Abstract
The conversion of photocatalytic methane into methanol in high yield with selectivity remains a huge challenge due to unavoidable overoxidation. Here, the photocatalytic oxidation of CH4 into CH3OH by O2 is carried out on Ag-decorated facet-dominated TiO2. The {001}-dominated TiO2 shows a durable CH3OH yield of 4.8 mmol g−1 h−1 and a selectivity of approximately 80%, which represent much higher values than those reported in recent studies and are better than those obtained for {101}-dominated TiO2. Operando Fourier transform infrared spectroscopy, electron spin resonance, and nuclear magnetic resonance techniques are used to comprehensively clarify the underlying mechanism. The straightforward generation of oxygen vacancies on {001} by photoinduced holes plays a key role in avoiding the formation of •CH3 and •OH, which are the main factors leading to overoxidation and are generally formed on the {101} facet. The generation of oxygen vacancies on {001} results in distinct intermediates and reaction pathways (oxygen vacancy → Ti–O2• → Ti–OO–Ti and Ti–(OO) → Ti–O• pairs), thus achieving high selectivity and yield for CH4 photooxidation into CH3OH.
Similar content being viewed by others
Introduction
Direct conversion of CH4 into methanol (CH3OH) is one of the most promising methods for methane optimization and utilization. However, CH4 is a very stable and inert molecule due to its negligible electron affinity, low polarizability, and high bonding energy for C–H (the first dissociation energy at 439 kJ mol−1). Thus, high temperatures and pressures are normally required to activate C–H bonds, which greatly increases capital investment and gives rise to operational risks and environmental problems1. Photocatalysis is a potential way to drive CH4 oxidation by utilizing photon energy instead of thermal energy. Upon excitation of semiconducting photocatalysts by photons, a series of highly active oxygen-containing radicals formed in photocatalytic CH4 oxidation can readily activate the C–H bond at room temperature2,3,4,5,6. However, the activation energy of the C–H bond in CH4 is much higher than that in the product (CH3OH)7. Thus, after the first C–H bond of CH4 is activated by active free radicals to form methyl or methoxy species, these species are easier to be activated and oxidized than CH4, eventually resulting in overoxidation of CH3OH to produce CO and CO28. Especially for gas-phase CH4 oxidation, the product CH3OH can easily adsorb onto the surface of photocatalyst (such as TiO2 and ZnO loaded with various cocatalysts) and become overoxidized to CO and CO29,10,11.
Due to unavoidable overoxidation, achieving high activity along with high selectivity at the same time is barely realized in the photocatalytic oxidation of CH4 to CH3OH12,13,14. The enhancement of one usually sacrifices the other. It has been found that in aqueous-phase CH4 oxidation, water can promote the desorption of products from active sites to avoid serious overoxidation15,16. Recently, Ma and Tang et al.17 reported direct photocatalysis of CH4 into CH3OH using a FeOx/TiO2 catalyst with H2O2 as the oxidant in an aqueous-phase system, resulting in a high CH3OH selectivity of ~90% but a relatively low yield of ~352 µmol g−1 h−1. However, the relatively high cost of H2O2 greatly limits its commercial application. Using O2 instead of H2O2 as the economically viable oxidant would represent substantial progress towards the oxidation of CH41. In this case, Ye and coworkers2 used molecular O2 to photooxidize CH4 on Au/ZnO with a total organic compound selectivity of 95%. However, the CH3OH product tends to be overoxidized by •OH radicals formed in the reaction, which results in a CH3OH selectivity of less than 27% with a yield of 2.0 mmol g−1 h−1. More recently, Ye and coworkers used a CrOx-decorated Au/TiO2 photocatalyst to reduce the formation of •OH radicals in the reaction18, which increased the selectivity of CH3OH to ~50% with a yield of 2.5 mmol g−1 h−1. As such, the overoxidation of CH3OH should be partly attributed to the formation of •OH radicals. Furthermore, for the reported catalysts, CH4 can react with the photoinduced holes to form •CH3 on the surface, and the •CH3 can react with O2 and superoxide (O2•−) to mainly form CH3OOH rather than CH3OH2,6,19. It should be noted that CH3OOH can readily decompose into HCHO and H2O2,9. Therefore, as long as •CH3 and •OH exist in photocatalytic CH4 oxidation, it is difficult to improve the selectivity of CH3OH unless a new reaction pathway is introduced to reduce the formation of •CH3 and •OH through rational catalyst design. It has been reported that the intermediate photocatalytic species are closely related to the arrangement and coordination of the surface atoms on different crystal facets20,21,22,23,24.
In this work, two types of anatase TiO2, {001} or {101}-dominated TiO2 with Ag cocatalysts, are studied for CH4 photooxidation by O2. The {001}-dominated TiO2 shows a durable CH3OH yield of 4.8 mmol/g/h and a selectivity of ~80%, which are much higher values than those obtained on {101}-dominated TiO2. A comprehensive study by operando IR, ESR, and NMR reveals that the initial generation of oxygen vacancies on {001} by photoinduced holes can avoid the formation of •CH3 and •OH in the following reaction steps (Ti–O2• → Ti–OO–Ti and Ti–(OO) → Ti–O• pairs) to significantly reduce overoxidation. The scarcity of photogenerated oxygen vacancies on {101} leads to the formation of •CH3 and •OH, which is the main factor for overoxidation. This study provides a strategy to avoid overoxidation in reforming CH4 to CH3OH using other photocatalysts by controlling the generation of photogenerated oxygen vacancies.
