Abstract
Rheumatoid arthritis (RA) is an autoimmune disease affecting synovial joints where different CD4+ T cell subsets may contribute to pathology. Here, we perform single cell sequencing on synovial CD4+ T cells from anti-citrullinated protein antibodies (ACPA)+ and ACPA- RA patients and identify two peripheral helper T cell (TPH) states and a cytotoxic CD4+ T cell subset. We show that the adhesion G-protein coupled receptor 56 (GPR56) delineates synovial CXCL13high TPH CD4+ T cells expressing LAG-3 and the tissue-resident memory receptors CXCR6 and CD69. In ACPA- SF, TPH cells display lower levels of GPR56 and LAG-3. Further, most expanded T cell clones in the joint are within CXCL13high TPH CD4+ T cells. Finally, RNA-velocity analyses suggest a common differentiation pathway between the two TPH clusters and effector CD4+ T cells. Our study provides comprehensive immunoprofiling of the synovial CD4+ T cell subsets in ACPA+ and ACPA- RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA), which affects around 1% of the global population, is an autoimmune disease characterized by articular cartilage and bone erosion leading to physical disability, pain, and decreased quality of life. Joint damage is particularly exacerbated in the subset of RA patients who present with antibodies against citrullinated proteins (ACPA)1,2. Although the use of biologics has revolutionized the treatment of RA, 30–40% of patients fail to respond to treatment stressing the need for novel therapeutic targets3. The activity of CD4+ T cells in RA pathogenesis is evidenced by the HLA-DRB1 association with ACPA+ RA4. Expanded patient-specific T-cell clones have been identified in the synovium and synovial fluid (SF) of RA patients using bulk TCRβ repertoire analysis5 but without any connection to their effector function or antigen reactivities. Over the years, several CD4+ T-cell subsets, including Th1 and Th17 cells have been described in the synovial joints of RA patients6. Recently, PD-1high MHC-II+ (major histocompatibility complex class II) peripheral helper T cells (TPH) were identified in the synovial fluid and tissue of seropositive RA patients7,8,9 where they are proposed to facilitate B cell recruitment and activation through CXCL13 and IL-21 production; however, there is still no satisfying marker that defines the TPH subset. Furthermore, it is also unclear how this population is generated in the synovial joint. We have recently identified CD4+ T cells with cytotoxic features in SF of RA patients carrying the PTPN22 1858T risk allele variant10. This finding, as well as previous studies reporting expanded cytotoxic CD4+ CD28null T cells in the blood of RA patients11,12, suggests that cytotoxic CD4+ T cells might contribute to RA. However, a transcriptomic and clonality analysis of cytotoxic CD4+ T cells in the context of previously described CD4+ T-cell subsets, is still missing. The identification of T-cell effector functions is deemed important since it can lead to new therapeutic strategies.
Here, we performed 5´single-cell sequencing in combination with TCRαβ sequencing on peripheral blood (PB) and synovial fluid (SF) from ACPA+ and ACPA− RA patients to investigate CD4+ T-cell subsets. We demonstrate that clonal expansions are prominent amongst CXCL13high TPH, Treg, effector and cytotoxic CD4+ T cells in RA SF. We also describe two TPH CD4+ T-cell clusters expressing different levels of CXCL13. Finally, we find that TPH cells express the G-protein-coupled receptor GPR56, the inhibitory receptor LAG-3, and the tissue-resident memory (TRM) receptors CXCR6 and CD69 implicating that these T cells are maintained in the ACPA+ RA synovial joint. Our data provide a comprehensive immunoprofiling analysis of pathogenic CD4+ T cells at the site of inflammation in ACPA− and ACPA+ RA.
Results
Cytotoxic CD4+ T cells are enriched in ACPA+ RA synovial fluid
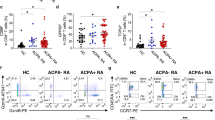
CD4+ T cells with cytotoxic function share common functional characteristics with NK and CD8+ T cells, including the expression of cytolytic proteins PRF1 (perforin-1), GZMB (granzyme B)13 and NKG7 (natural killer cell granule protein 7)14. Both transcription factors Eomes15,16 and Hobit17 are expressed in circulating human cytotoxic T cells. GPR56 is an adhesion G-protein-coupled receptor encoded by the ADGRG1 gene expressed on human circulating NK, CD8+, and CD4+ cytotoxic cells18. Synovial fluid mononuclear cells (SFMC) from ACPA− (n = 9) and ACPA+ (n = 12) RA patients were screened for the expression of these cytotoxic effector molecules and transcription factors in both CD4+ and CD8+ T cells by flow cytometry (Fig. 1, Supplementary Figs. 1a and 2, and Supplementary Data 1). ACPA+ SFMC presented with a significantly increased frequency of GZMB+ PRF1+, Hobit+, NKG7high, and GPR56+ CD4+ cells (Fig. 1a, b). No significant difference was observed for expression of GZMA and Eomes. Among CD8+ T cells, Hobit expression was increased in ACPA+ SFMC and a similar trend was observed for the expression of GZMA, GZMB, and NKG7 without reaching statistical significance (Supplementary Fig. 2). No significant difference was observed in the expression of PRF1 and GPR56 on CD8+ T cells in ACPA+ versus ACPA− RA. The frequency of GZMB+ PRF1+ CD4+ T cells in SF positively correlated with CCP (cyclic citrullinated peptide) positivity (Fig. 1c). In ACPA+ synovial fluid, GPR56 identified a distinct CD4+ T-cell population with a frequency that also correlated with CCP positivity (Fig. 1d).
a Representative flow cytometry dot plot staining of effector molecules, receptors and transcription factors associated with cytotoxic functions in CD4+ T cells from synovial fluid (SF) from ACPA− (upper panel) and ACPA+ (lower panel) RA patients, quantified in b, (ACPA−, n = 7–9) (ACPA+ , n = 10–12). Line represents median, two-tailed Mann–Whitney U test, P = 0.0072 (%GZMB+PRF1+), ns (%GZMA), P = 0.0018 (%Hobit), ns (%Eomes), P = 0.0036 (%NKG7-high), P = 0.0007 (%GPR56), ns: not significant. c Correlation between the frequency of GZMB+ PRF1+ in CD4+ T cells in SF and the level of serum anti-CCP (cyclic citrullinated peptide) antibodies, n = 21, Spearman two-tailed test, P = 0.0070. d Correlation between the frequency of GPR56 in CD4+ T cells in SF and the level of anti-CCP (cyclic citrullinated peptide) antibodies, n = 21, Spearman two-tailed test, P < 0.0001. a–d Data are from a pool of nine independent experiments where a circle is a single replicate. Blue dots indicate ACPA− RA SF and red dots indicate ACPA+ RA SF.
