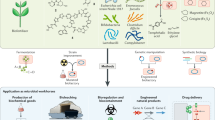
Abstract
A central goal of synthetic biology is to predictably and efficiently reprogram living systems to perform computations and carry out specific biological tasks. Although there have been many advances in the bio-computational design of living systems, these advances have mainly been applied to microorganisms or cell lines; programming animal physiology remains challenging for synthetic biology because of the system complexity. Here, we present a bacteria-animal symbiont system in which engineered bacteria recognize external signals and modulate animal gene expression, twitching phenotype, and fat metabolism through RNA interference toward gfp, sbp-1, and unc-22 gene in C. elegans. By using genetic circuits in bacteria to control these RNA expressions, we are able to program the physiology of the model animal Caenorhabditis elegans with logic gates. We anticipate that engineered bacteria can be used more extensively to program animal physiology for agricultural, therapeutic, and basic science applications.
Similar content being viewed by others
Introduction
Recent advances in synthetic biology have resulted in highly programmable bacteria and mammalian cells that perform specific functions1. At the bacterial level, researchers have created logic functions and memory in living bacteria for bacteria and community oscillation, programmed bacteria to respond to signals, automated the circuit design process, and expanded the repertoire of logic gates so that they can recognize a variety of signals, including light and nutrients1,2,3. At the level of mammalian cells, researchers have engineered CAR-T cells and other mammalian cell types with switching logic circuits to program cell performance4,5,6,7,8. Despite rapid advances and promising results in bio-computational design, the programming of higher organisms remains largely unexplored because the engineering of an entire organism requires highly complex and sometimes impossible tasks, such as the delivery of gene editing cascades9,10,11 or the engineering of whole embryos12,13. Nevertheless, such programming is worthwhile because it will yield a greater understanding than we have so far of how biological phenomena emerge from genetic and cellular events. The ability to change animal including insects and worm physiology would open up possibilities for agriculture, biomedical, and bioenvironmental applications.
Animals share a life-long relationship with microbes, which play significant roles in animal nutrition, immunity, behavior, and metabolism14,15,16,17. Engineering bacteria have demonstrated their potentials with pest control18,19, plant growth promotion20, human disease diagnosis21,22,23, and therapeutics24. However, little has been done in terms of transferring the decision-making logics from bacteria to the animals to program animal physiology. Here, using bacteria as an engineering platform, we present an efficient strategy to program Caenorhabditis elegans GFP expression, twitching phenotype, and fat metabolism by building synthetic logic circuits in bacteria. We used the nematode C. elegans as the animal host as it has been extensively used as a model system to elucidate mechanisms of interaction between prokaryotes and their hosts25,26. The information from bacteria was transferred to C. elegans through an orthogonal gene transfer called RNA interference (RNAi), a widely used technique in which double-stranded RNA is exogenously introduced into an organism, causing knockdown of a target gene27. RNA interference is a regulatory mechanism conserved in eukaryotes that plays key roles in numerous biological processes, including RNA stability and processing, biotic and abiotic stress response, and the regulation of morphological and developmental events28. Horizontal transfer of mobile RNAs between different species has been observed between pathogens/parasites and host animals, pathogens/parasites and host plants, and plants and animals28. In C. elegans, RNAi is easy to implement because RNA can be delivered by feeding the worms bacteria that express double-stranded RNA complementary to a C. elegans gene of interest29. The inhibitory RNA is delivered from the bacterial cell to C. elegans when C. elegans grind on the bacteria by pharynx and absorb the bacterial contents29. The absorbed inhibitory RNA will then be distributed throughout the worm body leading to RNA interference29,30.
In this work, we engineer bacteria to produce RNA, and by putting the RNA expression under the control of genetic circuits in bacteria, we program C. elegans green fluorescence protein (GFP) expression profiles, twitching phenotype, and worm fat storage with “AND” and “OR” logic gates. This study presents a platform for the programming of higher organisms by harnessing engineered prokaryotic species to exert effects on eukaryotic organisms (Fig. 1).