Results
Selective photocatalytic oxidation of CH4 to CH3OH
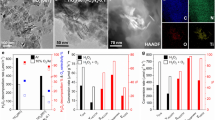
Two types of anatase TiO2 (Supplementary Fig. S1) with predominantly exposed {001} or {101} facets were prepared, called TiO2 {001} and TiO2 {101}, respectively. Silver (Ag) was chosen to decorate TiO2, which can trap photoinduced electrons to facilitate the separation and transfer of photoinduced carriers25,26,27. Typical oxide semiconductors, such as ZnO and TiO2, loaded with Ag cocatalysts have been demonstrated to exhibit good photocatalytic activity for CH4 activation10,28. Bare TiO2 (including TiO2 {001} and TiO2 {101}) and that with variable Ag loading were evaluated for photocatalytic CH4 oxidation with molecular O2 as an oxidant (CH4:O2 ratio = 20:1). As shown in Fig. 1A, the products of CH4 photooxidation on TiO2 {001} are CH3OH, HCHO, CO, and CO2. Among these products, the yield of CH3OH is 950 µmol gcat.−1 h−1 and the selectivity of CH3OH is 42%. However, only deep oxidation products (HCHO, CO, and CO2) are formed on TiO2 {101}, and the production of CH3OH is invisible (Fig. 1B). This is consistent with previous reports of CH4 photooxidation by O2 over TiO2 (P25) and ZnO2,9. As such, the {001} facet should be favorable for the formation of CH3OH in CH4 photooxidation by O2, while the {101} facet is inclined to undergo deep oxidation.
CH3OH selectivity and product yields for a series of Ag-loaded TiO2 photocatalysts with predominantly exposed A {001} facets (0–5.0%Ag/TiO2 {001}) and B {101} facets (0–3.0%Ag/TiO2 {101}) under 2 h of irradiation. Time course for CH3OH selectivity and product yields for C 3.2%Ag/TiO2 {001} and D 2.1%Ag/TiO2 {101} under irradiation. Reaction conditions: 10 mg Ag/TiO2, 100 mL water, 2 MPa CH4, 0.1 MPa O2, 25 °C, light source: 300 W Xe lamp, light intensity of 450 mW/cm2.
We further studied the effect of the Ag loading amount on the catalytic performance. Clearly, a series of Ag loadings on TiO2 can effectively improve the activity of CH4 photooxidation. For TiO2 {001}, a 2.5%Ag and 3.2%Ag loading exhibited a high CH3OH yield of 4.3 mmol gcat.−1 h−1 with a selectivity of ~70% and a yield of 4.8 mmol gcat.−1 h−1 with an ultrahigh selectivity of ~80%, respectively (Fig. 1A and Table 1). For TiO2 {101}, the 2.1%Ag loading shows an optimal CH3OH yield of 3.3 mmol gcat.−1 h−1 and a selectivity of ~68% (Fig. 1B and Table 1). Furthermore, with increasing irradiation time, the amounts of the total products (including CH3OH, HCHO, CO, and CO2) increase gradually with relatively stable CH3OH selectivity (74–80%) in CH4 photooxidation of 3.2%Ag/TiO2 {001}. (Fig. 1C and Table 1). Similar results can also be found for 2.5%Ag/TiO2 {001} (Supplementary Fig. S2). The optimal CH3OH yield and selectivity on 3.2%Ag/TiO2 {001} are much superior to those of most reported photocatalytic systems for CH4 oxidation to CH3OH (Supplementary Table S1). And we believe that this is the first study to achieve a high CH3OH yield and selectivity for CH4 photooxidation with O2 at room temperature. For 2.1%Ag/TiO2 {101}, although the amount of the deep oxidation product (HCHO, CO, and CO2) still increases with increasing irradiation time, the amount of CH3OH barely increases after 2 h of irradiation, which results in a significant decrease in CH3OH selectivity from 68% to 53% (Fig. 1D and Table 1). Therefore, it can be indicated that the mechanism for CH4 photooxidation by O2 on the {001} facet should be different from that on the {101} facet. The synergy between the cocatalyst Ag and the {001} facet can achieve selective and stable photocatalytic oxidation of CH4 into CH3OH by O2.
Characterization of Ag/TiO2 nanocatalysts
To further clarify the correlation between photocatalytic performance and the crystal facet on Ag/TiO2 nanocatalysts, high-resolution transmission electron microscopy (HRTEM, 3100FEF spectrometer) and X-ray photoelectron spectroscopy (XPS) were performed to validate the morphology and elemental structure of 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101}. According to the HRTEM results, TiO2 {001} exhibits a uniform square morphology with an average length of 54 nm and thickness of 4.6 nm (Fig. 2A), and TiO2 {101} possesses an octahedral morphology with an average size of 17 nm (Fig. 2D). The lattice spacing parallel to the lateral facets and the top is 3.56 and 2.38 Å, corresponding to the {101} and {001} facets of anatase TiO2, respectively (Fig. 2B–E). As such, it can be found that both TiO2 {001} and TiO2 {101} nanoparticles coexpose {001} and {101} facets, and the percentage of dominant facets should be ~87% of the {001} facet in TiO2 {001}, and ~92% of the {101} facet in TiO2 {101}. Furthermore, HRTEM clearly shows that the Ag cocatalyst with a particle size of ~1.5 nm was selectively photodeposited onto the {101} facet of 2.5%Ag/TiO2 {001} as shown in Fig. 2A, B. The chemical states for the Ag species on 2.5%Ag/TiO2 {001} were characterized by XPS (Fig. 2F), and the Ag 3d5/2 peak observed at 368.0 eV can be associated with metal Ag nanoparticles. Similarly, on 2.1% Ag/TiO2 {101}, the Ag cocatalyst was also selectively photodeposited onto the {101} facet. However, due to the larger {101} facet, Ag can be dispersed better and smaller (<1.0 nm) than that on 2.5%Ag/TiO2 {001} (Fig. 2D, E), which is reflected redshift observed for the Ag 3d5/2 peak (0.2 eV) in the XPS spectrum, as shown in Fig. 2F. According to the XPS results in Supplementary Fig. S3, titanium atoms exist in the form of Ti4+–O bonds in both TiO2 {101} and TiO2 {001}29, as confirmed by the Ti 2p3/2 and 2p1/2 XPS peaks at 458.8 and 464.5 eV, and the O 1s XPS spectra show two peaks at 530.0 and 531.4 eV ascribed to lattice oxygen in Ti–O2−–Ti bonds and OH groups on the surface of TiO230. In addition, no fluorine or chlorine atoms are present on Ag/TiO2 catalysts. The elemental composition of Ag and Ti was studied by energy dispersive spectroscopy (EDS), as shown in Supplementary Fig. S4. In addition, according to N2 adsorption and desorption experiments (Supplementary Fig. S5), the BET surface area of TiO2 {101} (100.6 m2/g) is slightly higher than that of TiO2 {001} (84.5 m2 g−1). Due to the particle accumulation, both TiO2 {101} and TiO2 {001} are porous with similar pore diameters 14.3 and 14.9 nm, respectively. As such, compared with TiO2 {101}, the higher photocatalytic activity and selectivity on TiO2 {001} can be mainly ascribed to crystal plane and elemental structure rather than surface area and porosity.
