Abstract
A fully conjugated azacorannulene dimer with a large π-surface (76π system) was successfully synthesized from a fully conjugated bifunctional polycyclic aromatic azomethine ylide. This molecule represents an example of diaza[80]fullerene (C78N2) fragment molecule bearing two internal nitrogen atoms. X-ray crystallography analysis shows its boat-shaped structure with two terminal azacorannulenes bent in the same direction. The molecular shape leads to unique selective association with a dumbbell-shaped C60 dimer (C120) over C60 through shape recognition. Owing to its large π-surface and a narrow HOMO–LUMO gap, the azacorannulene dimer exhibits red fluorescence with a quantum yield of up to 31%. The utilization of the fully conjugated bifunctional azomethine ylide is a powerful method for the bottom-up synthesis of large multiazafullerene fragments, providing a step towards the selective total synthesis of multiazafullerenes.
Similar content being viewed by others
Introduction
Heterofullerene is a class of fullerenes in which one or more of its carbon atoms are substituted by heteroatoms such as nitrogen, boron, and phosphorous1,2,3,4. Since the substitution of carbon atoms within fullerene frameworks by heteroatoms is a feasible way to adjust its electronic and chemical properties, heterofullerenes are expected to find numerous potential applications in superconductors, optoelectronics, and organic semiconductors5,6,7. As such, heterofullerenes have been an important synthetic target for organic chemists. In contrast to the established methods of synthesizing fullerenes8,9, the synthetic process of heterofullerenes has long been a challenge10. The only successfully synthesized and isolated heterofullerene is an azafullerene, which contains nitrogen atoms within its framework. In 1995, Wudl et al. reported the first synthesis of azafullerene C59N in its dimeric form11. However, thus far, no multiazafullerene has been successfully synthesized and isolated on a macroscopic scale12, presumably due to its “isomeric problem”13,14,15. For instance, attempts to synthesize diazafullerene C58N216,17 leads to the generation of 23 possible isomers18,19,20. Hence, currently the synthesis and isolation of a single isomer is still an open challenge.
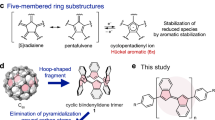
Encouraged by the success for the “bottom-up” synthesis of C60 from well-designed aromatic precursors21, researchers have sparked off immense interest amongst the “bottom-up” synthesis of multiazafullerenes from azafullerene precursors. This synthetic approach will allow for a controlled and selective introduction of nitrogen atoms into fullerenes22,23,24,25. However, appropriate synthetic protocols to synthesize nitrogen-embedded polycyclic aromatic molecules as large azafullerene fragments are still lacking26,27,28,29. As far as we are aware, only a handful of multiazafullerene fragments have been reported30,31,32,33,34. As shown in Fig. 1a, triazasumanene 130 and “hydrazinobuckybowl” 232 are considered to be partial fragments of C60-xNx. Meanwhile, the molecular fragments of higher multiazafullerenes are scarce. To our knowledge, the examples include chrysaorole 331 and a corannulene molecule fused with two π-extended pyrroles35. It is worth noting that a pyrrolo[3,2-b]pyrrole-cored nanographene36,37 may be a good precursor for the synthesis of “isomeric multiazafullerenes”, which contain heptagon as well as pentagon and hexagon. In this regard, multiazafullerene fragment molecules are an attractive target, given the fact that the synthesis of multiazafullerene C80-xNx (x ≥ 2) has not been achieved. During our continuous investigations on the synthesis of azafullerene fragment molecules38,39,40,41, we succeeded in achieving the bottom-up synthesis of diaza[80]fullerene fragment t-Bu4C72H24N2 (4a in Fig. 1b). This polycyclic aromatic molecule provides the largest π-surface of a [80]fullerene fragment bearing multiple heteroatoms.