Results
Using RNA in ingested bacteria to silence GFP in C. elegans through RNAi
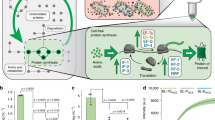
We started with the genetically engineered strain C. elegans SD1084, which expresses nuclear-localized SUR-5::GFP31. We intended to use the chemical inducer IPTG to silence GFP expression in this C. elegans strain through the worms’ ingestion of engineered bacteria. To achieve that, we put gfp dsRNA synthesis under the control of a bidirectional lac promoter, which induces the synthesis of RNA in E. coli under IPTG induction. While many E. coli strains including HT115(rnc-), BL21(DE3), and OP50(xu363) have been utilized to produce RNA for RNAi in worm32,33, within which HT115(rnc-) lacks RNase III to produce most dsRNA32 and BL21 has the lowest RNA producing levels32. We started with BL21 because the relatively lower dsRNA production is correlated with less RNAi efficiency in C. elegans and thus can potentially provide a dynamic range of RNAi for a complete “ON” and OFF” state32. We used BL21(DE3) throughout this work. By feeding these induced plasmid carrying BL21(DE3) to C. elegans, we aimed to trigger gfp RNAi, which silences gfp in C. elegans31 (Fig. 2a). We expected that the differences between the “ON” and “OFF” states of the RNAi in C. elegans would be large enough for us to measure. However, the first round of experiments with full-length gfp dsRNA led to gfp silencing under both the induced (i.e., with IPTG) and the uninduced (i.e., without IPTG) conditions (Supplementary Fig. 1). We hypothesized that the leaky lac promoter already produced enough dsRNA to silence the worm gfp without IPTG. Rather than solve the problem by tuning the strength of the lac promoter, which is a less desirable approach because tuning all promoters for genetic circuits in the future is highly laborious, we decided to work on the intermediates between the bacteria and the worm to achieve an appropriate dynamic range.
a Schematic of engineered E. coli triggering RNAi in C. elegans through feeding. b Quantification of C. elegans GFP fluorescence intensity controlled by bacteria yielded single-stranded gfp RNA of various lengths with no IPTG and 1 mM IPTG. c Optical and fluorescent images of C. elegans SD1084 GFP expression programmed by bacterial single-stranded 200 bp gfp RNA (ss200) with no IPTG and 1 mM IPTG. Scale bar, 100 µm. The experiment was repeated three times independently for a total of 15 worms with similar results. d C. elegans SD1084 GFP intensity change caused by varying IPTG concentration-induced RNA synthesis. (***P < 0.001 using two-sided t-test. Error bars represent the mean ± standard deviation for n = 15 worms over three independent experiments). Source data are available in the Source Data file.
Systemic RNAi in C. elegans requires systemic RNA interference deficiency-1 (SID-1) protein for the transport of RNA34,35. SID-1 has been shown to have a lower binding affinity for shorter RNA sequences34 leading to lower RNAi efficiency. We hypothesized that we could achieve the right dynamic range of RNAi by reducing the length of the RNA and thus transporting less RNA into the host with less RNAi efficiency. We constructed plasmids for RNA synthesis with lengths ranging from 100 bp to 400 bp in double-stranded as well as single-stranded format (Supplementary Fig. 2). After feeding the bacteria containing these plasmids to the worms, we found that single-stranded RNA sequences of 200 bp, 300 bp, and 400 bp achieved distinguishable “ON” and “OFF” status of GFP expression in C. elegans with and without IPTG (Fig. 2b). We picked the single-stranded 200 bp gfp RNA for subsequent experiments (Fig. 2c).