Reactive intermediate by operando and in situ characterization
To gain insight into the different photocatalytic mechanisms for CH4 photooxidation on the {001} and {101} facets of TiO2, reactive intermediates were detected by operando ATR-FTIR experiments on 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101} in the aqueous phase. The change in the difference spectra before light irradiation was also taken as the reference and indicator. It is certain at first that all the spectra observed under different conditions showed no change before light irradiation (Supplementary Figs. S6–S9). For the 2.5%Ag/TiO2 {001} photocatalyst in the aqueous-phase saturated with Ar and O2 (Ar + O2) (Fig. 3A in 1st column), light irradiation led to the appearance of several peaks located at 943, 842, and 812 cm−1. The peaks in the 800–1000 cm−1 region were specifically assigned in previous reports31,32 to the O–O stretching of a surface peroxo species Ti–(OO) at 943 cm−1 32,33, Ti–OOH at 842 cm−1 31, and Ti–OO–Ti at 812 cm−1 32,34,35,36. To validate this assignment, operando FTIR experiments were performed for the adsorption of H2O2 on the surface of TiO2 {001} over time, and it was confirmed that the signal in the region of 800–1000 cm−1 corresponds to free and adsorbed H2O2 (Supplementary Fig. S10). The intensities of the peaks at 943 and 812 cm−1 increase immediately with the light irradiation time and drop down quickly after the light is turned off, suggesting that the intermediate species of Ti–(OO) and Ti–OO–Ti are photoinduced and metastable. Besides, these intermediate species show few changes with time under the light. However, when CH4 was introduced into the aqueous-phase system instead of Ar (CH4 + O2), both peaks at 943 and 812 cm−1 decreased significantly with light irradiation (Fig. 3A in 2nd column), suggesting the consumption of intermediate species (Ti–(OO) and Ti–OO–Ti) in the presence of CH4. Instead, two new peaks appear in the region of 1000–1100 cm−1, which can be assigned to the C–O stretching modes of CH3OH and Ti–O–CH337.
A Operando ATR-FTIR matrix for 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101} with a 4 mL aqueous phase in a (Ar + O2) and (CH4 + O2) atmospheres before, during, and after light irradiation. B In situ ESR spectra for 2.5%Ag/TiO2 {001} (left) and 2.1% Ag/TiO2 {101} (right) with 300 µmol H2O loading in O2 (bottom) and (CH4 + O2) (upper) atmospheres before and during light irradiation. We fit the ESR data with the easyspin programs. C Ex situ 17O NMR spectra for 2.5%Ag/TiO2 {001} (upper) and 2.1% Ag/TiO2 {101} (lower) in a (CH4 + 17O2) atmosphere before and after light irradiation. The signals marked by asterisks are spinning sidebands. We fit the 17O NMR data with the Dimfit programs.
Compared with the 2.5%Ag/TiO2 {001} photocatalyst, no peak appears in the 800–1000 cm−1 region for the 2.1%Ag/TiO2 {101} photocatalyst in the aqueous phase with Ar + O2 during light irradiation (Fig. 3A in 3rd column). It is suggested that the intermediates of Ti–(OO) and Ti–OO–Ti are not photoinduced on 2.1%Ag/TiO2 {101}. A similar change in spectral behavior is observed for 2.1%Ag/TiO2 {101} in the aqueous phase with CH4 + O2 during light irradiation (Fig. 3A in 4th column), except for the presence of the peaks in the region of 1000–1100 cm−1 due to CH3OH and Ti–O–CH3 formation during light irradiation. Notably, the peaks in the region of 1000–1100 cm−1 on 2.1%Ag/TiO2 {101} are much weaker than those on 2.5%Ag/TiO2 {001} (Fig. 3A in 2nd and 4th columns) indicating that CH4 activation on the {001} facet should make it easier to form CH3OH and Ti–OCH3. In addition, a new peak located at 966 cm−1 can be assigned to the O–O stretching mode of CH3OOH (Fig. 3A in 4th column)38.
We used in situ ESR spectroscopy to follow the formation of paramagnetic intermediates on the {001} and {101} facets (Fig. 3B). For 2.5%Ag/TiO2 {001} with 300 µmol H2O loading in a 24 µmol O2 atmosphere, two sets of ESR signals appeared upon light irradiation (Fig. 3B, left). The strong signal at g = 1.9996 is associated with oxygen vacancies arising from trapped photoinduced holes at surface oxygen. According to previous reports39,40, the signal for orthorhombic symmetry at gzz = 2.023, gyy = 2.007, and gxx = 2.000 is ascribed to surface superoxide (Ti–O2•) sites. O2•− is usually stabilized on a metallic cationic site so that the electrostatic interaction splits the 2π* antibonding orbitals by a certain amount (δ) due to the local cationic crystal field. The gzz value can be measured by the equation gzz = ge + 2λ/δ, where λ is the spin–orbit coupling constant of oxygen40. As such, gzz = 2.023 indicates that O2•− is stabilized at the Ti sites for the oxygen vacancies. Combined with the operando FTIR results, the surface superoxide can be converted into surface peroxo species by the reduction of photoinduced electrons. However, when 30 µmol CH4 was introduced into the O2–Ag/TiO2 {001} system ((CH4 + O2)–Ag/TiO2 {001}), both ESR signals decreased obviously upon light irradiation, indicating the consumption of paramagnetic intermediates (oxygen vacancies and Ti–O2•) in the presence of CH4. Compared with 2.5%Ag/TiO2 {001}, no surface superoxide appears for 2.1%Ag/TiO2 {101} with a 300 µmol H2O loading in a 24 µmol O2 atmosphere during light irradiation (Fig. 3B, right), except for a small number of oxygen vacancies associated with the signal at g = 1.9996. Furthermore, the amount of oxygen vacancies does not change in the presence of 30 µmol CH4, indicating that oxygen vacancies are not involved in CH4 photooxidation by O2 on 2.1%Ag/TiO2 {101}. In addition, the pair of signals at g = 2.03 and 1.96 should be assigned to the background signal of in situ ESR tube (Supplementary Fig. S11).