Results and discussion
Synthesis and characterization
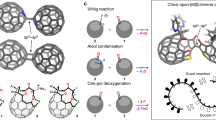
Our synthetic strategy shown in Fig. 2 started with the bromination of 2,7-diaminopyrene 542,43 to afford 1,3,6,8-tetrabromopyrene-2,7-diamine (6) in 96% yield. Subsequently, a palladium-catalyzed Suzuki-Miyaura cross-coupling reaction of 6 with an arylboronic acid 7 afforded the corresponding tetraarylated compound 8 in 40% yield. Afterward, an intramolecular cyclization of 8 by treatment with hydrogen chloride followed by air oxidation generates bifunctional iminium salt 9 in 55% yield as a mixture of regioisomers. Following the successful synthesis of iminium salt 9, 1,3-dipolar cycloaddition with 2,2’,6-trichlorodiphenylethyne followed by oxidation with DDQ under ambient air was performed to generate fused pyrrole 10 in 20% yield. Finally, an intramolecular cyclization of 10 was carried out in the presence of Pd(OAc)2, (t-Bu)2MeP ∙ HBF4, and DBU39 to obtain 4a in 37% yield. It is worth noting that 4a should be stored under inert atmosphere due to its sensitivity to oxygen in a solution state, which is comparable to the corannulene/azacorannulene hybrid molecule in our previous report38. The structure of 4a was confirmed by spectroscopic analysis. The 1H NMR spectrum exhibited three singlets, two doublets, and one triplet in aromatic region as well as one singlet in the aliphatic region, which are consistent with the C2v symmetric structure of 4a. In HRMS spectrum, an m/z value of 1145.4867, corresponding to an ion mass of C88H61N2 (m/z = 1145.4835), was observed as a major signal.
The structure of 4a was further confirmed by X-ray diffraction analysis of its single crystals, which were obtained by slow evaporation from its benzene/diethyl ether solution under argon atmosphere (Fig. 3). The ORTEP structure shows a boat-shaped structure with a fusion of two bowl-shaped azapentabenzocorannulene (APBC) moieties linked by a naphthalene unit. The two terminal azacorannulene bowls are bent in a syn-conformation. The central pyrene unit bends to give quadruple [4]helicene structures, in which two helicenes have a screw sense of P while the other two have M (Fig. 3a). The average interplanar angle between two terminal benzene rings (shown red and blue) of four [4]helicene units was determined to be 33.4°. This angle is larger than a substituted [4]helicene (25.1°)44 but is smaller than a hexabenzocoronene (42.5°)45. Due to the helicene structure, the central pyrene moiety is no longer planar. The dihedral angle between planes formed by C7-C69-C70-C67 and C9-C71-C72-C65 was determined to be 20.5°. The bowl depths, defined as the average perpendicular distance from the mean planes of the hub pyrrole rings (N1-C1-C2-C3-C4 and N2-C35-C36-C37-C38) to each summit atoms of C14, C32, C42 and C60, was determined to be 1.92 Å (Fig. 3b). The bowl depth is deeper than that of APBC (1.38–1.73 Å)26, which is attributed to the steric repulsion between hydrogen atoms in the [4]helicene structure. In the packing structure, two molecules of 4a are packed as a dimeric form with a convex-to-convex π-π interaction (Fig. 3c). The shortest atomic distance between the two molecules is 3.30 Å, which is similar to that of a pentagon- and heptagon-embedded azabuckybowl (3.27 Å)37 and that of a pentagon- and heptagon-embedded nanographene (3.28 Å)46. These results indicate the presence of a strong intermolecular interaction.
