas of UC or SqD. Blood and/or normal tissues (benign lymph nodes procured at the time of cystectomy) were used as a source of germline DNA. Immunohistochemistry for GATA3 (L50-823, Biocare), FOXA1 (HNF-3α/β [C-20]: sc-6553, Santa Cruz), PPARG (D69, Cell Signaling), and PD-L1 (SP263, Ventana) was performed using the antibodies specified. In total, 42 tumor regions of the primary bladder tumor from 21 patients (one UC and one SqD region per tumor), from 16 male and 6 female patients, age range 52–83 years, underwent whole-exome sequencing (WES). Among the 21 tumors, 12 UC-SqD pairs were of sufficient quality for whole transcriptome profiling (RNA-Seq). One additional tumor sample from a male patient was subjected to single-cell RNA-Seq. The gender distribution in this cohort is consistent with the overall disease prevalence (bladder cancer is 3–4 times more common in men than in women)13.
Whole exome sequencing
Whole exome sequencing was performed by the MSKCC Center for Molecular Oncology (CMO). Briefly, DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s modified protocol and captured using Human whole exome Sureselect (Agilent). Libraries were then sequenced on a HiSeq2500 using 100 bp pair-end mode. The average numbers of read pairs per sample was 78, 99, and 105 million for the normal, UC, and SqD regions, respectively. Average duplication rates were 0.08%, 0.23%, and 0.35% for normal, UC, and SqD, respectively. A comprehensive data analysis pipeline (TEMPO, https://ccstempo.netlify.app), developed by the MSK Center for Molecular Oncology (CMO) team, was used to perform read alignment, variant calling, mutational signature analysis, copy number and clonality analysis, neoantigen prediction, microsatellite instability analysis.
For clonality analysis, the Clonality R package (v1.40.0)16 was applied to test whether a pair of tumors from the same patient were clonal (derived from a common precursor) or independent (distinct primary tumors). When analyzing somatic mutation data, as was performed here, the Clonality R package applies a statistical test developed by Ostrovnaya et al.17. In brief, a conditional likelihood model is applied to test the null hypothesis that the tumors are independent. The model only uses loci where at least one of the two tumors has a mutation. Marginal mutation frequencies are estimated from an external reference, and in this analysis the frequencies were derived from mutations observed in the TCGA BLCA cohort13. A generalized likelihood ratio test is performed based on the number of observed mutations common to both tumors or those that appear in exactly one of the two tumors. The Clonality output is presented in Supplementary Table 2.
For phylogeny analysis, evolutionary relationships of mutation phylogeny between UC and SqD regions were inferred using the union of somatic mutations called in any of the paired samples, a normal sample with none of these mutations was added for each patient. Package “Analyses of Phylogenetics and Evolution” (R package “ape”, v5.4-1) was used to infer the phylogenetics relationship based on the cluster results of the mutation table of the UC, SqD and the assumed normal sample.
RNA-Sequencing
RNA from formalin fixed paraffin embedded tumor tissue was extracted using the RNeasy mini kit (Qiagen; Valencia, CA) according to manufacturer’s instructions. After ribogreen quantification and quality control using a Agilent BioAnalyzer, 500 ng to 2 μg of total RNA underwent polyA selection and Truseq library (TruSeq™ RNA Sample Prep Kit v2) preparation. Briefly, samples were fragmented for 2 min at 94 °C before undergoing first strand and second strand cDNA synthesis. Libraries were amplified with 10 cycles of PCR and size-selected for fragments between 400 and 550 bp with a Pippin prep instrument (Sage Science). Samples were barcoded and run on a Hiseq 2500 in a 50 bp paired end run, using the TruSeq SBS Kit v3 (Illumina). Sequence reads were aligned and counted for each gene using the RSEM algorithm (v1.2.25) with the STAR alignment program (v2.5.0)66. An average of 30 million paired reads were generated per sample. Reads normalization and differentially expressed gene were analyzed using DESeq2 (v1.30.0)67. Gene pathway analysis was performed using GSEA (v.2.2.0)68. Immune cell fractions were inferred using both the GSVA (v1.44.2)68 and CIBERSORTX (v1.0)69 programs.