Ex-situ 17O MAS NMR experiments were used to follow the transfer and evolution of oxygen in CH4 photooxidation by 17O2 on TiO2 photocatalysts (Fig. 3C). For 2.5%Ag/TiO2 {001} in a 24 µmol 17O2 and 30 µmol CH4 atmosphere, three NMR signals appeared after 0.5 h of light irradiation. According to the previous reports41, the resonances at 480–570 ppm should arise from three coordinated oxygen species in the bulk of TiO2; the peaks at higher frequencies (600–750 ppm) can be assigned to two coordinated oxygen species on the surface of TiO2; the signals at much lower frequencies (100–300 ppm) can be attributed to hydroxyl groups (Ti–OH); the signals at −100–10 ppm can be attributed to adsorbed H2O. As such, the signals observed at −24 and 180 ppm are associated with H217O and surface terminal hydroxyl (Ti–17OH), respectively (Fig. 3C, upper). Due to the deshielding effect of CH3, the 17O NMR signal of Ti–OCH3 should shift to a lower field than that of Ti–OH. Accordingly, we assign the 17O NMR signal at 263 ppm to Ti–OCH3 (Fig. 3C, upper), which is in good agreement with the formation of –OCH3 in the operando IR experiment for CH4 oxidation. Similar results are obtained on 2.1%Ag/TiO2 {101} in a 24 µmol 17O2 and 30 µmol CH4 atmosphere before and after light irradiation (Fig. 3C, lower). However, the 17O signals at 180 ppm and 263 ppm are much lower than those for 2.5%Ag/TiO2 {001}, indicating that the O transfer efficiency from O2 to Ti–OCH3 and Ti–OH on the {001} facet in the photoreaction is much higher than that on the {101} facet. Combined with the operando FTIR and in situ ESR results, this can be attributed to the difference in the photocatalytic mechanism between the {001} and {101} facets, rather than the difference in the catalytic activity, because of the difference in reactivity (Fig. 1) is far from matching the difference in the O transfer efficiency.
Distinct photocatalytic mechanism of CH4 oxidation by O2 on the {001} facet
To detect the direct participation of CH4 and O2 in the formation of CH3OH, isotope labeling NMR experiments using 13CH4 and 17O2 were conducted under 80 kPa 13CH4 mixed with 20 kPa 17O2 on 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101} in 50 mL water for 4 h. Figure 4A shows the 1H NMR spectra for the product obtained from photocatalytic CH4 oxidation in various atmospheres. For 2.5%Ag/TiO2 {001} in a 12CH4 and 16O2 atmosphere, the strong peak at 3.26 ppm corresponds to 12CH3OH, and the weak peak at 3.77 ppm corresponds to trace 12CH3OOH. Using 13CH4 instead of 12CH4, both peaks split into two peaks due to 1H–13C J coupling (~140 Hz) for the methyl groups in the formed 13CH3OH (3.40 and 3.12 ppm) and 13CH3OOH (3.62 and 3.91 ppm) (Fig. 4A, upper). Similar results are also obtained on 2.1%Ag/TiO2 {101} (Fig. 4A, lower). It can be indicated that the product CH3OH indeed originates from CH4 conversion on both 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101}. Interestingly, when the reaction was carried out on 2.5%Ag/TiO2 {001} in a 13CH4 and 17O2 atmosphere, the FWHM (full width at half maximum) for the 13CH3OH signal obviously increased (Fig. 4A, upper). It should be noted that the FWHM of the signal for dissolved 13CH4 (0.20 and −0.04 ppm) in the 13CH4 and 17O2 atmosphere is consistent with that of dissolved 13CH4 in the 13CH4 and 16O2 atmosphere (Fig. 4A, upper). Thus, the widening of the 13CH3OH signal is due to weak J coupling (1.96 Hz) between the methyl proton and 17O, rather than any error related to the spectrometer and operation. On the other hand, when the reaction was carried out on 2.1% Ag/TiO2 {101} in a 13CH4 and 17O2 atmosphere, the FWHM for the signal for the product 13CH3OH barely increased compared with that of the product 13CH3OH in a 13CH4 and 16O2 atmosphere (Fig. 4A, lower). Similar results were also found from the 13C NMR spectra (Fig. 4B). For Ag/TiO2 in the 13CH4 and 16O2 atmosphere, the two 13C NMR signals at 48.97 and 65.03 ppm can be attributed to 13CH3OH and 13CH3OOH, respectively, which further proves that the product CH3OH indeed originated from CH4 conversion on both 2.5%Ag/TiO2 {001} and 2.1% Ag/TiO2 {101}. When the reaction was carried out on Ag/TiO2 in a 13CH4 and 17O2 atmosphere, the obvious peak broadening, originating from 13C–17O J coupling only occurred for the signal for 13CH3OH generated by 2.5%Ag/TiO2 {001} (Fig. 4B, upper) but was barely observed for the signal for 13CH3OH generated by 2.1% Ag/TiO2 {101} (Fig. 4B, lower). Combined with the results from 1H NMR experiments, it can be indicated that the oxygen in the CH3OH product mainly originates from the O2 in the photocatalytic CH4 oxidation on the {001} facet, while the oxygen in the CH3OH product should mainly originate from H2O and the surface oxygen of TiO2 in the photocatalytic CH4 oxidation on the {101} facet.