Single-cell sequencing map of CD4+ T-cell subsets in RA
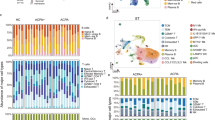
To further characterize cytotoxic CD4+ T cells in SFMC, we performed 10X 5´single-cell sequencing in combination with TCRαβ sequencing on purified CD4+ T cells from paired SFMC and peripheral blood mononuclear cells (PBMC) from 11 ACPA+ RA patients and 4 ACPA− patients (Fig. 2a and Supplementary Data 1). After quality control (Supplementary Figs. 3 and 4a), we obtained transcriptomic data from a total of 166,944 cells. Unsupervised analysis of this transcriptomic data generated 12 total clusters that were annotated based on both known cell-type markers and enrichment for cell-type-specific gene modules (Fig. 2b, c, Supplementary Figs. 4b, c and 5a, b, and Supplementary Data 2 and 3). Differentially-expressed genes (DEGs) were also compared to clusters described in PB of healthy donors19 and the Azimuth reference single-cell mapping database20,21 using the EnrichR interface22 (Supplementary Fig. 5c). Together, using this approach we identified a naive CD4+ T-cell cluster (cluster 1) expressing high levels of SELL, CCR7, and IL7R23, central memory CD4+ T cells (cluster 3) expressing LTB and KLF2; effector CD4+ T cells (cluster 4) expressing KLRB1 and GZMA; Tregs (cluster 5) expressing FOXP3 and IL2Ra24; proliferating CD4+ T cells (cluster 11) expressing MKI67 and STMN1; cytotoxic CD4+ T cells (cluster 6) expressing cytotoxicity related genes including NKG7, GZMH, PRF1 and ZNF683 (Hobit) among others; activated CD4+ T cells (cluster 12) expressing CD38 and HLA-DR; SESN3high memory/effector CD4+ T cells (cluster 7) and EGR1high naive CD4+ T cells (cluster 10). Interestingly, SESN3high CD4+ T cells presented a cytokine signature matching with IL-2, TNF, or IL-6 signatures depending on the comparative datasets (Supplementary Fig. 5c). Cells from cluster nine expressed mitochondrial encoding genes and were named Humanin CD4+ T cells as previously described in psoriatic arthritis patients25. Finally, based on the expression of CXCL13, MAF, TOX, PRDM1, TIGIT and PDCD17,9,26, two TPH cell clusters were subclassified as CXCL13high TPH (cluster 2) and CXCL13low TPH (cluster 8). We further conducted our analysis on paired PB and SF compartment from four ACPA− and four ACPA+ RA patients (Fig. 2b, see “Single-cell sequencing of RA samples” in “Methods” and Supplementary Fig. 3). As expected, naive and central memory CD4+ T-cell frequencies were decreased in SF as compared to PB (Fig. 2d). Humanin and EGR1high CD4+ T-cell cluster frequencies were also decreased in SF. The frequency of Tregs was increased in SF as previously described by us and others27,28. Similarly, CXCL13high TPH, CXCL13low TPH, effector CD4+, proliferating CD4+, and activated CD4+ T-cell clusters frequencies were all increased in SF as compared to PB. Of note, the cytotoxic CD4+ T-cell cluster was equally frequent in PB and SF. These differences remain significant even when exclusively looking at the memory T-cell compartment (Supplementary Fig. 6a). No significant differences were observed between ACPA− and ACPA+ patients (Supplementary Fig. 6b, c). Of note, the frequency of the different clusters was not significantly different in patients undergoing corticosteroid, methotrexate, or biologics treatments at the time of sampling (Supplementary Fig. 7a). Hence, single-cell transcriptomic data identify several CD4+ T-cell subsets, including a subset of cytotoxic CD4+ T cells and two distinct TPH clusters in SF of RA patients.
a Technical workflow including CD4+ T-cell enrichment and 10X 5′ single-cell sequencing coupled to TCRαβ sequencing on 15 paired PB and SF from RA patients (11 ACPA+, 4 ACPA−). b UMAP displaying 12 CD4+ T-cell clusters in combined SF and PB (upper panel, n = 15 RA (11 ACPA+, 4 ACPA−), n = 166,944 cells), SF (middle panel, n = 8 RA (4 ACPA+, 4 ACPA−), n = 31,089 cells) and PB (lower panel, n = 8 RA (4 ACPA+, 4 ACPA−), n = 48,167 cells). c Heatmap showing selected differentially-expressed genes (DEGs) in the different CD4+ T-cell clusters in combined PB and SF, P < 0.01, model-based analysis of single-cell transcriptomics (MAST). d Frequencies of CD4+ T-cell clusters in PB and SF from n = 8 RA patients (4 ACPA+, 4 ACPA−). Circle indicates ACPA+ RA patients, triangle indicates ACPA− patients. Line represents median, two-tailed Mann–Whitney U test.
GPR56 delineates TPH CD4+ T cells in ACPA+ RA synovial fluid
Since GPR56 expression was upregulated on 26% of CD4+ T cells in SF of ACPA+ RA patients (Fig. 1a, b), we further investigated ADGRG1 (encoding GPR56) in the single-cell dataset. In PB, ADGRG1 was mainly expressed by cytotoxic CD4+ T cells (cluster 6), that are also characterized by the expression of GZMA, GZMB, NKG7, PRF1 and ZNF683 (Hobit) (Supplementary Fig. 8a–c). At the protein level, flow cytometry experiments validated that, in circulating CD4+ T cells, GPR56 expression correlates with the expression of cytotoxic effector molecules PRF1, GZMB, GZMA, NKG7, and the transcription factors Eomes and Hobit (Supplementary Fig. 8d–f) confirming a previous report18. Still, a fraction of circulating GPR56+ CD4+ T cells expressed CXCL13 after CD3/CD28 stimulation (Supplementary Fig. 9). Interestingly, in SF, ADGRG1 expression was mainly detected in CXCL13high TPH (cluster 2), CXCL13low TPH (cluster 8) and proliferating CD4+ T cells (cluster 11) (Fig. 3a, Supplementary Fig. 10a–c). We further examined the expression of known TPH-associated markers7,9,26 and observed that ADGRG1 expression overlapped with the expression of PDCD1 (encoding PD-1), CXCL13, HLA-DRB1, MAF, ICOS, PDRM1 (encoding BLIMP-1), and TOX in the two TPH states (Fig. 3a). At the RNA level, the two TPH clusters were characterized by different levels of ADGRG1, PDCD1, CXCL13, HLA-DRB1, MAF, and PDRM1 (Supplementary Fig. 11a, b). In particular, in ACPA+ SF, CXCL13, PDCD1, and ADGRG1 were increased in CXCL13high TPH whereas PRDM1 was increased in CXCL13low TPH cells. The expression of TOX was not significantly different between the two subsets (Supplementary Fig. 11a). The same differences were also observed in TPH cells from ACPA− SF, in particular for ADGRG1 and PRDM1 (Supplementary Fig. 11b). Flow cytometry experiments confirmed previous reports7,9 showing an increased frequency of PD-1high and PD-1 expression on CD4+ T cells in ACPA+ as compared to ACPA− RA patients (Fig. 3b and Supplementary Fig. 12). In SF, GPR56 expression defines the subset of TPH cells (Fig. 3b) as illustrated by the increased expression of GPR56 on PD-1high as compared to PD-1neg CD4+ T cells in both ACPA+ and ACPA− RA patients. ACPA− TPH cells, however, expressed lower levels of GPR56 as their ACPA+ counterparts (Fig. 3b). Of note, these frequencies were not different depending on the treatment at the time of sampling (Supplementary Fig. 7b). We confirmed that the two TPH subsets could be distinguished based on different levels of GPR56 expression within PD-1high CD4+ T cells (Fig. 3c, gating strategy in Supplementary Fig. 1b). PD-1high GPR56+ CD4+ T cells expressed a higher level of MHC-II, PD-1, and CXCL13 as compared to PD-1high GPR56low CD4+ T cells (Fig. 3c–e). However, PD-1high GPR56+ CD4+ T cells also expressed higher levels of BLIMP-1 although PDRM1 gene expression was lower in this subset (Fig. 3e, lower panel and Supplementary Fig. 11a, b). While the frequency of PD-1high GPR56+ CD4+ T cells was lower in ACPA− SF (Fig. 3c, left panel), they also display increased CXCL13, MHC-II, and PD-1 expression as compared to their PD-1high GPR56low counterparts (Fig. 3c–e). GPR56 was not detected on cytotoxic CD4+ T cells in SF (Supplementary Fig. 10d, e) and the expression of ADGRG1 was low in SF cytotoxic CD4+ T cells (Supplementary Fig. 10b, c). In NK cells, the expression of GPR56 is regulated by cleavage and endocytosis after activation29 and a similar phenomenon could also contribute to the absence of GPR56 on cytotoxic CD4+ T cells in SF. We, therefore, investigated GPR56 expression after three hours of CD3/CD28 beads or PMA/ionomycin stimulation on PBMC and SFMC from RA patients. While three hours of CD3/CD28 stimulation had no effect on GPR56 expression, PMA/ionomycin induced downregulation of GPR56 surface expression on PB and SF CD4+ T cells (Supplementary Fig. 13). These experiments show that rapid GPR56 downregulation can also occur on GPR56+ CD4+ T cells through TCR-independent mechanisms. Altogether, these data indicate that, while GPR56 is mainly expressed on cytotoxic CD4+ T cells in PB, it also delineates the subset of PD-1high CXCL13high TPH cells which is increased in ACPA+ RA SF.