Single-cell RNA sequencing
For single-cell RNA sequencing, tumor tissue was processed immediately after removal from the bladder. Briefly, tumor tissue was cut into small pieces (<1 mm in greatest dimension) and incubated in 1 ml media supplied with enzymes from the tumor dissociation kit (Miltenyi, Cat: 130-095-929) for 30 min on a 37 °C shaker. Subsequently, 10 ml DMEM media was added to dilute the suspension, then a 40-μm cell mesh was used to filter the suspension. After centrifugation at 250 g for 5 min, the supernatant was discarded, and the cells were washed with DMEM media twice. The pellet was resuspended in 1 ml of calcium- and magnesium-free PBS containing 0.04% weight/volume BSA. Finally, 10 µl of suspension was counted under an inverted microscope with a hemocytometer. Trypan blue was used to quantify live cells.
Following the manufacturer’s protocol, the Chromium Single cell 3′ Reagent v3 kit was used to prepare barcoded scRNA-seq libraries. Single-cell suspensions were loaded onto a Chromium Single-Cell Controller Instrument (10x Genomics) to generate single-cell gel beads in emulsions (GEMs). To capture 10,000 cells per library, approximately 15,000 cells were added to each channel. After generation of GEMs, reverse transcription reactions were engaged to generate barcoded full-length cDNA. Next, cDNA was amplified, fragmented, end-repaired, A-tailed, and ligated to an index adaptor, and then the library was amplified. The library was sequenced on a HiSeq X Ten platform (Illumina) to generate 150 bp paired-end reads.
Sequencing reads were aligned to GRCh38 by Cell Ranger (10x genomics, v3.0) and analyzed with the Seurat package v4.070. Cells with UMI numbers <1000 or with over 10% mitochondrial-derived UMI counts were considered low-quality cells and were removed. Doublets were predicted using DoubletFinder, v2.0 and excluded after which 4768 cells remained for downstream analysis. Data normalization, dimension reduction (RunUMAP), and cluster identification (FindClusters) were performed with default parameters. To map differentiation of the tumor cell subpopulations, pseudotime analysis was performed using the Slingshot package in R71 with default parameters.
Expression and immune subtype assignment
Macrodissected areas of SqD and UC were assigned to TCGA molecular subtypes by nearest centroid analysis using the BLCAsubtyping package in R [https://github.com/cit-bioinfo/BLCAsubtyping] (v. 2.1). Immune subtypes were assigned by nearest centroid analysis and the ClaNC package (v1.1). For the immune subtype portion of the analysis, we randomly selected 1000 non-bladder cancer cases from the TCGA dataset. Subtypes for these had been assigned in the study by Thorsson et al., supplemental data42. We then generated centroids for the C1-C4 immune subtypes from these cases using RNA sequencing data applied to ClaNC, with each centroid defined by 500 genes. SqD and UC areas separated by macrodissection were assigned to the nearest centroid, using correlation-based distance.