The efficiency of RNAi can be manipulated by changing the RNA concentration27,34, which can be controlled by the concentration of inducers. Using the 200-bp design, we tested the dynamic range of the RNAi. We achieved a silencing of the GFP in C. elegans along a gradient, such that 81% of GFP in C. elegans was silenced when 15 μM of IPTG was supplied on the plate (Fig. 2d and Supplementary Fig. 3A). To further support our observation on the dose-dependent silencing effect, quantitative reverse transcription PCR (RT-qPCR) was used to measure the RNA synthesis levels at different IPTG concentrations. The bacterial RNA concentration is consistent with the worm RNAi efficiency (Supplementary Fig. 3B). What is worth mentioning here is that the C. elegans strain SD1084 we picked express nuclear-localized SUR-5::GFP31 in all cells with a flag-tagged GES-1 (a carboxylesterase) protein co-overexpressed31. Since neuronal genes are more difficult to target with RNAi36, we usually see ~20% residual fluorescence on our “OFF” stage, which should come from the neuronal GFP that were not RNAi silenced.
Programming GFP expression with logic gates
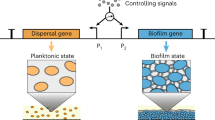
Logic gates, the building blocks of genetic circuits, can process complex signals and trigger downstream reactions1. Here we put gfp RNA synthesis under the control of an “AND” or an “OR” gate and transferred the programming languages to C. elegans through RNAi.
An “AND” gate can be built by utilizing the split T7 polymerase (T7 RNAP)37. T7 RNAP is a single-subunit RNA polymerase that drives transcription by acting on its cognate promoter, the T7 promoter PT7. By splitting T7 RNAP between amino acids 179 and 180, a transcriptional AND gate is created whereby both fragments of the split protein are needed to drive transcription from PT737. We used two orthogonal promoters, pBAD and pTet, to control T7 RNAP N-terminal and C-terminal expression, respectively. Both l-arabinose (Ara) and anhydrotetracycline (aTc), the respective inducers of these promoters, are needed to induce synthesis of gfp ssRNA coding strand fragment in the bacteria. The gfp RNA fragment will then lead to RNAi in the worm after the worm grinds the bacteria open and absorb the RNA fragment. With this system, worm GFP expression was silenced by >70% in the presence of both signals (Ara and aTc) whereas GFP was still expressed (>95%) with either no signal or only one of the two signals (Fig. 3a and Supplementary Fig. 4A). The RNA levels synthesized in the bacteria were consistent with the AND gate profile that was observed in the worm experiment (Supplementary Fig. 4C).
Single-stranded 200-bp gfp RNA synthesis in E. coli BL21, controlled by a AND gate or b OR gate, interferes with GFP expression in C. elegans that have ingested the bacteria. (***P < 0.001 using two-sided t-test with Bonferroni correction. Error bars represent the mean ± standard deviation for n = 15 worms over three independent experiments) Source data are available in the Source Data file.
For the OR gate, the pTet and pBAD were arranged in tandem to control the synthesis of full-length T7 polymerase, which transcribes single-stranded RNA against GFP in engineered bacteria under the control of promoter PT7. Activation of either pTet or pBAD was expected to lead to GFP silencing in C. elegans. We observed that the addition of one or both of the inducers led to >90% reduction in C. elegans GFP intensity, while the no-inducer control group only had a 24% reduction (Fig. 3b and Supplementary Fig. 4B). Similar to the AND gate, the RNA levels measured by RT-qPCR was consistent with the OR gate profile (Supplementary Fig. 4D).
Programmed C. elegans physiology with logic gates
After demonstrating that C. elegans GFP expression could be programmed with bacterial genetic circuits, we programmed essential gene silencing that affects C. elegans physiology. First, we programmed the twitching phenotype in C. elegans by feeding unc-22 RNA producing bacteria to worm to interfere with the UNC-22 protein expression through RNAi in the worm. Since unc-22 encodes a polypeptide involved in locomotion35, we were able to program the twitching phenotype in C. elegans. Using similar approaches as those described above for GFP, we screened out double-stranded 400 bp and 200 bp unc-22 RNA for the “AND” and “OR” gates of worm twitching phenotype (Fig. 4a). The twitching phenotype was characterized by observing the C. elegans body movement under a microscope immediately after the worms were anesthetized by levamisole38. The AND gate was constructed by using the same genetic circuit-carrying plasmid and replacing the gene of interest with unc-22. Only the worms grown on plates with both inducers showed the twitching phenotype. In all, 100% of the worms exhibited twitching phenotype in the presence of both signals while 0% of the worms on the other three plates (no inducers plate, aTc only plate, and Ara only plate) showed twitching phenotype. Using the same OR gate but replacing the GFP in the OR genetic circuits with unc-22, 100% of the worms grown on plates with one or both inducers showed twitching phenotype (Fig. 4b and Supplementary Fig. 5).