a 2-D dot plots showing the expression of selected genes in the different CD4+ T-cell clusters in ACPA+ and ACPA− SF (circle size indicates the percentage of cells expressing, color intensity indicates average expression) (n = 4 ACPA+, n = 4 ACPA− RA SF). b Representative flow cytometry dot plot of GPR56 expression within PD-1high (TPH) and PD-1− (non-TPH) CD4+ T cells in ACPA+ (upper panel) and ACPA− (lower panel) RA SF, quantified in n = 9 ACPA− SF and n = 12 ACPA+ SF, P = 0.0006 (%PD-1high), P < 0.0001 (%GPR56 in PD-1− versus PD-1high in ACPA+), P < 0.0001 (%GPR56 in PD-1− versus PD-1high in ACPA−), P = 0.0007 (%GPR56 in PD-1high in ACPA− versus ACPA+). c Left panel, representative flow cytometry dot plot of the two TPH states (PD-1highGPR56low and PD-1highGPR56+) and quantification of their frequency in SF, ns (%PD-1highGPR56low), P = 0.0005 (%PD-1highGPR56+) in ACPA+ versus ACPA−. Middle panel, MHC-II MFI: P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56low), P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56+) in ACPA− SF; P = 0.0001 (PD-1−GPR56− versus PD-1highGPR56low), P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56+), P = 0.0192 (PD-1highGPR56low versus PD-1highGPR56+) in ACPA+ SF; P = 0.0381 (PD-1highGPR56low in ACPA− versus ACPA+), ns (PD-1highGPR56+ in ACPA− versus ACPA+). Right panel, PD-1 MFI: P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56low), P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56+) in ACPA− SF; P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56low), P < 0.0001 (PD-1−GPR56− versus PD-1highGPR56+), P = 0.0059 (PD-1highGPR56low versus PD-1highGPR56+) in ACPA+ SF; P = 0.0491 (PD-1highGPR56low in ACPA− versus ACPA+), P = 0.0056 (PD-1highGPR56+ in ACPA− versus ACPA+). (n = 9 ACPA−, n = 11 ACPA+, MHC-II) (n = 9 ACPA−, n = 12 ACPA+, PD-1). d Representative flow cytometry dot plots of CXCL13 and BLIMP-1 expression within PD-1−GPR56− (non-TPH) and PD-1highGPR56low and PD-1highGPR56+ (2 TPH states) in CD4+ T cells in ACPA+ and ACPA− RA SF, quantified in e in n = 5 ACPA− SF and n = 5 ACPA+ SF. Upper panel, %CXCL13: P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56low), P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56+), P = 0.0079 (PD-1highGPR56low versus PD-1highGPR56+) in ACPA− SF; P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56low), P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56+), P = 0.0317 (PD-1highGPR56low versus PD-1highGPR56+) in ACPA+ SF; ns (PD-1highGPR56+ in ACPA− versus ACPA+). Lower panel, %BLIMP-1: P = 0.0159 (PD-1−GPR56− versus PD-1highGPR56low), P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56+), ns (PD-1highGPR56low versus PD-1highGPR56+) in ACPA− SF; P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56low), P = 0.0079 (PD-1−GPR56− versus PD-1highGPR56+), P = 0.0079 (PD-1highGPR56low versus PD-1highGPR56+) in ACPA+ SF; ns (PD-1highGPR56+ in ACPA− versus ACPA+). b, c Data are from a pool of nine independent experiments where a circle is a single replicate. e Data are from a pool of five independent experiments where a circle is a single replicate. b, c, e Line represents median, two-tailed Mann–Whitney U test. Blue dots indicate ACPA− RA SF and red dots indicate ACPA+ RA SF. ns not significant, MFI mean fluorescence intensity.
GPR56+ CD4+ T cells display distinct tissue-resident memory receptors
Besides being an inhibitory receptor, PD-1 is also included in the signature associated to T-cell tissue residency30 and implicated in follicular helper T-cell maintenance in germinal centers31. We therefore assessed the expression of known tissue-resident memory (TRM) T-cell receptors: ITGA1 (CD49a), CD69, CXCR6 but also CX3CR1 which is downregulated on TRM cells30. We also evaluated the expression of the inhibitory receptor LAG-3 which is often co-expressed with PD-1 on tumor-infiltrating lymphocytes32. In ACPA+ SF, we found that LAG-3 was mainly expressed on TPH cells (cluster 2 and 8) and proliferating T cells (cluster 11) (Fig. 4a). A similar tendency was also observed for the expression of CXCR6 in SF. Since we previously observed that GPR56 delineates the subset of TPH cells, we assessed the expression of these receptors in the context of GPR56 by flow cytometry. LAG-3, CXCR6, and CD69 frequencies were enriched on synovial GPR56+ CD4+ T cells (Fig. 4b, c, gating strategy in Supplementary Fig. 1c) in ACPA+ SF. In contrast, CD49a frequency was higher on GPR56− CD4+ T cells. In ACPA− SF, GPR56+ CD4+ T cells also showed a higher frequency of CXCR6, CD69, and LAG-3 as compared to GPR56− CD4+ T cells. However, LAG-3 frequency was higher on CD4+ T cells in ACPA+ SF as compared to ACPA− SF (Fig. 4d) and correlated with CCP positivity (Fig. 4e). In CD8+ T cells, CD49a was the only receptor for which an increased frequency was observed in ACPA+ SF (Supplementary Fig. 14). These data indicate that, in SF, GPR56 expression correlates with the concomitant expression of a distinct set of tissue-resident memory receptors on CD4+ T cells: CXCR6 and CD69 and the inhibitory receptor LAG-3.