ChIP-Seq
Cells were fixed with 1% formaldehyde for 15 min and quenched with 0.125 M glycine. Chromatin was isolated by the addition of lysis buffer, followed by disruption with a Dounce homogenizer. Lysates were sonicated, and the DNA sheared to an average length of 300–500 bp. Genomic DNA (Input) was prepared by treating aliquots of chromatin with RNase, proteinase K, and heat for de-crosslinking, followed by ethanol precipitation. Pellets were resuspended, and the resulting DNA was quantified on a NanoDrop spectrophotometer. Extrapolation to the original chromatin volume allowed quantitation of the total chromatin yield. An aliquot of chromatin (30 μg) was precleared with protein A agarose beads (Invitrogen). Genomic DNA regions of interest were isolated using 4 μg of antibody against H3K27ac (Active Motif, cat# 39133) or FOXA1 (Abcam, cat# ab5089). Complexes were washed, eluted from the beads with SDS buffer, and subjected to RNase and proteinase K treatment. Crosslinks were reversed by incubation overnight at 65 °C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation. Quantitative PCR (QPCR) reactions were carried out in triplicate on specific genomic regions using SYBR Green Supermix (Bio-Rad). The resulting signals were normalized for primer efficiency by carrying out QPCR for each primer pair using Input DNA. Illumina sequencing libraries were prepared from the ChIP and Input DNAs by the standard consecutive enzymatic steps of end-polishing, dA-addition, and adaptor ligation. Steps were performed on an automated system (Apollo 342, Wafergen Biosystems/Takara). After a final PCR amplification step, the resulting DNA libraries were quantified and sequenced on an Illumina NextSeq 500 (75 nt reads, single end). Reads were aligned to the human genome (hg38) using the Bowtie2 algorithm (default settings). Quality and adapter trimming of raw data was performed using trim galore (v0.6.5). Duplicate reads were removed by PICARD MarkDuplicate and only uniquely mapped reads (mapping quality >= 25) were used for further analysis (average deduplicated 23 million reads per sample). Peak locations were determined using the MACS2 algorithm (v2.1.0) with a cutoff of p-value = 1e−7. Peaks that were on the ENCODE blacklist of known false ChIP-Seq peaks were removed. Differentially modified H3K27ac loci were identified by the DiffBind package (v3.0.15). Peak annotation was performed using the Homer program (v4.11). Promoter region cis-element prediction was performed using the universal motif package (v1.6.3).
Immunofluorescence staining
Multiplex immunofluorescence (mIF) was performed for the following markers: PD-L1 (1:400, E1L3N, Cell Signaling), CD4 (1:200, EPR6855, abcam), CD8 (1:400, C8/114B, Cell Signaling), CD56 (1:2, MRQ-42, Cell MARQUE), CYP27A1 (1:600, EPR7529, abcam), and p63 (1:200, D9L7L, Cell Signaling). The tissue was de-paraffinized and underwent heat-mediated antigen retrieval in citrate buffer prior to triple labeling with the Opal Multiplex Immunostaining kit (Perkin Elmer, Waltham, MA). Briefly, slides were rinsed with TBST for 10 min, incubated in 1% bovine serum albumin for 30 min at room temperature (RT), and incubated with the anti-CD4 primary antibody for 2 h followed by washing with TBST (3 × 2 min). Slides were incubated with secondary antibody (Genomic technology co. LTD, Shanghai, China) for 30 min at RT, washed with TBST (3 × 2 min), incubated with the Opal 520 fluorophore working solution (1:300) for 10 min at RT, and washed with TBST (3 × 2 min). Slides then underwent heat-mediated antigen retrieval and antibody removal in citrate buffer. After cooling, the process was repeated for two additional rounds for labeling with anti-CD8, CD56, CYP27A1, PD-L1, and p63, followed by secondary labeling with the respective fluorophore working solution [i.e, Opal 570 (1:300), Opal 650 (1:500), Opal 690 (1:300) and Opal 620 (1:1000), respectively, (PerkinElmer, Waltham, MA)] as described above. Next, nuclei were labeled with DAPI for 10 min in a humid chamber, washed with dH2O, and coverslips were mounted with glycerin. Upon completion of multiplex IF staining, the slides were imaged using the Vectra 3.0 spectral imaging system (Perkin-Elmer). The chromogenic IHC-stained slides were scanned by using the bright field protocol, and the uniplex and multiplex IF staining was imaged by using the fluorescence protocol at 10 nm λ from 420 nm to 720 nm, to extract fluorescent intensity information from the images. A similar approach was used to build the spectral library using the InForm 2.2.1 image analysis software (PerkinElmer).
Cell culture and Western Blot analysis
Human bladder cancer cells (UM-UC-1, UM-UC-3) were purchased from ATCC, and authenticity was confirmed by short tandem repeat (STR) analysis20 or by MSK-IMPACT analysis72. All cell lines are routinely screened for mycoplasma. Cells were cultured in Minimal Essential Medium (UM-UC-1, UM-UC-3) supplemented with 10% FBS. Western blotting was performed as described previously20. Primary antibodies were used as follows: FOXA1 (1:500, ab23738, Abcam), PD-L1 (1:1000, ab213524, abcam), GAPDH (14C10) (1:1000, #2118, Cell signaling).