As in the previous reports2,7,9,42, the primary step of CH4 activation on typical oxide semiconductors should involve a reaction with surface O− radical ions. When a TiO2 {101} sample is illuminated under UV irradiation, the surface oxygen (Ti–O–Ti) captures one photoinduced hole to form Ti–O•2,3,4. As O− is present on typical TiO2 and ZnO2,9, Ti–O• can easily react with CH4 and H2O to form •CH3 and •OH:
which was proven by ESR for 2.1% Ag/TiO2 {101} in an aqueous solution (Supplementary Fig. S12). The active •CH3 can couple with the surface O− and •OH to form Ti–OCH3 and CH3OH, as evidenced in the IR range of 1000–1100 cm−1 (Fig. 3A in 4th column). This is the reason why the oxygen in the CH3OH product mainly originates from H2O and Ti–O–Ti rather than O2 in the photocatalytic CH4 oxidation on 2.1% Ag/TiO2 {101} (Fig. 4A, B). The O2 can be reduced by photoinduced electrons to form superoxide anion radicals (O2•−), which can easily react with •CH3 to form CH3OOH associated with the IR signal at 966 cm−1 (Fig. 3A in 4th column)2,9,42:
CH3OOH can readily decompose into formaldehyde (HCHO) and H2O2,9, and HCHO and CH3OH can be overoxidized to CO2 and H2O by •OH radicals. Thus, as long as •CH3 and •OH radicals occur in photocatalytic CH4 oxidation, overoxidation should be unavoidable.
It has been found that the Ti–O–Ti bond angles of the {001} facets are more distorted than those of the {101} facets21, and there are much more pentacoordinated Ti4+ sites present on the {001} facets than that on the {101} facet21,24. All this leads to a higher surface energy (0.90 J m−2) on {001} facets than that (0.44 J m−2) on {101} facets23. In order to further prove the difference of the activity of oxygen sites and the formation of intermediates (oxygen (O) vacancy and surface peroxide species) on {001} and {101} facet of TiO2, the theoretical calculation has been performed (Supplementary Fig. S13). The energy (Ef) required for the formation of O vacancy on {001} facet is much lower than that on the {101} facet, and the energy (Eads) released by O2 adsorption on O vacancy of {001} facet is much higher than that on O vacancy of {101} facet. In one word, the energy (2.717 eV) required for the formation of peroxide intermediate by O2 adsorption on {001} facet is much lower than that (3.983 eV) on {101} facet. Therefore, it can be concluded that, firstly, the O centers of the {001} facet are more active than those of the {101} facet, which is favorable for the formation of O vacancies on the {001} facet upon light irradiation, as confirmed by the ESR signal at g = 1.9996 in Fig. 3B; secondly, it should be easier to form peroxide intermediates by O2 adsorption on {001} facet, as confirmed by the operando FTIR experiment in Fig. 3A.
Thus, unlike CH4 activation on the {101} facet, the primary step of CH4 activation on the {001} facet should be the oxidation of surface oxygen by photoinduced holes to form oxygen vacancies:
which results in a distinct catalytic mechanism appearing on the {001} facet (Fig. 4C). The oxygen vacancy can stabilize the superoxide radical (O2•−), which should be formed by O2 reduction by photoinduced electrons, as in reaction 3, to form surface superoxide (Ti–O2•):
which is associated with the ESR signals at gzz = 2.023, gyy = 2.007, and gxx = 2.000 (Fig. 3B). The surface superoxide can capture photoinduced electrons to form two types of surface peroxides (Ti–OO–Ti and Ti–(OO)):
which corresponds to the appearance of O–O stretching bands at 812 and 943 cm−1 that show increased intensity with light irradiation time in the operando FTIR experiment, respectively (Fig. 3A in 1st column). With the presence of CH4, both Ti–OO–Ti and Ti–(OO) decrease significantly upon light irradiation (Fig. 3A in 2nd column). Theoretically, the dissociation barrier for the surface peroxides into Ti–O• pairs on the {001} facet of anatase TiO2 is 1.0–1.4 eV, which can be overcome by the photon energy available from UV and visible light irradiation43:
The Ti–O• pairs can split CH4 to generate adjacent surface methoxyl and hydroxyl groups (Ti–OCH3 HO–Ti), which are associated with the NMR signals observed 263 and 180 ppm, respectively (Fig. 3C), subsequently releasing CH3OH:
This should be the reason why the oxygen in the CH3OH product mainly originates from O2 rather than H2O and Ti–O–Ti on 2.5% Ag/TiO2 {001} (Fig. 4A, B), with almost no •CH3 and •OH generated in the photocatalytic CH4 oxidation on 2.5% Ag/TiO2 {001} (Supplementary Fig. S12). Obviously, this reaction path can effectively hinder the formation of CH3OOH. According to the ESR spectra (Supplementary Fig. S12), there are no •OH species present in the photocatalytic CH4 oxidation on 2.5% Ag/TiO2 {001}, which can greatly reduce the overoxidation of CH3OH by •OH2. In addition, the presence of water in the reactions could promote the desorption of methoxyl/methanol from the surface of catalysts into an aqueous solution to avoid overoxidation of methanol to CO and CO215,16,17, since the product CH3OH can physically or chemically adsorb onto the photocatalyst surface4, and can be further oxidized with the surface-active species (such as Ti–O•, peroxides, and superoxide) to form CO and CO29,10. Thus, the aqueous phase is important to improve CH3OH selectivity in a photocatalytic CH4 oxidation reaction.
The proposed mechanism can further shed light on the results obtained for the photocatalytic activity and selectivity relative to the Ag loading amount on both TiO2 {001} and TiO2 {101}. As observed in Fig. 1A, B, the initial increase in Ag loading can increase the photocatalytic activity and selectivity for both TiO2 {001} and TiO2 {101}. To get insight into the role of Ag, the operando ATR-FTIR experiments on TiO2 {001} and TiO2 {101} have been performed (Supplementary Fig. S14). Similar to 2.5%Ag/ TiO2 {001}, light irradiation also led to two types of surface peroxo intermediates (Ti–OO–Ti and Ti–(OO)) for the TiO2 {001} in the aqueous phase saturated with Ar and O2 (Ar + O2). On the other hand, similar to 2.1%Ag/TiO2 {101}, almost no peak appears in the 800–1000 cm−1 region for the TiO2 {101} in the aqueous phase with Ar + O2 during light irradiation. It can be indicated that the formation of surface peroxo intermediates mainly occurred on the {001} facet of TiO2. The in situ ESR experiments were used to follow the formation of paramagnetic intermediates on TiO2 {001} and TiO2 {101} (Supplementary Fig. S15). Different from 2.5%Ag/ TiO2 {001}, no surface superoxide (Ti–O2•) was observed on both TiO2 {001} and TiO2 {101} in the presence of 300 μmol H2O and 24 μmol O2 during light irradiation, while a small number of oxygen vacancies was formed on TiO2 {001}. It can be indicated that Ag loading contributes to the formation of Ti–O2• on TiO2 {001}. This may have two reasons: firstly, Ag as a cocatalyst can trap the photogenerated electrons to reduce molecular O2 to form O2− species; secondly, the electron trapping on cocatalyst Ag can effectively improve the separation of photogenerated electrons and holes, which can lead to the increase of O vacancy formation arisen from the oxidation of surface oxygen by photogenerated hole on the {001} facet of TiO2 (Fig. 3B). As a result, the observable O2− species is stabilized at the O vacancy on the {001} facet of TiO2 loaded with Ag (Fig. 3B). In addition to the role of Ag in separating the electron-hole pairs to increase the activity, more Ag loading can reduce the exposed area of the {101} facet since the Ag is mainly photodeposited on the {101} facet. Instead, the {001} facet will continue to be exposed to the solution, resulting in superior selectivity. However, excessive loading of Ag will decrease the activity because of the intrinsic light absorption by Ag, while the selectivity can remain high since the {001} facet can still be exposed to the solution.