a 2-D dot plots showing the expression of selected genes in the different CD4+ T-cell clusters in ACPA+ and ACPA− SF (circle size indicates the percentage of cells expressing, color intensity indicates average expression) (n = 4 ACPA+, n = 4 ACPA− RA SF). b Representative flow cytometry dot plots showing the expression of LAG-3, CXCR6, CD69, CD49a, and CX3CR1 on GPR56+ and GPR56− CD4+ T cells in ACPA+ RA, quantified in ACPA+ and ACPA− SF in c) P < 0.0001 (%LAG-3), P = 0.0052 (%CXCR6), P < 0.0001 (%CD69), P < 0.0001 (%CD49a), P = 0.0379 (%CX3CR1) in GPR56− versus GPR56+ in ACPA+ SF; P = 0.0002 (%LAG-3), P = 0.0006 (%CXCR6), P = 0.0078 (%CD69), ns (%CD49a), P = 0.0262 (%CX3CR1) in GPR56− versus GPR56+ in ACPA− SF; P < 0.0001 (%LAG-3) in GPR56+ in ACPA− versus ACPA+; P = 0.0052 (MFI LAG-3), P = 0.0007 (MFI CXCR6), P = 0.0002 (MFI CD69), P = 0.0196 (MFI CD49a) in GPR56− versus GPR56+ in ACPA+ SF; ns (MFI LAG-3), P = 0.0002 (MFI CXCR6), P = 0.0002 (MFI CD69), ns (MFI CD49a) in GPR56− versus GPR56+ in ACPA − SF; (n = 10 ACPA+, n = 8 ACPA−, LAG-3, CXCR6, CD69, CD49a) (n = 8 ACPA+, n = 7 ACPA−, CX3CR1). White dots indicate GPR56− CD4+ T cells and black dots indicate GPR56+ CD4+ T cells. d Expression of LAG-3, CXCR6, CD69, CD49a and CX3CR1 on CD4+ T cells in SF; P = 0.0005 (%LAG-3 in ACPA− versus ACPA+); (n = 8 ACPA−, n = 10 ACPA+, LAG-3, CXCR6, CD69, CD49a) and (n = 7 ACPA−, n = 8 ACPA+, CXC3CR1). Blue dots indicate ACPA− RA SF and red dots indicate ACPA+ RA SF. c, d Line represents median, two-tailed Mann–Whitney U test. Data are from a pool of eight independent experiments where a circle is a single replicate. e Correlation between the frequency of LAG-3 in CD4+ T cells in RA SF and the levels of anti-CCP (cyclic citrullinated peptide) antibodies (n = 18), P < 0.0001, Spearman two-tailed test. ns not significant.
Clonally expanded CXCL13high TPH CD4+ T cells in RA synovial fluid
Next, we examined the expanded TCR clones in relation to the aforementioned CD4+ T-cell clusters (Supplementary Data 4 and 5). A clone was defined by at least 2 cells sharing the same CDR3 amino acid sequence from both TCRα and TCRβ chains (Supplementary Data 6a). Of note, TCR recovery was low in patient RA12 which was excluded from further clonality analysis. In SF, the most expanded clones were found in CXCL13high TPH (median frequency 45.7%), Treg (median frequency 28.7%), effector CD4+ (median frequency 11.3%) and cytotoxic CD4+ (median frequency 7.2%) T-cell clusters (Fig. 5a–c and Supplementary Data 6b). In all four ACPA+ RA patients CXCL13high TPH cells contained most of the expanded clones. In two out of three ACPA− RA patients (RA8, RA9), Tregs represented the most expanded clones in SF (Fig. 5b, left panel, Fig. 5c, left panel and Supplementary Data 6b). This observation prompted us to analyze the frequency of FOXP3+ CD4+ T cells which was slightly increased in ACPA− SF (Fig. 5d). FOXP3+ CD4+ T-cell frequency in SF was not different depending on treatment at the time of sampling (Supplementary Fig. 7b). In blood, most of the expanded clones originated from central memory CD4+ T cells (median frequency 45.8%) and cytotoxic CD4+ T cells (median frequency 32.6%) (Fig. 5b, right panel, Fig. 5c, right and Supplementary Data 6c) irrespective of their ACPA status.
a UMAP plots showing expanded clones in ACPA+ (n = 4) and ACPA− (n = 4) RA PB and SF, colored based on sample size. Orange dots indicate small clones (1e-4 < X ≤0.001), purple dots indicate medium size clones (0.001 < X ≤0.01), green dots indicate large size clones (0.01 < X ≤0.1). Each dot displays a cell. b Stacked barplots displaying the phenotype of the SF (left) and PB (right) expanded clones (n ≥ 2 cells) in each RA patient, quadrant represents an individual clone, total number of clones per patient are indicated above each column. c Frequency of expanded clones within each CD4+ T-cell cluster in SF (left panel) and PB (right panel) for each RA patient (n = 4 ACPA+, 3 ACPA−). d Frequency of FOXP3+ cells among CD4+ T cells in ACPA− (n = 9) and ACPA+ (n = 12) RA SF, P = 0.027. Data are from a pool of nine independent experiments where a circle is a single replicate. Blue dots indicate ACPA− RA SF and red dots indicate ACPA+ RA SF. Line represents median, two-tailed Mann–Whitney U test.
Overall, these data show that in SF of RA patients most of the expanded clones are found within the CXCL13high TPH, the Tregs, the effector and the cytotoxic CD4+ T-cell subsets. In blood, the expanded T-cell clones were mostly found within cytotoxic and central memory CD4+ T cells.
The two TPH subsets are functionally related
Further, we examined the overlapping of all CDR3 sequences between SF CD4+ T-cell subsets that would be indicative of a possible shared differentiation pathway (Fig. 6a, b). Of note, no common CDR3 sequences were observed across patients. In SF, CXCL13high TPH showed clonal overlap with CXCL13low TPH (Jaccard Index (JI):0.038), proliferating (JI:0.031), effector (JI:0.023) and cytotoxic CD4+ T cells (JI:0.013) (Fig. 6b). SF cytotoxic CD4+ T cells showed also clonal overlap with effector (JI:0.016) and proliferating CD4+ T cells (JI:0.013). CXCL13low TPH presented a clonal overlap with CXCL13high TPH (JI:0.038), proliferating (JI:0.028), effector (JI:0.014), and cytotoxic CD4+ T cells (JI:0.007). SF Tregs showed clonal overlap with proliferating CD4+ T cells (JI:0.02). We then focused our analysis on expanded clones in CXCL13high TPH, Tregs, effector CD4+ T cells and cytotoxic CD4+ T cells (Supplementary Data 7). A median of 59% of the CXCL13high TPH expanded clones shared CDR3 sequences with cells from other clusters (either clones or unique cells) (Supplementary Data 6d). These shared sequences were identified in all patients. A median frequency of 28% of these clones was shared with proliferating CD4+ T cells, 25% with CXCL13low TPH, 19% with effector CD4+ T cells, and 6% with cytotoxic CD4+ T cells (Fig. 6c and Supplementary Data 6e). Treg clones shared a median of 42% and 17% of their CDR3 sequences with proliferating CD4+ T cells and CXCL13high TPH cells, respectively. Effector CD4+ T-cell clones shared a median of 26% and 10% of their CDR3 sequences with CXCL13high TPH and cytotoxic CD4+ cells, respectively. Cytotoxic CD4+ T-cell clones shared a median of 26% and 6% of their CDR3 sequences with CXCL13high TPH and effector CD4+ T cells. Of note, for effector and cytotoxic CD4+ T cells, sharing was primarily coming from two patients (RA10, and RA11, Supplementary Data 6e and 7). Cytotoxic CD4+ T cells from PB and SF also presented clonal overlap (JI:0.036). To further understand the cell fate of CXCL13low TPH cells in the context of cytotoxic CD4+ T cells, we sorted GPR56low PD-1high CD4+ TPH cells and stimulated with CD3/CD28 beads (gating strategy in Supplementary Fig. 1D). We observed an increased PD-1 expression and a tendency towards increased GPR56 and BLIMP-1 expression (Fig. 6d). Perforin-1 expression, however, did not dramatically increase. Of note, CXCL13 did not increase after TCR stimulation, possibly due to the lack of additional inducers such as TGFβ and IL-2 neutralizing conditions as previously described26. To further evaluate cell differentiation across clusters, we performed an RNA-velocity analysis (scVelo) which estimates future cell states based on the ratio of detected spliced and unspliced mRNAs transcripts33. Unsurprisingly, we observed a progression from naive CD4+ T cells into central memory and effector CD4+ T cells validating our approach (Fig. 6e). In ACPA+ SF, both CXCL13low TPH and effector CD4+ T cells led to CXCL13high TPH cells (Fig. 6e, right panel). This transition from CXCL13low to CXCL13high TPH was less obvious in ACPA− SF (Fig. 6e, left panel). In addition, the cytotoxic CD4+ T-cell final differentiation stage was originating from SESN3+ and effector CD4+ T cells in both ACPA− and ACPA+ RA. Hence, effector CD4+ T cells project into two independent branches leading to TPH in one direction and cytotoxic CD4+ T cells in the other direction. Tregs were connected to central memory and proliferative CD4+ T cells. These lines of evidence suggest that CXCL13low and CXCL13high TPH in SF are functionally and developmentally related, possibly originating from effector CD4+ T cells while cytotoxic CD4+ T cells potentially arise from other effector CD4+ T cells independent of the TPH state.