To establish UM-UC-1 FOXA1 knockout (KO), UM-UC-1 (2 × 105) cells were transfected with 2.5 mg of HNF-3alpha CRISPR/Cas9 KO plasmid (Santa Cruz, sc-400743) using lipofectamine3000 (Thermo fisher scientific). Three GFP-positive cells were sorted in a single well of 96-well plate containing 100 ml of medium by Aria II cell sorter (BD Biosciences). Sorted cells were expanded and knockout of FOXA1 in UM-UC-1 cells was confirmed by western blot analysis.
Transient transfection
One day before transfection, 2 × 105 UM-UC-3 or UM-UC1 cells were plated in wells of 6 well plates. The next day, attached cells were transfected with pCMV6-Entry (Origene; CMV empty vector) or pCMV6-FOXA1 (Origene; RC206045) or pCMV6-IRF1 (Origene; RC203500) using lipofectamine3000. After 48 h, RNA and protein were collected using the RNeasy kit (Qiagen) and lysed using RIPA buffer (Thermo fisher scientific) containing protease inhibitor (Roche) according to manufacturer’s protocol.
Regulon analysis
We inferred the FOXA1 regulons using the RTN package (v2.13.2)73,74. We estimated single-sample regulon activity by a two-tailed gene set enrichment analysis (GSEA-2T), which was used to sort samples of the TCGA BLCA13 and Sjödahl et al.49. cohorts. The GSEA-2T was performed in R75 using the RTN73 and RTNsurvival packages (v1.20.0)76. For the Kaplan-Meier analysis, we stratified the cohort into 2 groups—positive and negative regulon activity—and evaluated differences between the groups using a Logrank test.
Gene expression correlation analysis
Gene expression data of each cancer cohort in the Cancer Genome Atlas (TCGA) project were analyzed by TCGAbiolinks package (v2.24.3). The expression correlations between CD274 and FOXA1 were calculated based on the normalized reads using cor.test function in R with Spearman method. The results of correlation factors and p-values were summarized, and Bonferroni adjusted p-values were calculated by p.adjust function.
DNA pulldown assay
DNA pulldown assays were performed similar to as described in ref. 77, with the following modifications. 5′-biotinylated probes used corresponded to a region of the promoter of the human CD274 gene, which includes FOXA1 and ISRE motifs. The following 5′ biotinylated DNA probes were used in this study. Wild-type CD274 (forward 5′-ACTGACATGTTTCACTTTCTGTTTCATTTC; reverse 5′-GAAATGAAACAGAAAGTGAAACATGTCAGT), Mutant (forward 5′-ACTGACACGTCGCACCGACCGTCGCACGAC; reverse 5′-GTCGTGCGACGGTCGGTGCGACGTGTCAGT), scrambled (forward 5′-CGAGCGATCGAGCGATCGAGCGATCGAGCG; reverse 5′-CGCTCGATCGCTCGATCGCTCGATCGCTCG). DNA probes were annealed in annealing buffer (10 mM Tris (pH8.0), 50 mM NaCl, 1 mM EDTA (pH8.0)) at 95 C for 5 min, and then cooled to room temperature. Annealed probes were then added to streptavidin-coated beads (Thermo Fisher Scientific). Probe-conjugated beads were incubated in blocking buffer (20 mM Tris (pH8.0), 15% glycerol, 0.05% NP40) containing 50 mg of sonicated salmon sperm DNA. Beads were washed 3 times with 200 ml of blocking buffer (no salmon sperm DNA) and incubated at 4 C overnight with nuclear lysates. Nuclear lysates of UM-UC1 cells, UM-UC1 cells after 18 h treatment with IFNγ (100 ng/ml) (R&D systems) or vehicle control (distilled water), or FOXA1 KO UM-UC1 cells were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). After incubation, beads were washed 3 times with 200 μl of blocking buffer without salmon sperm DNA. After washing, bound proteins were eluted with 50 μl of elution buffer (1xNuPAGE LDS sample buffer, 10% mercaptoethanol). Samples were run on 4–12% NuPAGE gel (Thermofisher Fisher Scientific) and were immunoblotted using primary antibodies for FOXA1 (1:500, ab23738, Abcam) and IRF1 (D5E4) (1:1000, #8478, Cell signaling).