Conformational analysis
The conformation of molecule 4b, in which t-butyl groups of 4a are replaced by hydro groups, was analyzed by density functional theory (DFT) calculations at the B3LYP/6-31G(d) level of theory. Overall, there are 10 possible conformational isomers which are formed by combinations of the direction of two bowls (syn/anti) and the helicity of four [4]helicene units (P/M), whilst disregarding all enantiomers. Optimization starting from all the ten possible conformers resulted in eight local minimums. The Gibbs free energy values (kcal mol−1) relative to the most stable conformer are summarized in Supplementary Fig. 26. The most stable conformer was found to be syn-III (Fig. 4), which is in agreement with that observed in the X-ray diffraction analysis (Fig. 3) and is opposite to that of a similar bisdibenzocorannulene47. The relative energies of the other 7 conformers range within 5.6 kcal/mol. The transition states of some interconversions were also calculated. The conversion of syn-III into syn-II, which corresponds to the helicene flipping of one [4]helicene unit, has an activation energy of 5.4 kcal/mol. The energy of a bowl inversion (syn-III to anti-II) was calculated to be 15.1 kcal/mol. Considering the reasonably low activation barriers for these interconversions, all the 8 conformers which gave local minimums can equilibrate at room temperature in solution state.
Molecular properties
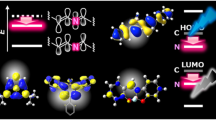
The optical properties of 4a were assessed by absorption and emission spectroscopy (Fig. 5a). The green solution of 4a in hexane, dichloromethane, and dimethyl sulfoxide exhibit comparable absorption bands at 300–720 nm. In dichloromethane, for instance, two major absorption peaks were observed at 447 and 650 nm, which are much larger than those of the parent APBC26 due to its extended π-conjugation system. A time-dependent DFT (TD-DFT) computation at the B3LYP/6-31G(d) level (Supplementary Table 4) indicates that the strong absorption band at around 650 nm corresponds to a HOMO-LUMO transition. To determine the HOMO-LUMO gap experimentally, the electrochemical properties of 4a were investigated by cyclic voltammetry (CV) measurements (Supplementary Fig. 25). Compound 4a showed two overlapped quasi-reversible oxidation peaks at E = ca. +0.16 and +0.25 V (vs. Fc/Fc+), while it showed one reversible reduction peak at E = − 1.77 V. The experimental HOMO-LUMO gap of 4a was determined to be 1.94 eV, which is highly consistent with the absorption at 650 nm. Moreover, 4a exhibits red fluorescence and indicates a positive solvatofluorochromic effect with maximum emission wavelengths (λem) at 665 nm (hexane), 692 nm (dichloromethane), and 706 nm (dimethyl sulfoxide) with quantum yields of ΦF = 0.31, 0.22, and 0.15, respectively. Since APBC shows a comparable fluorescence quantum yield of ΦF = 0.24 in dichloromethane26, the extended π-conjugation of 4a does not significantly affect its fluorescence quantum yield. The observed solvatofluorochromic phenomenon would be induced by the presence of intramolecular charge transfer due to donor-acceptor-donor nature of the molecule. Based on the fact that the HOMO is distributed all over the molecule including the two pyrrole moieties and that LUMO is mainly delocalized at the central pyrene moieties (Fig. 5b), the system involves the APBC cores as a donor moiety and the pyrene unit as an acceptor moiety. The lower fluorescence quantum yields in more polar solvents (Supplementary Table 2) as well as the redox properties in cyclic voltammetry also support our rationale48.
The aromaticity of 4b was characterized by nucleus independent chemical shift (NICS) analysis using DFT calculation at the B3LYP/6-31G(d) level of theory (Fig. 6). The large negative NICS values for the inner pyrrole core (−18.7 ppm) and its four outer benzene rings (−10.1 to −9.7 ppm) show that they are aromatic (Fig. 6a), which is in accordance with those of the reported APBC (Fig. 6b; −18.7 and −10.1 to −9.8 ppm)26. In addition, the central pyrene fragment in 4b has comparable NICS values (−9.8 and −4.6 ppm) with pyrene (Fig. 6c; −12.7 and −5.1 ppm). The anisotropy of the induced current density (ACID) plot of 4b in Fig. 6d shows that the central pyrene moiety has typical ring currents, in which two 6π benzene rings are connected by two carbon–carbon double bonds. In the azacorannulene moiety, clockwise (diamagnetic) 26π ring currents flowing along the core pyrrole moiety and the four outer benzene rings were observed, which substantiates the aromaticity by NICS calculation. These results show that the fusion of two APBC moieties does not significantly change their aromaticity.