a Chord diagrams showing the interconnectivity between CD4+ T cells sharing the same CDR3 (paired TCRα, and TCRβ chains) amino acid sequences within CD4+ T-cell clusters in RA SF (left panel) and between compartments in selected clusters (right panel) (n = 4 ACPA−, n = 4 ACPA+). b Jaccard overlap quantifications for clonal overlap between cell clusters across compartments (n = 4 ACPA−, n = 4 ACPA+). c Percentage of shared expanded clones (n ≥ 2 cells) shared with selected subsets among total cell clones, n = 7 RA patients (n = 4 ACPA+, 3 ACPA−). d Representative flow cytometry dot plots showing the expression of PD-1, GPR56, CXCL13, BLIMP-1, and PRF1 in control (upper panel) or after 48 h CD3/CD28-beads activation of TPH cell states (lower panel) (PD-1highGPR56low and PD-1highGPR56+ from ACPA+ SF), quantified in n = 3 ACPA+ RA patients. d Data are from a pool of three independent experiments where a circle is a single replicate. Line indicates median, two-tailed Wilcoxon paired test, P = 0.0005 (MFI PD-1 on PD-1highGPR56low after activation), P = 0.0740 (MFI PD-1 on PD-1highGPR56+ after activation), P = 0.0869 (MFI GPR56 on PD-1highGPR56low after activation), P = 0.0276 (MFI GPR56 on PD-1highGPR56+ after activation), P = 0.0563 (% BLIMP-1 on PD-1highGPR56low after activation), P = 0.0775 (% BLIMP-1 on PD-1highGPR56+ after activation), P = 0.019 (% PRF1 on PD-1highGPR56low after activation). e Velocity plots showing the phenotype directionality between the different CD4+ T-cell clusters in RA split in ACPA− (left panel) and ACPA+ (right panel) (n = 4 ACPA−, n = 11 ACPA+, pool PB and SF). f Graphical summary showing the proposed developmental link between the two TPH subsets, effector and cytotoxic CD4+ T cells in SF as well as the identified receptors.
Discussion
Leveraging single-cell technology of CD4+ T cells in PB and SF of RA patients, we dissected the TPH CD4+ T-cell subset at the single-cell level showing that it is composed of two independent clusters with different CXCL13 expression levels. So far, the TPH CD4+ T-cell subset was only defined by high expression of PD-1 and MHC-II while more accurate markers were still lacking. We show that GPR56 clearly delineates the CXCL13high TPH subset and associates with the expression of tissue-resident memory receptors CXCR6 and CD69 and the inhibitory receptor LAG-3. We also identify an increased frequency of cytotoxic CD4+ T cells in ACPA+ RA SF. Finally, we show that CXCL13high TPH, Treg, T effector, and cytotoxic CD4+ T cells are clonally expanded in RA SF. Together, these data provide a refined characterization of TPH and cytotoxic CD4+ T cells, two important CD4+ T-cell subsets in RA (Fig. 6f).
In synovial tissue of ACPA+ RA patients, TPH CD4+ cells produce CXCL137,9,34, localize with B cells and have the ability to induce in vitro plasma cell differentiation through IL-21 production7. TPH cells are defined by high expression of PD-1 and MHC-II, the cytokines CXCL13, IL-21, and IL-10, and the transcription factors BLIMP-1, TOX, MAF, and SOX47,9,26. TPH CD4+ T-cell differentiation is induced by TGFβ in IL-2 neutralizing conditions26. Single-cell sequencing identified two distinct clusters of TPH cells: CXCL13low TPH cells and CXCL13high TPH cells. We postulated that these two clusters correspond to two states of differentiation: CXCL13low TPH CD4+ T cells could be the precursors of the more differentiated cytokine-producing CXCL13high TPH CD4+ T cells. In line with this hypothesis, common TCR sequences were observed between CXCL13low TPH cells and CXCL13high TPH cells clusters suggesting a common origin between these two subsets. CD3/CD28 stimulation of GPR56low PD-1high TPH cells also induced an increased PD-1 upregulation compatible with this scenario. Finally, RNA velocity and TCR clonality analysis show a connection between effector memory CD4+ T cells and CXCL13high TPH, indicating that the precursors of TPH cells are probably contained within the effector CD4+ T-cell population which is enriched in synovial fluid.
CXCL13high TPH CD4+ T cells were also detected in ACPA− RA SF but at a much lower frequency than in ACPA+ patients. In ACPA− patients, these few CXCL13high TPH also presented an increased expression of bona fide TPH markers (MHC-II, PD-1, CXCL13, BLIMP-1) but lower levels of LAG-3, PD-1, and GPR56 than in ACPA+ patients. The expression of these inhibitory receptors might be a sign of persistent T-cell activation in the ACPA+ synovial joint. We also identified clonally expanded Tregs in SF of two ACPA− RA patients and a slight increase in Treg frequency. Altogether, these data suggest that the differentiation towards CXCL13high TPH cells is increased in the joint of ACPA+ RA as compared to ACPA− RA patients. In line with this hypothesis, the RNA-velocity analysis of ACPA+ CD4+ T cells revealed a trajectory from CXCL13low to CXCL13high TPH. An increased frequency of PD-1high CD4+ T cells was recently identified in leukocyte-rich RA synovial tissues8. We confirm the presence of this subset also in SF with an increased frequency in ACPA+ RA patients. While SF analysis allows a refined analysis of many immune cells and the downstream clonality analysis, a limitation is that these samples are originating from patients with long disease duration and under immunosuppressive drugs. In our dataset, we did not find any specific association with ongoing medication but, interestingly, the only ACPA− patient whose SF presented CXCL13high TPH cell clonal expansion was not under any immunosuppressive medication at time of sampling. Longitudinal studies will inform about the possible effects of current treatments on the expansion and maintenance of these CD4+ T-cell subsets.
Expanded TCR clones have been identified in synovial tissues and fluid from RA patients using bulk TCRβ sequencing5. In blood, expanded cytotoxic CD4+ CD8null cells present with a bias in their TCRVβ usage35,36. We found that expanded clones in the blood were originating from the CD4+ cytotoxic T-cell subset. Clonally expanded cytotoxic CD4+ T cells were also observed in RA SF, although at a lower degree than the CXCL13high TPH cells. Overlap clonality between cytotoxic clones in PB and SF suggest their recirculation. Expansions of cytotoxic CD4+ T cells are frequently observed in chronic viral infections, and they are capable of directly killing EBV-infected B cells in an MHC-II-dependent manner37. Our data show that cytotoxic CD4+ T cells are enriched in SF of ACPA+ RA patients and correlate with CCP titers, suggesting that these cells are mainly implicated in the subset of autoantibody-positive RA. Still, so far, their autoreactive properties have not been investigated in the context of citrulline immunity. The differentiation processes that lead to the differentiation of cytotoxic CD4+ T cells are not completely elucidated, but IL-238, IL-1539, and type I IFNs40 contribute to their development. The RNA-velocity analysis suggests that cytotoxic CD4+ T cells originate from effector CD4+ T cells and from the subset of SESN3+ memory T cells. Although the memory SESN3+ CD4+ T-cell subset has not been extensively studied before, it has been identified in skin biopsies from patients with psoriasis41. Interestingly, we detected cytokine-related signatures in SESN3+ CD4+ T cells which could be implicated in their differentiation into cytotoxic CD4+ T cells. Further studies are warranted to dissect the differential pathways leading to cytotoxic CD4+ and CXCL13high TPH cells, two CD4+ T-cell types enriched in ACPA+ RA.