Discussion
In summary, we report the photocatalytic oxidation of CH4 into CH3OH by molecular O2 on anatase TiO2. To compare the microstructure and catalytic mechanism on the {001} and {101} facets of TiO2, two types of TiO2 with predominantly exposed {001} or {101} facets were prepared. By selectively photodepositing a Ag cocatalyst onto the {101} facet of TiO2 to facilitate the separation and transfer of photoinduced carriers, the CH3OH yield can be promoted significantly. According to studies based on operando FTIR, in situ ESR, and NMR techniques, completely different catalytic mechanisms exist for CH4 photooxidation by O2 on the {001} and {101} facets. It was found that oxygen vacancies on {001} facets are generated in a straightforward manner by photoinduced holes and that these photogenerated oxygen vacancies can stabilize superoxide radicals (Ti–O2•). Ti–O2• can capture photoinduced electrons to form surface peroxides (Ti–OO–Ti and Ti–(OO)), and the surface peroxides dissociate into Ti–O• pairs, which can split CH4 to release CH3OH directly. This distinct catalytic mechanism effectively avoids the formation of •CH3 and •OH, which are the main factors leading to overoxidation and are generally formed on the {101} facet. Thus, the optimized {001} facet-dominated TiO2 sample shows an impressively high CH3OH yield of 4.8 mmol g−1 h−1 with high CH3OH selectivity of ~80%. This study will provide a strategy to avoid overoxidation in CH4 reforming to CH3OH in other photocatalysts by controlling the generation of photoinduced oxygen vacancies.
Methods
Sample preparation
The anatase TiO2 with predominantly exposed {001} facets (TiO2 {001}) were prepared according to the previous report44. 25 mL of Ti(OBu)4 and 4 mL of hydrofluoric acid solution were mixed in a dried Teflon autoclave with a capacity of 100 mL, and then kept at 180 °C for 24 h. After being cooled to room temperature, the white powder was separated by high-speed centrifugation and washed with ethanol, 0.1 M NaOH aqueous solution, and deionized water several times to remove F− on the surface.
The anatase TiO2 with predominantly exposed {101} facets (TiO2 {101}) were prepared according to the previous report45. Firstly, for the preparation of Ti(OH)4 precursor, 6.6 mL of TiCl4 was added to aqueous HCl (0.4 mol L−1) drop by drop under strong stirring in an ice bath to obtain an aqueous TiCl4. This TiCl4 aqueous was then added to aqueous NH3·H2O (5.5 wt.%) drop by drop under stirring. White Ti(OH)4 precipitate could be formed during the process. Afterward, the aqueous NH3·H2O (4.0 wt.%) was added to adjust the pH value to 6–7. After aging at room temperature for 2 h, the suspension was centrifuged, and the precipitate was washed with ethanol, 0.1 M NaOH aqueous solution, and deionized water several times to remove Cl− on the surface.
4.0 g the fresh Ti(OH)4 precursor was first dispersed in the mixture of 30 mL deionized water and 30 ml isopropanol. After stirring and ultrasonic treatment, the suspension was transferred to a 100 mL Teflon-lined autoclave and heated for 24 h at 180 °C. The products were collected by centrifugation and washed with deionized ethanol one time and water three times. According to the EDX of TiO2 {101} and TiO2 {001} (Supplementary Fig. S16), there is no fluorine or chlorine atoms present on these TiO2.
The Ag-loaded TiO2 catalyst was prepared by in situ photodeposited reactions of AgNO3 with TiO2. Briefly, 0.4 g of the prepared TiO2 was suspended in an anaerobic aqueous solution containing deionized water (20.0 mL), CH3OH (5.0 mL), and a certain number of AgNO3. After 0.5 h irradiation under 300 W Xe lamp, the products were collected via centrifugation, then washed by water and dried at 60 °C.
Characterization
A homemade spectral cell for attenuated total reflectance (ATR) Fourier transform infrared spectroscopy (FTIR) experiments is shown in Supplementary Fig. S1732. An internal reflection element (IRE) made of ZnSe (size: 50 mm × 20 mm × 3 mm, incident angle: 60°) was obtained from Pier Optics Co., Ltd. Japan. 20 mL TiO2 suspensions of 5 mg mL−1 in ethanol were drop casted on the IRE surface and dried in air. The IRE with TiO2 coating was then set into the homemade spectral cell. 4 mL of water was purged by the gas for different purposes and was then injected into the cell. Before the Operando ATR-FTIR experiments, the cell was kept in dark for 5 min.
An FTIR spectrometer (FT/IR-6300, Jasco Inc.) with liquid nitrogen cooled MCT detector was used and purged by dry nitrogen before ATR-FTIR experiments. The absorbance spectra ranged from 4000 to 500 cm−1 were obtained by repeated 32 scans with a resolution of 4 cm−1 and a processing time of 30 s. The background was monitored and recorded in every minute of 5 min before the light illumination. Thereafter, the spectra were obtained at every minute of 10 min with light illumination and after light illumination.