Statistics and reproducibility
Cell line experiments were performed in triplicate at least two times. No statistical method was used to predetermine sample size. No data were excluded from the analyses. The experiments were not randomized. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All somatic mutational calls and CNAs along with accompanying clinical data will be available for analysis and visualization in the cBioPortal for Cancer Genomics (https://cbioportal.mskcc.org/study/summary?id=blca_cmo_06155_2016). Raw whole exome sequencing data have been deposited in the Database of Genotypes and Phenotypes under dbGaP Accession phs001783.v4.p1. Due to informed consent requirements related to the genomics results uploaded to dbGaP, this data is made available through controlled-access. Data access is provided by dbGaP Authorized Access [https://dbgap.ncbi.nlm.nih.gov/aa/wga.cgi?page=login] upon request. Use of the data must be related to Cancer. Requestor agrees to make results of studies using the data available to the larger scientific community. Use of the data includes methods development research (e.g., development of software or algorithms). According to the dbGaP agreement outlines, access to the requested dataset(s) is granted for a period of one (1) year, with the option to renew access or close-out a project at the end of that year. Details on how to obtain authorized access from dbGaP can be retrieved through this link [https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/GetPdf.cgi?document_name=GeneralAAInstructions.pdf].
The RNASeq data generated in this study (including bulk RNA-Seq and scRNA-Seq raw data) and ChIP-Seq data generated from the UM-UC-1 isogenic cells have been deposited in the GEO database under accession code GSE172433.
Source data are provided with this paper. Figures associated with raw data include Figs. 1–7 and Supplementary Figs. 2–8, 11 and 12. The source data for Figs. 5a, b, 7a, d, e, h, and Supplementary Figs. 10a–d are provided as a Source Data file.
The remaining data are available within the Article, Supplementary Information or Source Data file.
The publicly available TCGA data used in this study are available in the Genomic Data Commons database accessible thorough this link [https://gdc.cancer.gov] and in the TCGA publication page accessible through this link [https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga]13. The Lund University (Sjödahl et al.) data used in this study are deposited at the Gene Expression Omnibus under access GSE3289449. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
Change history
23 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41467-022-35644-0
References
Loehrer, P. J. Sr. et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992).
von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005).
De Santis, M. et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 30, 191–199 (2012).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Zibelman, M., Ramamurthy, C. & Plimack, E. R. Emerging role of immunotherapy in urothelial carcinoma-Advanced disease. Urol. Oncol. 34, 538–547 (2016).
Snyder, A. et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis. PLoS Med. 14, e1002309 (2017).
Amin, M. B. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod. Pathol. 22, S96–S118 (2009).
Chalasani, V., Chin, J. L. & Izawa, J. I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 3, S193–S198 (2009).
Linder, B. J. et al. The impact of histological reclassification during pathology re-review–evidence of a Will Rogers effect in bladder cancer? J. Urol. 190, 1692–1696 (2013).
Shah, R. B. et al. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: impact of mandatory central pathology review at a large referral hospital. Urol. Oncol. 31, 1650–1655 (2013).
Al-Ahmadie, H. A. et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat. Genet. 48, 356–358 (2016).
Chang, M. T. et al. Small-cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin. Cancer Res 24, 1965–1973 (2018).
Robertson, A. G. et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540–556.e25. (2017).
Kamoun, A. et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 77, 420–433 (2020).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med 23, 703–713 (2017).
Ostrovnaya, I., Clonality: Clonality testing. 2022. R package version 1.44.0. https://www.bioconductor.org/packages/release/bioc/html/Clonality.html.
Ostrovnaya, I., Seshan, V. E. & Begg, C. B. Using somatic mutation data to test tumors for clonal relatedness. Ann. Appl. Stat. 9, 1533–1548 (2015).
Consortium, I.T.P.-C.A.O.W.G. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 14, 7 (2013).