Host-guest chemistry
During the investigation on the application of 4a, we discovered its interesting shape-recognition behavior in host-guest chemistry. Inspired by the previous reports of azabuckybowls being utilized as buckycatchers49,50, association behavior of 4a with C60 and a dumbbell-shaped C60 dimer (C120)51 was examined by fluorescence titration. As shown in Fig. 7a, the addition of C120 into a diluted solution of 4a in 1,2-dichlorobenzene resulted in the gradual decrease of its fluorescence intensity. Based on the Benesi–Hildebrand equation, the association constants of 4a toward C60 and C120 were determined to be Ka(C60) = 4.5 × 102 M−1 and Ka(C120) = 2.9 × 103 M−1 respectively (Fig. 7b), which indicate that 4a favors C120 over C60 by one order of magnitude. The Job’s plot for the emission intensity indicates the formation of 1:1 supramolecular assembly of 4a and C120 (Supplementary Fig. 21). It is worth noting that comparable association constants of APBC with C60 and C120 were observed (Fig. 7b), thus showing no distinct selectivity. These results strongly indicate that the boat-shaped structure of 4a recognizes the dumbbell-shape of C120 during the association process in solution, leading to the higher association constant for C120 (Fig. 7c).
a Fluorescence spectra of 4a upon titration with C120. b Association constants of host molecules (4a and APBC) and guest molecules (C60 and C120) determined by fluorescence titration. c One of the possible association modes of 4a and C120 determined by DFT calculation at the B3LYP/6-31G(d) level with Grimme’s D3 dispersion correction.
In summary, we have demonstrated the bottom-up synthesis of a diaza[80]fullerene fragment molecule 4a. The large nitrogen-containing polycyclic aromatic molecule has a boat-shaped structure which can be viewed as the fusion of two bowl-shaped APBC moieties linked by a fused naphthalene unit. Conformational studies showed that a butterfly-butterfly conformer where the two azabuckybowls bend in the same direction is the most stable, which is consistent with that observed in X-ray diffraction analysis. The unique molecular shape leads to preferable association with a dumbbell-shaped C60 dimer (C120) over C60 through shape recognition. Theoretical analysis revealed the presence of a narrow HOMO-LUMO band gap, resulting in a strong absorption band at around 650 nm. The optical measurement exhibits a red fluorescence and solvatofluorochromic behavior. Importantly, the utilization of fully conjugated bifunctional polycyclic aromatic azomethine ylide 9 in a bottom-up synthetic approach provides a practical method for the selective synthesis of large multiazafullerene fragments.
Methods
Experimental procedure
The synthesis of 4a is as follows: to a mixture of 10 (10 mg, 7.3 μmol), palladium diacetate (4.9 mg, 22 μmol) and di-t-butyl(methyl)phosphonium tetrafluoroborate (16 mg, 66 μmol) were added 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU; 0.5 ml) and N,N-dimethylacetamide (DMA; 2.0 ml). The mixture was stirred for 19 h at 160 °C. After cooling to room temperature and dilution with toluene (5 ml), the mixture was washed with water (3 × 5 ml), dried over sodium sulfate, filtered, and concentrated in vacuo. The resulting mixture was purified by silica gel column chromatography with hexane/dichloromethane (4/1) to obtain 4a as a dark green solid (3.1 mg, 2.7 μmol, 37%). Full experiment details can be found in the Supplementary Information.
Theoretical calculations
All calculations were performed by using Gaussian 16 (revision A.03) program52 by the B3LYP method53,54 with the 6-31G(d) basis set55,56 for structure optimization, vibrational frequency, time-dependent density functional theory, NICS, and ACID calculations. Grimme’s D3 dispersion correction57 was used to investigate the association of 4b with C120. Molecular geometries and transition state (TS) structures were optimized without any symmetry assumptions. Intrinsic reaction coordinate calculations were also performed for all TSs to ensure their true nature. All thermodynamics were obtained by utilizing the standard conditions at 298 K and 1 atm. Energies are presented as ΔG in kcal/mol.