17O solid-state NMR spectra were acquired at 18.8 T on the Bruker Avance III spectrometers, equipped with a 4 mm double-resonance probe. The Larmor resonance frequencies for the 1H and 17O resonances were 800.4 and 108.5 MHz, respectively. 17O MAS NMR spectra were acquired using a typical π/2 pulse length of 2.5 μs, with a recycle delay of 1.0 s and a 1H decoupling field strength of 130 kHz. The experiments were carried out with a MAS frequency of 13.5 kHz. 120,000 scans were accumulated to acquire each spectrum. The chemical shifts of 17O resonance signals were referred to as liquid H217O. Prior to ex-situ 17O NMR measurements, CH4 (30 μmol) and 17O2 (20μmol) were introduced into a glass ampule containing 0.05 g TiO2 catalyst under vacuum at the liquid N2 temperature, and then the glass ampule was sealed off. The photoreaction was performed in the sealed ampule under successive irradiation by a 300 W Xe lamp, and then the ampule was transferred into the rotor for the ex situ NMR measurements.
A homemade spectral cell for in situ electron spin resonance (ESR) experiments is shown in Supplementary Fig. S18. In situ ESR experiments were carried at X-band using a JOEL FA 2000 spectrometer. The microwave frequency was 9.1 GHz, the modulation amplitude was 0.1 mT, the microwave power was 5 mW and the experimental temperature was 25 °C. The g values of the radical species were referenced to Mn-marker. The Mn-marker is Mn2+ in the CaO with g = 2.0009.
Photocatalytic measurements
The photocatalytic methane oxidation reaction tests were conducted in a 230 mL batch reactor equipped with a quartz window to allow light irradiation. 10 mg catalyst was dispersed in 100 mL water by ultrasonication for 10 min. Then, the mixture was added into a glass cell with a volume of 30 mL, and the glass cell was placed in the batch reactor. The actual working volume decreased to 100 mL. The batch reactor was purged with O2 (purity, 99.99995%) for 15 min to exhaust air. After that, the reactor vessel was pressurized with 0.1 MPa O2 and 2 MPa CH4 (purity, 99.9995%). Subsequently, the reactor was loaded into a cold-water bath, and the solution was stirred at 1200 rpm. 300 W Xe lamp was used as the light source with wavelength ranging from 300 to 500 nm and the light intensity of 450 mW cm−2. A thermocouple was inserted into the solution to directly detect the temperature of the liquid solution. During the reaction process, the temperature of the liquid solution was maintained at 25 °C. After the reaction, the reactor was cooled in an ice bath to a temperature below 10 °C. Then the gas product was collected, and the concentrations of gas products were analyzed by gas chromatograph (GC, Shimadzu) equipped with methanizer and flame ionization detector. The liquid phase of the reaction mixture product was collected by centrifugation. The liquid product was analyzed by nuclear magnetic resonance spectroscopy (NMR) and the colorimetric method.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Ravi, M., Ranocchiari, M. & van Bokhoven, J. A. The direct catalytic oxidation of methane to methanol—a critical assessment. Angew. Chem. Int. Ed. 56, 16464–16483 (2017).
Song, H. et al. Direct and selective photocatalytic oxidation of CH4 to oxygenates with O2 on cocatalysts/ZnO at room temperature in water. J. Am. Chem. Soc. 141, 20507–20515 (2019).
Liu, F. et al. Transfer channel of photoinduced holes on a TiO2 surface as revealed by solid-state nuclear magnetic resonance and electron spin resonance spectroscopy. J. Am. Chem. Soc. 139, 10020–10028 (2017).
Yang, L. et al. Surface water loading on titanium dioxide modulates photocatalytic water splitting. Cell Rep. Phys. Sci. 1, 100013 (2020).
Volodin, A. M. & Cherkashin, A. E. ERS spectrum of methyl radicals on ZnO surface. React. Kinet. Catal. Lett. 18, 243–246 (1982).
Maldotti, A., Molinari, A. & Amadelli, R. Photocatalysis with organized systems for the oxofunctionalization of hydrocarbons by O2. Chem. Rev. 102, 3811–3836 (2002).
Latimer, A. A. et al. Understanding trends in C–H bond activation in heterogeneous catalysis. Nat. Mater. 16, 225–229 (2017).
Song, H. et al. Solar-energy-mediated methane conversion. Joule 3, 1606–1636 (2019).
Chen, X. et al. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 7, 12273 (2016).
Yu, X., De Waele, V., Löfberg, A., Ordomsky, V. & Khodakov, A. Y. Selective photocatalytic conversion of methane into carbon monoxide over zinc-heteropolyacid-titania nanocomposites. Nat. Commun. 10, 700 (2019).
Li, Z., Pan, X. & Yi, Z. Photocatalytic oxidation of methane over CuO-decorated ZnO nanocatalysts. J. Mater. Chem. A 7, 469–475 (2019).
Murcia-López, S., Villa, K., Andreu, T. & Morante, J. R. Partial oxidation of methane to methanol using bismuth-based photocatalysts. ACS Catal. 4, 3013–3019 (2014).
Villa, K., Murcia-López, S., Andreu, T. & Morante, J. R. Mesoporous WO3 photocatalyst for the partial oxidation of methane to methanol using electron scavengers. Appl. Catal. B 163, 150–155 (2015).
Murcia-López, S. et al. Controlled photocatalytic oxidation of methane to methanol through surface modification of beta zeolites. ACS Catal. 7, 2878–2885 (2017).
Latimer, A. A., Kakekhani, A., Kulkarni, A. R. & Nørskov, J. K. Direct methane to methanol: the selectivity–conversion Limit and design strategies. ACS Catal. 8, 6894–6907 (2018).
Lustemberg, P. G. et al. Direct conversion of methane to methanol on Ni-Ceria surfaces: metal–support interactions and water-enabled catalytic conversion by site blocking. J. Am. Chem. Soc. 140, 7681–7687 (2018).
Xie, J. et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species. Nat. Catal. 1, 889–896 (2018).
Song, H. et al. Selective photo-oxidation of methane to methanol with oxygen over dual-cocatalyst-modified titanium dioxide. ACS Catal. 10, 14318–14326 (2020).