Warrick, J. I. et al. FOXA1, GATA3 and PPAR cooperate to drive luminal subtype in bladder cancer: a molecular analysis of established human cell lines. Sci. Rep. 6, 38531 (2016).
Damrauer, J. S. et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014).
Bernot, K. M., Coulombe, P. A. & McGowan, K. M. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. J. Invest. Dermatol. 119, 1137–1149 (2002).
Bragulla, H. H. & Homberger, D. G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 214, 516–559 (2009).
Delva, E., Tucker, D. K. & Kowalczyk, A. P. The desmosome. Cold Spring Harb. Perspect. Biol. 1, a002543 (2009).
Huynh, J. et al. Interferon Regulatory Factor 6 promotes Keratinocyte differentiation in response to Porphyromonas gingivalis. Infect. Immun. 85, 5 (2017).
Nohara, K. et al. Expression of kallikrein-related peptidase 13 is associated with poor prognosis in esophageal squamous cell carcinoma. Gen. Thorac. Cardiovasc. Surg. 66, 351–357 (2018).
Chung, C. H. et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5, 489–500 (2004).
Bai, Y. et al. CASC15 contributes to proliferation and invasion through regulating miR-766-5p/ KLK12 axis in lung cancer. Cell Cycle 18, 2323–2331 (2019).
Liu, B. et al. Analysis of gene coexpression network reveals prognostic significance of CNFN in patients with head and neck cancer. Oncol. Rep. 41, 2168–2180 (2019).
Segura, S. et al. Identification of differentially expressed genes in actinic keratosis samples treated with ingenol mebutate gel. PLoS One 15, e0232146 (2020).
Khanom, R. et al. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS One 11, e0161163 (2016).
Suwanwela, J. & Osathanon, T. Inflammation-related genes are upregulated in surgical margins of advanced stage oral squamous cell carcinoma. J. Oral. Biol. Craniofac. Res 7, 193–197 (2017).
Dai, F. et al. The global expression profiling in esophageal squamous cell carcinoma. Genomics 109, 241–250 (2017).
Busfield, S. J. et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics 66, 213–216 (2000).
Savci-Heijink, C. D. et al. The role of desmoglein-3 in the diagnosis of squamous cell carcinoma of the lung. Am. J. Pathol. 174, 1629–1637 (2009).
Monica, V. et al. Desmocollin-3: a new marker of squamous differentiation in undifferentiated large-cell carcinoma of the lung. Mod. Pathol. 22, 709–717 (2009).
Osei-Amponsa, V. et al. Hypermethylation of FOXA1 and allelic loss of PTEN drive squamous differentiation and promote heterogeneity in bladder cancer. Oncogene 39, 1302–1317 (2020).
Reddy, O. L. et al. Loss of FOXA1 drives sexually dimorphic changes in urothelial differentiation and is an independent predictor of poor prognosis in bladder cancer. Am. J. Pathol. 185, 1385–1395 (2015).
DeGraff, D. J. et al. Loss of the urothelial differentiation marker FOXA1 is associated with high-grade, late-stage bladder cancer and increased tumor proliferation. PLoS One 7, e36669 (2012).
Choudhury, K. R. et al. A robust automated measure of average antibody staining in immunohistochemistry images. J. Histochem. Cytochem 58, 95–107 (2010).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Higgs, B. W. et al. Interferon Gamma Messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with Durvalumab. Clin. Cancer Res 24, 3857–3866 (2018).
Jorgovanovic, D. et al. Roles of IFN-gamma in tumor progression and regression: a review. Biomark. Res. 8, 49 (2020).
Salerno, E. P. et al. Human melanomas and ovarian cancers overexpressing mechanical barrier molecule genes lack immune signatures and have increased patient mortality risk. Oncoimmunology 5, e1240857 (2016).
Pai, S. I., Cesano, A. & Marincola, F. M. The paradox of cancer immune exclusion: immune oncology next frontier. Cancer Treat. Res. 180, 173–195 (2020).
Cascone, T. et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 27, 977–987 e4 (2018).
Liu, Y. et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat. Med. 20, 272–282 (2014).
Sjodahl, G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012).