Data availability
The data supporting the findings of the current study are available within the paper and its Supplementary Information or from the corresponding author upon request. The crystallographic data for compound 4a have been deposited with the Cambridge Crystallographic Data Centre under deposition number 2103521 [https://www.ccdc.cam.ac.uk/solutions/csd-core/components/csd/].
Change history
05 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41467-022-29706-6
References
Hummelen, J. C., Bellavia-Lund, C. & Wudl, F. Heterofullerenes. Top. Curr. Chem. 199, 93–134 (1999).
Hirsch, A. & Nuber, B. Nitrogen heterofullerenes. Acc. Chem. Res. 32, 795–804 (1999).
Hirsch, A. & Brettreich, M. Heterofullerenes. In Fullerenes—Chemistry and Reactions 59–373 (Wiley-VCH, 2005).
Vostrowsky, O. & Hirsch, A. Heterofullerenes. Chem. Rev. 106, 5191–5207 (2006).
Xie, R.-H. et al. Tuning spectral properties of fullerenes by substitutional doping. Phys. Rev. B. 69, 201403 (2004).
Kumashiro, R. et al. Azafullerene (C59N)2 thin-film field-effect transistors. Appl. Phys. Lett. 84, 2154–2156 (2004).
Kaneko, T., Li, Y., Nishigaki, S. & Hatakeyama, R. Azafullerene encapsulated single-walled carbon nanotubes with n-type electrical transport property. J. Am. Chem. Soc. 130, 2714–2715 (2008).
Krätschmer, W., Lamb, L. D., Fostiropoulos, K. & Huffman, D. R. Solid C60: a new form of carbon. Nature 347, 354–358 (1990).
Mojica, M., Alonso, J. A. & Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 26, 526–539 (2013).
von Delius, M. & Hirsch, A. Heterofullerenes: doped buckyballs. In Chemical Synthesis and Applications of Graphene and Carbon Materials (eds Antonietti, M. & Müllen, K.) 191–216 (Wiley-VCH, 2017).
Hummelen, J. C., Knight, B., Pavlovich, J., González, R. & Wudl, F. Isolation of the heterofullerene C59N as its dimer (C59N)2. Science 269, 1554–1556 (1995).
Otero, G. et al. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nature 454, 865–868 (2008).
Chen, Z., Zhao, X. & Tang, A. Theoretical studies of the substitution patterns in heterofullerenes C60-xNx and C60-xBx (x = 2 − 8). J. Phys. Chem. A 103, 10961–10968 (1999).
Chen, Z., Reuther, U., Hirsch, A. & Thiel, W. Theoretical studies on the substitution patterns in heterofullerenes C70-xNx and C70-xBx (x = 2–10). J. Phys. Chem. A 105, 8105–8110 (2001).
Sharma, H., Garg, I., Dharamvir, K. & Jindal, V. K. Structural, electronic, and vibrational properties of C60-nNn (n = 1–12). J. Phys. Chem. A 113, 9002–9013 (2009).
von Delius, M., Hauke, F. & Hirsch, A. Evaluation of an intramolecular approach for the synthesis of the elusive C58N2 heterofullerene family. Eur. J. Org. Chem. 2008, 4109–4119 (2008).
Huang, H. et al. Synthesis of an azahomoazafullerene C59N(NH)R and gas-phase formation of the diazafullerene C58N2. Angew. Chem. Int. Ed. 52, 5037–5040 (2013).
Chen, Z. et al. Calculations on all possible isomers of the substituted fullerenes C58X2 (X = N, B) using semiempirical methods. J. Chem. Soc. Faraday Trans. 94, 2269–2276 (1998).