GPR56 is an adhesion G-protein-coupled receptor encoded by the ADGRG1 gene implicated in numerous migration/adhesion processes including neuron migration42 and tumor growth inhibition43. In NK cells, GPR56 is an inhibitory receptor binding to the tetraspanin CD8129. We confirmed data from a previous report showing that GPR56 is also a marker of peripheral cytotoxic CD4+ cells18. At the site of inflammation, however, we discovered that GPR56 was highly expressed on TPH cells in ACPA+ RA patients and could be used to identify TPH CD4+ T cells. In SF CD4+ T cells, GPR56 expression correlated with the expression of the inhibitory receptors LAG-3 and PD-1 as well as CXCR6 and CD69, receptors expressed on tissue-resident memory T cells30. Similarly, in multiple sclerosis, CD8+ T cells from active lesions exhibit a tissue-resident memory phenotype (CD69, CD103, PD-1, CD49a) associated with an intermediate expression of GPR56 without granzyme B expression44. In mice, Blimp-1 and Hobit mediates the transcriptional program associated with T-cell tissue residency45. In ACPA+ SF, BLIMP-1 was expressed on TPH CD4+ cells presenting with tissue-resident memory receptors whereas Hobit was only expressed on cytotoxic CD4+ T cells suggesting that, BLIMP-1 could drive tissue residency of TPH CD4+ T cells in the synovial joint. GPR56 might be implicated in the migration and/or maintenance of TPH CD4+ T cells in the synovial joints where its ligand remains to be determined. In SF, cytotoxic CD4+ T cells expressed low levels of ADGRG1 and had no detectable GPR56 surface expression. We showed that similar to what has been described in NK cells29, PMA/Ionomycin stimulation induced a rapid GPR56 downregulation on GPR56+ CD4+ T cells. In vivo, the inflammatory milieu might therefore contribute to GPR56 downregulation on SF cytotoxic CD4+ T cells.
In summary, we identify two clusters of TPH CD4+ T cells and observe clonally expanded CXCL13high TPH, Tregs, effector, and cytotoxic CD4+ T cells in RA SF. We show that GPR56 is a receptor that should be used to identify CXCL13high TPH CD4+ cells. Future studies aiming at understanding the function of GPR56 in TPH CD4+ and cytotoxic CD4+ T cells will contribute to evaluating the feasibility of targeting this receptor in ACPA+ RA.
Methods
Patient and samples
This study was approved by the research ethics committee of Karolinska University Hospital, Sweden and all patients signed informed consent according to the Declaration of Helsinki. All patients had established disease, based on the 1987 criteria from the American College of Rheumatology. Synovial fluid from ACPA− (n = 13) and ACPA+ (n = 16) RA patients were collected at several visits at the Rheumatology Clinic of Karolinska University Hospital, Stockholm (Supplementary Data 1). In n = 12 ACPA− and n = 10 ACPA+ RA patients, paired PBMCs were also available. PBMC and SFMC were isolated by Ficoll separation (GE Healthcare) and cryopreserved in fetal bovine serum (FBS) with 10% DMSO in liquid nitrogen.
Flow cytometry
In total, 1 × 106 PBMC and 1 × 106 SFMC were labeled with LIVE DEAD Fixable NEAR IR Dead Cell Stain Kit (Invitrogen, ref: L10119) and further stained with fluorescently labeled antibodies (Supplementary Data 8). ACPA− and ACPA+ SF and PB were always run in the same experiment. Depending on the number of recovered cells, 2–5 flow cytometry panels were run (Supplementary Data 9). For intracellular stainings, cells were fixed and permeabilized using the FOXP3 permeabilization buffer kit (ebioscience, ref: 00–5523) according to the manufacturer´s protocol. In some conditions, 1 × 106 PBMC and 1 × 106 SFMC were activated with CD3/CD28 dynabeads (ThermoFischer Scientific, ref: 11131D, one bead/cell) or Phorbol-12-myristate-13-acetate (PMA, Calbiochem, ref: 10,524400 ng/mL) plus Ionomycin (Calbiochem, ref: 1,407950 µM) in RPMI 10% FBS in the presence of Brefeldin-A (Sigma-Aldrich, ref: B6542, 5 µg/mL) for 3 h. Samples were run on the BD LSR Fortessa and flow cytometry data were analyzed with the FlowJo software (version 10.07.1) (gating strategies, Supplementary Fig.1)
Sorting and T-cell activation
SFMC (n = 3, ACPA+) were labeled with LIVE DEAD Fixable NEAR IR Dead Cell Stain Kit (Invitrogen, ref: L10119) and further stained with fluorescently labeled antibodies (Supplementary Data 8, Supplementary Data 9, panel 6, Supplementary Fig. 1). GPR56lowPD-1high and GPR56+PD-1high TPH were subsequently cell sorted on the BD Influx. Sorted cells were incubated with CD3/CD28 dynabeads (1bead/cell, ThermoFischer, ref: 11131D) in RPMI 10%FBS for 48 h. Brefeldin-A (Sigma-Aldrich, ref: B6542, 5 µg/mL) was added during the last 4 h. Samples were then processed as described in “Flow cytometry”.
Statistical analysis
Flow cytometry results were analyzed with the Prism 7 software (GraphPad, San Diego, CA, USA). Statistical tests performed are indicated in the figure legends.
Single-cell RNA sequencing of RA samples
CD4+ T cells were enriched from cryopreserved PBMC and SFMC samples using the EasySep human CD4 Positive Selection Kit II (STEMCELL Technologies, Catalog# 17852) (Supplementary Fig. 3). In experiment 10X-1 (Supplementary Data 1), for samples from RA1–7, following CD4+ T-cell isolation, cells were counted, hashed with TotalSeq-C0251 (ref: 394661) and TotalSeq-C0253 (ref: 394665) anti-human antibodies, Biolegend) and pooled per patient (i.e., pool 1 = PB and SF from patient 1, pool 2 = PB and SF from patient 2, etc.). The pooled cells were then counted again and immediately loaded onto the chromium chip G using the standard protocol for the Chromium single-cell 5’ kit v1 (10x Genomics, Inc). Following Gel Bead-in Emulsion (GEM) generation, samples were processed using the standard manufacturer’s protocol. This pipeline was also used to process samples for experiment 10X-2 (Supplementary Data 1) with RA8–15 except for antibody hashing and the chromium kit version; each sample was processed independently (i.e., 1 sample/10x chromium lane; Supplementary Fig. 3) and cells were processed with the Chromium single-cell 5’ kit v2 (Dual Index). Once sequencing libraries passed standard quality control metrics, the libraries for RA1–7 were sequenced on Illumina NextSeq500/550 high output 75-cycle v2.5 kits with the following read structure: read1: 26, read2: 58, index 1: 8. Libraries for RA8–15 were sequenced on Illumina NextSeq500/550 high output 150-cycle v2.5 kits with the following read structure: read1: 26, read2: 90, index 1: 10, index 2: 10. Both sets of libraries were sequenced to obtain a read depth greater than 20,000 reads/cell for the gene-expression (GEX) libraries and greater than 5000 reads/cell for the V(D)J-enriched T Cell libraries.