Fu, X., et al., FOXA1 upregulation promotes enhancer and transcriptional reprogramming in endocrine-resistant breast cancer. Proc. Natl Acad. Sci. USA 116, 26823–26834 (2019).
Parolia, A. et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 571, 413–418 (2019).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Roe, J. S. et al. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 170, 875–888.e20 (2017).
Kim, J. et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080 (2017).
Hurtado, A. et al. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet. 43, 27–33 (2011).
Eeckhoute, J. et al. Cell-type selective chromatin remodeling defines the active subset of FOXA1-bound enhancers. Genome Res. 19, 372–380 (2009).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Moch, H., et al., WHO Classification of tumours of the urinary system and male genital organs. 4th ed. World Health Organization Classification of Tumours, ed. F. T. Bosman, et al. 2016, Lyon: International Agency for Research on Cancer.
Zaret, K. S. et al. Nuclear mobility and mitotic chromosome binding: similarities between pioneer transcription factor FoxA and linker histone H1. Cold Spring Harb. Symp. Quant. Biol. 75, 219–226 (2010).
Sekiya, T. et al. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev. 23, 804–809 (2009).
Cirillo, L. A. et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002).
He, Y., et al., FOXA1 overexpression suppresses interferon signaling and immune response in cancer. J. Clin. Invest. 131, e147025 (2021).
Willis, D. L. et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol. Oncol. 32, 826–832 (2014).
Lynch, S. P. et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur. Urol. 64, 307–313 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma. 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Irizarry, R. A. et al. Gene set enrichment analysis made simple. Stat. Methods Med. Res. 18, 565–575 (2009).
Chen, B. et al. Profiling Tumor-infiltrating immune cells with CIBERSORT. Methods Mol. Biol. 1711, 243–259 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Cheng, D. T. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Castro, M. A. et al. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat. Genet. 48, 12–21 (2016).
Fletcher, M. N. et al. Master regulators of FGFR2 signalling and breast cancer risk. Nat. Commun. 4, 2464 (2013).
R-Core-Team. R: A language and environment for statistical computing. 2019; Available from: https://www.R-project.org/.
Groeneveld, C. S. et al. RTNsurvival: an R/Bioconductor package for regulatory network survival analysis. Bioinformatics 35, 4488–4489 (2019).
Matthews, S. M. et al. The Crohn’s disease-associated SNP rs6651252 impacts MYC gene expression in human colonic epithelial cells. PLoS One 14, e0212850 (2019).
Acknowledgements
This work was supported by the Albert Institute for Bladder Cancer Care and Research (H.A.A, D.J.D.), Cycle for Survival (H.A.A, D.B.S.), the Mark Foundation (H.A.A., D.B.S.), Parker Institute for Cancer Immunotherapy (H.A.A.), the Samuel Waxman Cancer Research Foundation (Y.C., J.E.R), W81XWH-21-1-0916 from the Department of Defense (D.J.D.), RSG 17-233-01-TBE from the American Cancer Society (D.J.D.), W.W. Smith Charitable Trust (D.J.D.), Ruth Heisey Cagnoli Endowment in Urology at Penn State College of Medicine, Bladder Cancer Support Group at Penn State Health, R01 CA233899 (H.A.A.), P01CA221757 (H.A.A., D.B.S.), SPORE in Bladder Cancer P50CA221745 (H.A.A., D.B.S., D.F.B. J.E.R), the Sloan Kettering Institute for Cancer Research Cancer Center Support Grant P30CA008748, and the Marie-Josée and Henry R Kravis Center for Molecular Oncology (H.A.A., D.B.S.). The authors wish to acknowledge helpful discussions with Dr. Ryan Hobbs (Department of Dermatology, Pennsylvania State University College of Medicine) and Dr. Chris Norbury (Department of Microbiology and Immunology, Pennsylvania State University College of Medicine). The authors also wish to acknowledge Dr. Gregory Yochum (Associate Professor of Biochemistry, Pennsylvania State University College of Medicine) for providing protocols for the DNA affinity purification assay and the expert technical assistance of Dr. Yuka Imamura Kawasawa (Director, Genome Sciences Facility, Pennsylvania State University College of Medicine) and the MSK Integrated Genomics Organization (iGO).