Ostrowski, S., Jamróz, M. H., Rode, J. E. & Dobrowolski, J. C. On stability, chirality measures, and theoretical VCD spectra of the chiral C58X2 fullerenes (X = N, B). J. Phys. Chem. A 116, 631–643 (2012).
Nekoei, A.-R. & Hamzekhami, Z. H. Structural, electronic, vibrational and optical properties of all 23 isolated-pentagon rule isomers of C58N2 azafullerene; a DFT study. Comput. Theor. Chem. 1196, 113123 (2021).
Scott, L. T. et al. A rational chemical synthesis of C60. Science 295, 1500–1503 (2002).
Hou, X.-Q. et al. Bowl-shaped conjugated polycycles. Chin. Chem. Lett. 27, 1166–1174 (2016).
Stępień, M., Gońka, E., Żyła, M. & Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications. Chem. Rev. 117, 3479–3716 (2017).
Borissov, A., Maurya, Y. K., Moshniaha, L. & Wong, W.-S. Recent advances in heterocyclic nanographenes and other polycyclic heteroaromatic compounds. Chem. Rev. 122, 565–788 (2022).
Liu, J. & Feng, X. Bottom-up synthesis of nitrogen-doped polycyclic aromatic hydrocarbons. Synlett 31, 211–222 (2020).
Ito, S., Tokimaru, Y. & Nozaki, K. Benzene-fused azacorannulene bearing an internal nitrogen atom. Angew. Chem. Int. Ed. 54, 7256–7260 (2015).
Yokoi, H. et al. Nitrogen-embedded buckybowl and its assembly with C60. Nat. Commun. 6, 8215 (2015).
Tsefrikas, V. M., Greene, A. K. & Scott, L. T. 5-Azadibenzo[a,g]corannulene. Org. Chem. Front. 4, 688–698 (2017).
Zhou, L. & Zhang, G. A nanoboat with fused concave N-heterotriangulene. Angew. Chem. Int. Ed. 59, 8963–8968 (2020).
Tan, Q., Higashibayashi, S., Karanjit, S. & Sakurai, H. Enantioselective synthesis of a chiral nitrogen-doped buckybowl. Nat. Commun. 3, 891 (2012).
Myśliwiec, D. & Stępień, M. The fold-in approach to bowl-shaped aromatic compounds: synthesis of chrysaoroles. Angew. Chem. Int. Ed. 52, 1713–1717 (2013).
Higashibayashi, S., Pandit, P., Haruki, R., Adachi, S.-i. & Kumai, R. Redox-dependent transformation of a hydrazinobuckybowl between curved and planar geometries. Angew. Chem. Int. Ed. 55, 10830–10834 (2016).
Nakatsuka, S., Yasuda, N. & Hatakeyama, T. Four-step synthesis of B2N2-embedded corannulene. J. Am. Chem. Soc. 140, 13562–13565 (2018).
Zhu, G. et al. Modulating the properties of buckybowls containing multiple heteroatoms. Org. Chem. Front. 8, 727–735 (2021).
Tokimaru, Y., Ito, S. & Nozaki, K. Synthesis of pyrrole-fused corannulenes: 1,3-dipolar cycloaddition of azomethine ylides to corannulene. Angew. Chem. Int. Ed. 56, 15560–15564 (2017).
Mishra, S. et al. On-surface synthesis of a nitrogen-embedded buckybowl with inverse Stone–Thrower–Wales topology. Nat. Commun. 9, 1714 (2018).
Krzeszewski, M., Dobrzycki, Ł., Sobolewski, A. L., Cyrański, M. K. & Gryko, D. T. Bowl-shaped pentagon- and heptagon-embedded nanographene containing a central pyrrolo[3,2-b]pyrrole core. Angew. Chem. Int. Ed. 60, 14998–15005 (2021).
Tokimaru, Y., Ito, S. & Nozaki, K. A hybrid of corannulene and azacorannulene: synthesis of a highly curved nitrogen-containing buckybowl. Angew. Chem. Int. Ed. 57, 9818–9822 (2018).