Genomic alignment and digital-gene-expression generation
Following sequencing, the pooled GEX libraries were demultiplexed using bc2fastq (v2.17) with parameters (–minimum-trimmed-read-length = 10–mask-short-adapter-reads = 10–ignore-missing-positions–ignore-missing-controls) to generate FASTQ files for each patient. FASTQ files were then uploaded to Terra (www.app.terr.bio) where the raw sequencing data were mapped and quantified using STAR within the 10X Genomics Inc software package CellRanger on Cumulus (https://cumulus.readthedocs.io/en/latest/cellranger.html, snapshot 15, Cellranger 5.0.1, default parameters) with GRCh38-2020-A (https://cumulus.readthedocs.io/en/latest/cellranger.html) to generate UMI-collapsed, gene-by-cell expression matrices. For the RNA-velocity analysis, exonic and intronic alignment information was retrieved using velocyto on SevenBridges (https://www.sevenbridges.com/) and results visualized using scVelo33,46.
Data preprocessing and quality control
Aligned matrices were first filtered to remove low-quality barcodes, keeping only those with greater than 200 UMIs and less than 25% mitochondrial reads. After removing low-quality barcodes, cell doublets were then computationally removed using DoubletFinder47 with calculated doublet rates based on cell input for each sample. Using cell-type-defining markers, the cleaned datasets were filtered for non-CD4 T cells (e.g., monocytes, macrophages, CD8+ T, CD20+ B cells using CD14, CD8a, CD8b, and MS4A1). Finally, we attempted to deconvolute the multiplexed PBMC and SFMC cell populations, from RA1–7, using antibody hashes; however, due to sub-optimal tagging, we were unable to separate the compartment cell populations. To prevent this complication from occurring with RA8–15, we omitted antibody hashing from our pipeline and processed compartments separately to retain compartment-specific cell populations. Samples from RA1–15 were then merged accordingly for the subsequent unsupervised (Fig. 2b, left panel) and velocity analyses (Fig. 6e and Supplementary Fig. 3).
Unsupervised analysis and cell-type annotation
Using the Seurat19 (v4.0.0) package, filtered gene-by-cell matrices for RA1–15 were merged and then processed using a standard unsupervised workflow (i.e., normalization, scaling, dimensionality reduction, batch correction, cell clustering, and differential gene-expression analysis). First, the merged dataset was normalized and log-transformed (scaling factor = 10,000). The top 3000 highly variable genes (HVGs) were identified and used for scaling the data. During scaling, three sources of unwanted variation, cumulative UMI capture, cell cycling programs, and percent mitochondrial reads, were regressed out. Dimensionality reduction was then performed with principal component analysis (PCA) over the top 3000 variable genes and, using the JackStraw function in Seurat to identify statistically significant principal components (PCs, P < 0.05), as well as exploring individual PCs, we selected the top 70 principal components for visualization. Using these PCs, we corrected for patient-specific batch effects with the harmony48 package, and then proceeded with constructing the nearest neighbor graph and Uniform Manifold Approximation and Projection (UMAP) plots. Cells were clustered using Louvain clustering and the package clustree49 was used to generate a clustering tree and identify which resolution achieved stability. Differential gene-expression (DGE) analysis was then performed using the FindAllMarkers Seurat function with test.use set to ‘MAST’50. Finally, to further remove non-T cells that passed our initial screen, clusters with (1) low expression of CD3, (2) differentially expressed genes (DEGs) that are known markers of non-T cells, and (3) high enrichment scores for non-T-cell gene modules (MSigDB) were removed from the dataset; remaining cells were reanalyzed to reconstruct stable clusters for visualization and subsequent DGE analysis. Taking both the results of the DGE analysis and joint density approximations of known phenotypic markers, we identified 12 unique T-cell subsets: (1) naive CD4+ T cells (cluster 1; SELL+, CCR7+, IL7R+); (2) CXCL13high TPH (cluster 2; CXCL13+, ICOS+, CD3D+); (3) Central Memory CD4+ T cells (cluster 3; LTB+, KLF2+); (4) Effector CD4+ T cells (KLRB1+, GZMA+); (5) Tregs (cluster 5; FOXP3 + IL2Ra+); (6) Cytotoxic CD4+ T cells (cluster 6; NKG7+, GZMH+, PRF1+, and ZNF683+); (7) SESN3+ memory/effector CD4+ T cells (cluster 7; SESN3+); (8) CXCL13low TPH (cluster 8; CXCL13+, PRDM1+, CD3D+); (9) Humanin CD4+ T cells (cluster 9; MT-CO1+, MTRNR2L1+, MTRNR2L8+); (10) EGR1+ naïve CD4+ T cells (cluster 10; EGR1+); (11) Proliferating CD4+ T cells (cluster 11: MKI67+, STMN1+); (12) Activated CD4+ T cells (cluster 12; CD38+, HLA-DR+).
RNA velocity
The scVelo package, version 0.2.433,46 was used to model the RNA velocity of cells from volunteers RA1–15. Briefly, the RNA-velocity analysis leverages the expression relationship between spliced (exonic) and unspliced (intron-containing) RNA across selected variable genes to predict the directionality of transitions between cell types, as well as the putative driver genes of these transitions. Following the unsupervised analysis, the analyzed Seurat object was converted into an H5AD file using the SeuratDisk package [https://mojaveazure.github.io/seurat-disk/index.html], version 0.0.0.9011, and then imported into a jupyter notebook for preprocessing according to the scVelo standard pipeline: (1) genes were first normalized, selecting the top 3000 genes (pp.filter_and_normalize(adata,n_top_genes = 3000, enforece = T), (2) principal components, nearest neighbors in PCA space, and first and second-order moments of the nearest neighbors were calculated (n_pcs = 70, n_neighbors = 20), (3) using the dynamical model from scVelo, RNA velocities were then estimated (tl.recover_dynamics(adata, mode = ’dynamical’)) and (4) used to compute the velocities and velocity graph (tl.velocity and tl.velocity_graph). This approach was used for both ACPA+ and ACPA- RA cohorts.
TCR mapping
Following sequencing, V(D)J libraries for RA8–15 (experiment 10X-2, Supplementary Fig. 3, Supplementary Data 1) were demultiplexed using bc2fastq (v2.17.1.14) to generate FASTQ files for each sample, uploaded to terra, and mapped and quantified using the 10X Genomics Inc software package CellRanger on Cumulus (https://cumulus.readthedocs.io/en/latest/cellranger.html, snapshot 15, Cellranger 5.0.1, default parameters) to generate consensus annotation files for each sample. Using the scRepertoire51 package, sample-specific consensus annotation files were consolidated into a list of TCR sequencing results and then integrated with the Seurat object, containing RA8–15, using the combineExpression function. Clonotypes were called based on the CDR3 nucleotide sequence and VDJC gene (i.e., scRepertoire nomenclature “gene+nt”) and clonotype expansion were assigned based on the relative frequency. The interconnectivity between specific cell types, both within and between tissue compartments, were visualized using chord diagrams.
Reporting summary
Further information on research design and data analysis is available in the Nature Research Reporting Summary linked to this article.
Data availability
Sequence data have been deposited at the European Genome-phenome Archive (EGA), which is hosted by the EBI and the CRG, under accession number EGAS00001005241 (Dataset accession number: EGAD00001007586 https://ega-archive.org/studies/EGAS00001005241). Further information about EGA can be found on https://ega-archive.org “The European Genome-phenome Archive of human data consented for biomedical research”. All other data are available in the article and its Supplementary files or from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Codes are available at https://github.com/PfizerRD/Argyriou_Wadsworth_etal_2022.
References
Kroot, E. J. et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 43, 1831–1835 (2000).
Malmstrom, V., Catrina, A. I. & Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17, 60–75 (2017).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 18001 (2018).