Author information
Authors and Affiliations
Contributions
H.A.A, D.J.D, J.I.W, L.L.G, J.D.R, P.E.C., and R.A conceived the project. J.I.W, W.H, D.J.D, and H.A.A designed the study. D.J.D and H.A.A. supervised the project. J.I.W, W.H, H.Y, L.L.G, A.T.L, T.J.H, Y.L, D.J.D, and H.A.A collected data. J.I.W, W.H, H.Y, V.W, L.S, J.M.C, M.A.A.C, A.G.R (AG Robertson), F.K, I.O, T.A.C, D.J.D, and H.A.A analyzed data. W.H., V.W, F.K, I.O., and M.A.A.C. performed statistical and bioinformatic analyses. J.D.R., M.K., S.B.M., M.J., P.E.C., J.S, Y.B.C, A.G, S.J.S, S.W.F, S.K.T, K.K, J.T, N.K, S.P.G, T.N.C, T.A.C, Z.C, M.R, N.D.S, S.C, A.V, N.M, B.H.B, E.J.P, M.Y.T, G.I, J.E.R, D.F.B, M.K, S.B.M, M.J, J.A.T, V.E.R, Y.C, S.A.F, D.J.D, and H.A.A. provided clinical data and funding. J.I.W, W.H, H.Y, D.B.S, D.J.D, and H.A.A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.J.P. received honorarium from UpToDate, and received research funding from and is on the scientific advisory boards for Janssen Pharmaceuticals, Merck & Co. Inc., QED Therapeutics, UroGen Pharma and Steba Biotech. D.B.S. has consulted for/received honoraria from Pfizer, Loxo/Lilly Oncology, FORE Therapeutics, Vividion Therapeutics, Scorpion Therapeutics, Fog Pharma, and BridgeBio. D.J.D. received a research grant from Bristol-Myers-Squibb. H.A.A has consulted for Bristol-Myers-Squibb, AstraZeneca, Janssen Biotech, and Paige.ai. M.J. received research grant from AstraZeneca, Pfizer, and Eisai (drug only to Institution), and is on the advisory board for Seagen. J.S. is a consultant for Janssen Research & Development, LLC. S.A.F. received research support from AstraZeneca, Genentech/Roche, is a consultant/advisory board member for Merck and BioNTech, and owns stock/equity interest in Urogen, Allogene Therapeutics, Neogene Therapeutics, Kronos Bio, ByHeart, 76Bio, Vida Ventures, Inconovir, and Doximity. B.H.B is a consultant to Olympus corporation. D.F.B. reports personal fees from Bristol Myers Squibb and Merck; consulting/advisory role for Merck, Dragonfly Therapeutics, Fidia Farmaceutici S.p.A., and Bristol Myers Squibb Foundation; Travel/accommodations/expenses from Merck; and institutional research funding from Novartis, Merck, Bristol-Myers Squibb, AstraZeneca, Astellas Pharma, and Seattle Genetics/Astellas. Y.C. reports stock ownership in Oric Pharmaceuticals and sponsored Research from Foghorn Therapeutics. J.E.R is a Consultant for Seagen, Astellas, Bayer, AstraZeneca, QED Therapeutics, Merck, Genentech, Infinity, Gilead, Boehringer Ingelheim, Tyra, Mirati, Pfizer, EMD-Serono; reports sponsored research from Seagen, Astellas, Bayer, AstraZeneca, QED Therapeutics, Genentech; and received honoraria from Pfizer and EMD-Serono. The remaining authors declare no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks Qin Ma, Jean Paul Thiery, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Warrick, J.I., Hu, W., Yamashita, H. et al. FOXA1 repression drives lineage plasticity and immune heterogeneity in bladder cancers with squamous differentiation. Nat Commun 13, 6575 (2022). https://doi.org/10.1038/s41467-022-34251-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34251-3
This article is cited by
-
Tumour microenvironment as a predictive factor for immunotherapy in non-muscle-invasive bladder cancer
Cancer Immunology, Immunotherapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.