a Schematic of twitching phenotype RNAi in C. elegans. b C. elegans twitching phenotype in response to AND and OR gate programming through bacteria expressing 200 bp and 400 bp double-stranded unc-22 RNA. c Reduced fat storage due to sbp-1 silencing, indicated by Nile Red staining. Scale bar, 100 µm. The experiment was repeated three times independently for a total of 15 worms with similar results. d AND and OR gate-controlled C. elegans metabolism through sbp-1 RNA. (***P < 0.001 using two-sided t-test with Bonferroni correction. Error bars represent the mean ± standard deviation for n = 200 worms over three independent experiments for twitching phenotype, and n = 15 worms over three independent experiments for fat storage) Source data are available in the Source Data file.
Besides twitching phenotype, we programmed C. elegans metabolism through the sbp-1 gene. Previous studies have shown that sbp-1 facilitates fat storage, and silencing sbp-1 using RNAi leads to reduced body fat storage in C. elegans39. We modulated C. elegans fat storage by using sbp-1 RNAi (Fig. 4c); the modulation of fat storage was characterized by changes in Nile red staining. Because full-length sbp-1 RNA modulated C. elegans fat storage with a clear “ON” and “OFF” status after inducer concentration optimization (Supplementary Fig. 6C), RNA length optimization experiments were not performed as in the unc-22 experiment. When the worms grew on plates with bacteria containing plasmids for the AND gate and sbp-1 RNA synthesis, only the group that had both inducers showed a significant reduction in both worm body size and fat storage as indicated by a reduction in Nile red staining. For the engineered OR gate, the group without inducers had a body size and a level of fat storage similar to those of normal C. elegans (Fig. 4D and Supplementary Fig. 6). All other groups, which were exposed to either one or both of the inducers, showed an over 60% reduction in fat storage, demonstrating the successful OR gate programming of C. elegans fat storage.
Discussion
Although the technology for genetically engineering living organisms has advanced rapidly, the complex design of genetic circuits and gene editing in animals remain challenging because of the complexity of these systems. In contrast, genetic circuit design and editing have been highly successful in bacteria and mammalian cells. Bacteria affect the metabolism, immunity, and behavior of the animals they live in and on. We took advantage of the symbiosis between bacteria and animals to engineer prokaryotic cells as an indirect, alternative approach to programming animal physiology.
Using E. coli as the engineering platform and C. elegans as the host animal, we tuned C. elegans gene expression by changing the quantity of the RNA produced by the bacteria. The silencing of C. elegans gene expression was accomplished, through RNAi, with a reasonable dynamic range of programmable gene expression and physiology. We determined the effective dynamic range by screening out different lengths of RNA as mediators between bacterial and animal. An alternative way to tune the dynamic range is by changing the inducer concentration and incorporating T7 lysozyme to reduce basal RNA expression. This approach for basal level reduction as an alternative to shortening the RNA length is important because the logic gates can be tuned for enzyme expression rather than RNA synthesis. Furthermore, we applied genetic circuits to program C. elegans GFP expression, twitching phenotype, and fat storage through “AND” and “OR” logic gates. The fact that none of these worm phenotypes were affected by the inducers used in this study (l-arabinose and anhydrotetracycline) demonstrated that C. elegans underwent real programming by the engineered bacteria. The most striking advantage of this system is the ease with which system complexity can be extended by applying more complicated logic gates. Since bacteria have already been engineered with more complicated logic gates, memory, and sensing more signals, we should be able to program the animals with three or four input logic gates3 or extend the capability of animals to recognize external signals2,40,41 transmitted by these engineered bacteria.