Padyukov, L. et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann. Rheum. Dis. 70, 259–265 (2011).
Musters, A. et al. In rheumatoid arthritis, synovitis at different inflammatory sites is dominated by shared but patient-specific T cell clones. J. Immunol. 201, 417–422 (2018).
Chemin, K., Gerstner, C. & Malmstrom, V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front. Immunol. 10, 353 (2019).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Kobayashi, S. et al. A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium. Arthritis Rheum. 65, 3063–3072 (2013).
Chemin, K. et al. EOMES-positive CD4(+) T cells are increased in PTPN22 (1858T) risk allele carriers. Eur. J. Immunol. 48, 655–669 (2018).
Schmidt, D., Goronzy, J. J. & Weyand, C. M. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Investig. 97, 2027–2037 (1996).
Fasth, A. E., Cao, D., van Vollenhoven, R., Trollmo, C. & Malmstrom, V. CD28nullCD4+ T cells–characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand. J. Immunol. 60, 199–208 (2004).
Swain, S. L., McKinstry, K. K. & Strutt, T. M. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 12, 136–148 (2012).
Juno, J. A. et al. Cytotoxic CD4 T cells-friend or foe during viral infection? Front Immunol. 8, 19 (2017).
Knox, J. J., Cosma, G. L., Betts, M. R. & McLane, L. M. Characterization of T-bet and eomes in peripheral human immune cells. Front Immunol. 5, 217 (2014).
Raveney, B. J. et al. Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nat. Commun. 6, 8437 (2015).
Oja, A. E. et al. The transcription factor hobit identifies human cytotoxic CD4(+) T cells. Front. Immunol. 8, 325 (2017).
Peng, Y. M. et al. Specific expression of GPR56 by human cytotoxic lymphocytes. J. Leukoc. Biol. 90, 735–740 (2011).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Lozano, A. X. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. https://doi.org/10.1038/s41591-021-01623-z (2022).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Xie, Z. et al. Gene set knowledge discovery with enrichr. Curr. Protoc. 1, e90 (2021).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706, (2019).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Penkava, F. et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 11, 4767 (2020).
Yoshitomi, H. et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat. Commun. 9, 3762 (2018).
Cao, D. et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand. J. Immunol. 63, 444–452 (2006).
van Amelsfort, J. M., Jacobs, K. M., Bijlsma, J. W., Lafeber, F. P. & Taams, L. S. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 50, 2775–2785 (2004).
Chang, G. W. et al. The adhesion G protein-coupled receptor GPR56/ADGRG1 is an inhibitory receptor on human NK cells. Cell Rep. 15, 1757–1770 (2016).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Shi, J. et al. PD-1 controls follicular T helper cell positioning and function. Immunity 49, 264–274.e264 (2018).
Chihara, N. et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558, 454–459 (2018).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Manzo, A. et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. 58, 3377–3387 (2008).
Schmidt, D., Martens, P. B., Weyand, C. M. & Goronzy, J. J. The repertoire of CD4+ CD28− T cells in rheumatoid arthritis. Mol. Med. 2, 608–618 (1996).
Wagner, U. et al. Clonally expanded CD4+CD28null T cells in rheumatoid arthritis use distinct combinations of T cell receptor BV and BJ elements. Eur. J. Immunol. 33, 79–84 (2003).
Landais, E. et al. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 103, 1408–1416 (2004).
Brown, D. M., Kamperschroer, C., Dilzer, A. M., Roberts, D. M. & Swain, S. L. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 257, 69–79 (2009).
Mucida, D. et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14, 281–289 (2013).
Hua, L. et al. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J. Virol. 87, 11884–11893 (2013).
Hughes, T. K. et al. Second-strand synthesis-based massively parallel scRNA-Seq reveals cellular states and molecular features of human inflammatory skin pathologies. Immunity 53, 878–894.e877 (2020).
Piao, X. et al. G protein-coupled receptor-dependent development of human frontal cortex. Science 303, 2033–2036 (2004).
Yang, L., Friedland, S., Corson, N. & Xu, L. GPR56 inhibits melanoma growth by internalizing and degrading its ligand TG2. Cancer Res. 74, 1022–1031 (2014).
Fransen, N. L. et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730 (2020).
Mackay, L. K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e324 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Zappia, L. & Oshlack, A. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. Gigascience 7, https://doi.org/10.1093/gigascience/giy083 (2018).
McDavid, A., Finak, G. & Yajima, M. MAST: model-based analysis of single cell transcriptomics. R package version 1.16.0 https://github.com/RGLab/MAST/ (2020).
Borcherding, N., Bormann, N. L. & Kraus, G. scRepertoire: an R-based toolkit for single-cell immune receptor analysis. F1000Res 9, 47 (2020).
Acknowledgements
We thank the patients who donated samples and the medical staff at the Rheumatology Clinic of Karolinska University Hospital. Julia Boström, Gloria Rostvall, and Susana Hernandez Machado are acknowledged for organizing the sampling, storage, and administration of biomaterial. We thank Leonid Padyukov and Barbro Larsson for HLA-DR genotyping of the samples and Lena Israelsson for technical assistance with ACPA measurement. We thank Juan Sebastian Diaz Boada for bioinformatic technical assistance. Figure 6f was created using BioRender.com. This study is supported by grants from Dr. Margaretha Nilssons (K.C.), the Nanna Svartz (K.C., 2020-00349), the Börje Dahlin (K.C.) and the Ulla and Gustaf af Ugglas foundations (K.C., 2018-02627) as well as the Swedish association against rheumatism (K.C., R-969294). This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777537 (RTCure) (V.M.). The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
A.A., A.L., and C.G. performed and analyzed the flow cytometry experiments. M.H.W. performed the 10X experiments and led the single-cell analysis. S.M.C. performed preliminary 10X experiments and single-cell data analysis. A.H.H. diagnosed RA patients and collected clinical information. A.v.V. performed flow cytometry sorting. K.K. contributed to and oversaw the design, analysis, and interpretation of the single-cell experiments. A.W. contributed to the design of the study. V.M. contributed to the design of the study and the RA sample collection. A.A., M.H.W., and K.C. generated the figures. K.C designed the study, oversaw the analyses, and wrote the paper. All authors read, edited, and approved the paper.
Corresponding author
Ethics declarations
Competing interests
A.A., A.L., C.G., A.H.H., A.vV., V.M., and K.C. declare no competing interests. M.H.W., S.M.C., K.K., and A.W. are employees of Pfizer, Inc, Cambridge, MA 02139, United States.
Peer review
Peer review information
Nature Communications thanks Eva Tolosa, Dong Zhang and Femke Van Wijk for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Argyriou, A., Wadsworth, M.H., Lendvai, A. et al. Single cell sequencing identifies clonally expanded synovial CD4+ TPH cells expressing GPR56 in rheumatoid arthritis. Nat Commun 13, 4046 (2022). https://doi.org/10.1038/s41467-022-31519-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31519-6
This article is cited by
-
Single-cell characterisation of tissue homing CD4 + and CD8 + T cell clones in immune-mediated refractory arthritis
Molecular Medicine (2024)
-
Cytotoxic Tph subset with low B-cell helper functions and its involvement in systemic lupus erythematosus
Communications Biology (2024)
-
Citrulline immunity in RA: CD8+ T cells enter the scene
Nature Reviews Rheumatology (2023)
-
Transcriptomic profiling of programmed cell death 1 (PD-1) expressing T cells in early rheumatoid arthritis identifies a decreased CD4 + PD-1 + signature post-treatment
Scientific Reports (2023)
-
Research progress on the application of single-cell sequencing in autoimmune diseases
Genes & Immunity (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.