Agarwal, N. et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223–227 (2017).
Tian, N., Zhou, Z.-Y., Sun, S.-G., Ding, Y. & Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316, 732–735 (2007).
Selloni, A. Anatase shows its reactive side. Nat. Mater. 7, 613–615 (2008).
Yin, Y. & Alivisatos, A. P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437, 664–670 (2005).
Yang, H. G. et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638–641 (2008).
Pan, J., Liu, G., Lu, G. Q. & Cheng, H.-M. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew. Chem. Int. Ed. 50, 2133–2137 (2011).
Feng, N. et al. Understanding the high photocatalytic activity of (B, Ag)-codoped TiO2 under solar-light irradiation with XPS, solid-state NMR, and DFT calculations. J. Am. Chem. Soc. 135, 1607–1616 (2013).
Zhu, X. et al. Facet-selective deposition of a silver–manganese dual cocatalyst on potassium hexatitanate photocatalyst for highly selective reduction of carbon dioxide by water. Appl. Catal. B 274, 119085 (2020).
Gao, D. et al. Core-shell Ag@Ni cocatalyst on the TiO2 photocatalyst: one-step photoinduced deposition and its improved H2 evolution activity. Appl. Catal. B 260, 118190 (2020).
Yu, X. et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 5, 511–519 (2020).
Wang, Z. et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 6, 3007–3014 (2013).
Wang, Y., Feng, C., Zhang, M., Yang, J. & Zhang, Z. Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl. Catal. B 100, 84–90 (2010).
Nakamura, R. & Nakato, Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J. Am. Chem. Soc. 126, 1290–1298 (2004).
Nakamura, R., Imanishi, A., Murakoshi, K. & Nakato, Y. In situ FTIR studies of primary intermediates of photocatalytic reactions on nanocrystalline TiO2 films in contact with aqueous solutions. J. Am. Chem. Soc. 125, 7443–7450 (2003).
Ding, Q. et al. Unravelling the water oxidation mechanism on NaTaO3-based photocatalysts. J. Mater. Chem. A 8, 6812–6821 (2020).
Yamada, H. & Hurst, J. K. Resonance Raman, optical spectroscopic, and EPR characterization of the higher oxidation states of the water oxidation catalyst, cis,cis- (bpy)Ru(OH)2O4+. J. Am. Chem. Soc. 122, 5303–5311 (2000).
Root, D. E., Mahroof-Tahir, M., Karlin, K. D. & Solomon, E. I. Effect of protonation on peroxo-copper bonding: spectroscopic and electronic structure study of Cu-2((UN-O-)(OOH)(2+). Inorg. Chem. 37, 4838–4848 (1998).
Liu, H. F. & Frei, H. Observation of O-O bond forming step of molecular Co4O4 cubane catalyst for water oxidation by rapid-scan FT-IR spectroscopy. ACS Catal. 10, 2138–2147 (2020).
Chiarello, G. L., Ferri, D. & Selli, E. In situ attenuated total reflection infrared spectroscopy study of the photocatalytic steam reforming of methanol on Pt/TiO2. Appl. Surf. Sci. 450, 146–154 (2018).
Chen, Z. & Wang, C. Rate constants of the gas-phase reactions of CH3OOH with O3 and NOx at 293K. Chem. Phys. Lett. 424, 233–238 (2006).
Attwood, A. L., Murphy, D. M., Edwards, J. L., Egerton, T. A. & Harrison, R. W. An EPR study of thermally and photochemically generated oxygen radicals on hydrated and dehydrated titania surfaces. Res. Chem. Intermed. 29, 449–465 (2003).
Che, M. & Tench, A. J. Advances in Catalysis (eds Eley, D. D., Pines, H., & Weisz, P. B.) Vol. 32, 1–148 (1983).
Li, Y. et al. Distinguishing faceted oxide nanocrystals with 17O solid-state NMR spectroscopy. Nat. Commun. 8, 581 (2017).
Ito, T. & Lunsford, J. H. Synthesis of ethylene and ethane by partial oxidation of methane over lithium-doped magnesium oxide. Nature 314, 721–722 (1985).
Liu, L., Wang, Z., Pan, C., Xiao, W. & Cho, K. Effect of hydrogen on O2 adsorption and dissociation on a TiO2 anatase (001) surface. ChemPhysChem 14, 996–1002 (2013).
Han, X., Kuang, Q., Jin, M., Xie, Z. & Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 131, 3152–3153 (2009).
Liu, L. et al. Anion-assisted synthesis of TiO2 nanocrystals with tunable crystal forms and crystal facets and their photocatalytic redox activities in organic reactions. J. Phys. Chem. C 117, 18578–18587 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21673283, 21633004, and 21733013), JSPS KAKENHI Grant Number JP18H02065, Photoexcitonix Project in Hokkaido University, and Scholarship Fund of Chinese Academy of Sciences. We acknowledge Dr. Min Huang (Hubei University) for theoretical calculation.
Author information
Authors and Affiliations
Contributions
J.Y. and N.F. conceived the project. N.F. designed the studies and synthesized the TiO2 photocatalysts. N.F. and H.S. performed photocatalytic activity experiments. H.L. performed the in situ FTIR experiments. N.F., L.Y., and F.D. performed in situ ESR and NMR experiments. D.T. performed HRTEM experiments. N.F. and H.L. analyzed all the experimental data. N.F. and H.L. wrote the first draft of the manuscript. All authors interpreted the data and contributed to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, N., Lin, H., Song, H. et al. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat Commun 12, 4652 (2021). https://doi.org/10.1038/s41467-021-24912-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-24912-0
This article is cited by
-
Photocatalytic aerobic oxidation of C(sp3)-H bonds
Nature Communications (2024)
-
Insight into selectivity of photocatalytic methane oxidation to formaldehyde on tungsten trioxide
Nature Communications (2024)
-
Unusual facet and co-catalyst effects in TiO2-based photocatalytic coupling of methane
Nature Communications (2024)
-
Selective photocatalytic oxidation of methane to C1 oxygenates by regulating sizes and facets over Au/ZnO
Nano Research (2024)
-
Interfacial interaction promoted titanium oxide-based organic-inorganic nanoheterojunctions by chiral host-guest binding
Communications Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.