Nagano, T. et al. Functionalization of azapentabenzocorannulenes by fivefold C−H borylation and cross-coupling arylation: application to columnar liquid-crystalline materials. Chem. Eur. J. 24, 14075–14078 (2018).
Zhou, Z. et al. Stepwise reduction of azapentabenzocorannulene. Angew. Chem. Int. Ed. 58, 12107–12111 (2019).
Li, Q.-Q. et al. Diazapentabenzocorannulenium: a hydrophilic/biophilic cationic buckybowl. Angew. Chem. Int. Ed. 61, e202112638 (2022).
Coventry, D. N. et al. Selective Ir-catalysed borylation of polycyclic aromatic hydrocarbons: structures of naphthalene-2,6-bis(boronate), pyrene-2,7-bis(boronate) and perylene-2,5,8,11-tetra(boronate) esters. Chem. Commun. 2172–2174 (2005).
Merz, J. et al. Pyrene molecular orbital shuffle—controlling excited state and redox properties by changing the nature of the frontier orbitals. Chem. Eur. J. 23, 13164–13180 (2017).
Isobe, H., Hitosugi, S., Matsuno, T., Iwamoto, T. & Ichikawa, J. Concise synthesis of halogenated chrysenes ([4]phenacenes) that favor π-stack packing in single crystals. Org. Lett. 11, 4026–4028 (2009).
Xiao, S. et al. Molecular wires from contorted aromatic compounds. Angew. Chem. Int. Ed. 44, 7390–7394 (2005).
Fei, Y. et al. Defective nanographenes containing seven-five-seven (7–5–7)-membered rings. J. Am. Chem. Soc. 143, 2353–2360 (2021).
Li, B. et al. Synthesis and structural elucidation of bisdibenzocorannulene in multiple redox states. Angew. Chem. Int. Ed. 60, 19790–19796 (2021).
Grabowski, Z. R., Rotkiewicz, K. & Rettig, W. Structural changes accompanying intramolecular electron transfer: focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 103, 3899–4032 (2003).
Yokoi, H., Hiroto, S., Sakamaki, D., Seki, S. & Shinokubo, H. Supramolecular assemblies of a nitrogen-embedded buckybowl dimer with C60. Chem. Sci. 9, 819–824 (2018).
Yokoi, H., Hiroto, S. & Shinokubo, H. Reversible σ-bond formation in bowl-shaped π-radical cations: the effects of curved and planar structures. J. Am. Chem. Soc. 140, 4649–4655 (2018).
Wang, G.-W., Komatsu, K., Murata, Y. & Shiro, M. Synthesis and X-ray structure of dumb-bell-shaped C120. Nature 387, 583–586 (1997).
Frisch, M. J. et al. Gaussian 16, Revision A.03 (Gaussian, Inc., 2016).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular orbital methods. XII. further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Ditchfield, R., Hehre, W. J. & Pople, J. A. Self-consistent molecular-orbital methods. IX. an extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
This work was supported by Nanyang Technological University. The contributions from the NTU Center of High Field NMR in SPMS for NMR analyses and the NTU High Performance Computing Team for computing resources are gratefully acknowledged. We sincerely thanks Prof. Rei Kinjo and Mr. Kota Koshino (NTU) for their assistance in electrochemical analysis.
Author information
Authors and Affiliations
Contributions
S.I. directed and conceived the project. W.W. and F.H. performed all experimental work. Y.H. and S.I. performed the theoretical studies with DFT calculations. Y.L. performed the X-ray crystallography analyses. All the authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Hanindita, F., Hamamoto, Y. et al. Fully conjugated azacorannulene dimer as large diaza[80]fullerene fragment. Nat Commun 13, 1498 (2022). https://doi.org/10.1038/s41467-022-29106-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-29106-w
This article is cited by
-
Trichalcogenasupersumanenes and its concave-convex supramolecular assembly with fullerenes
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.