RNAi is especially useful in the area of pests control and worm physiology interference as a gene silencing tool. However, for higher organisms in which RNAi would not be sufficient to alter physiology, bacterial metabolites could be exploited to serve as mediators between bacterial and animal (e.g., bile acid42,43,44, butyrate45,46,47, colonic acid48, and nitrite49,50,51) to communicate between bacteria and higher organisms. Metabolites as intermediaries have broad applications for studying microbe-host symbiosis in situ; for example, they can be used as a drug delivery system. Indeed, bacterial metabolites operate as signaling molecules in the microbiome. Secondly, in this study we used synthetic inducers that were manually introduced into the host environment.
In summary, we have programmed C. elegans through the ingestion of engineered bacteria to exhibit changes in gene-expression and physiology. Our strategy could be extended to other bacteria or hosts, including fruit flies, zebrafish, plants, or mammals. The potential to program higher organisms using bacterial RNA or metabolites as intermediaries enabled us to study the bacteria-animal interaction.
Methods
Bacterial strains, worm strains, and growth conditions
Bacterial strain E. coli BL21 (DE3) was used for testing of constructed plasmids encoding the RNA production without logic gate control. E. coli BL21 was transformed with both the constructed logic gate and RNA production plasmids. E. coli OP50 was used for maintenance of C. elegans. Bacterial cultures were grown in LB media with appropriate antibiotics in a 37 °C shaking incubator overnight before use.
Wild-type Caenorhabditis elegans Bristol N2 and SD1084 (Caenorhabditis Genetics Center, Minnesota, USA) were cultured at room temperature on NGM (Nematode Growth Medium) agar plated with E. coli OP50 as described in WormBook52. Synchronized L1 worms were used in all RNAi treatment experiments. Synchronization was performed following the standard protocol52. Briefly, worms were allowed to grow on NGM plates with E. coli OP50 for 2–4 days then washed off the plates to collect a large number of gravid hermaphrodites, and eggs were isolated by bleach-NaOH lysis of the gravid worms. After 4X wash in M9 buffer to remove the residual bleach and NaOH, eggs were allowed to hatch in 10 mL of M9 buffer overnight and develop into starved L1, which were then used for the RNAi experiments.
Plasmid construction for RNA synthesis and logic gates
All plasmids were constructed using PCR and Gibson assembly and primers used are listed in Supplementary Table 1. Gene sequences used in this study are detailed in Supplementary Table 1. For double-stranded gfp and unc-22 RNA synthesis, the RNA sequence of interest was inserted between two T7/LacO for the synthesis of both complementary strands on a pET24a vector. (Using this method, pET24a-dsgfp, pET24a-dsgfp-100bp, pET24a-dsgfp-400bp, pET24a-dsunc-22-200bp, and pET24a-dsunc-22-400bp were constructed.) Additional T7 lysozyme and LacI were constitutively expressed under the control of the proD promoter for double-stranded sbp-1 RNAi to reduce background expression level for the construction of the plasmid pET24a-dssbp-1-proD-lysS-proD-lacI. We have added ProD promoter into the plasmid to overexpress promoter repressors, which improves the tightness of the pTet and pBAD promoters in the genetic circuit. We also used ProD to produce T7 lysozyme to reduce the background synthesis of RNA from lac promoter by binding with the T7 lysozyme to reduce the background transcription of RNA product53,54.
Plasmids for single-stranded RNA synthesis were constructed by putting the RNA sequence of interest downstream of one T7/Lac on a pET24a vector. (Plasmids pET24a-ssgfp-100bp and pET24a-ssgfp-400bp were synthesized this way)
The AND gate plasmid (pTet-Ara-Split-T7-AND-Gate-proD) was modified from plasmid pTSlb-wt by constitutively expressing the TetR and araC repressors under proD promoters. The C-terminus of T7 RNA polymerase was regulated by pBAD, and the N-terminus of the T7 RNA polymerase was regulated by pTet. The OR gate (pTet-Ara-OR-Gate-proD) was constructed similarly by putting the full-length T7 RNA polymerase under the control of the two promoters arranged (pBAD and pTet) in tandem.
Feeding C. elegans with the engineered bacteria for RNAi
E. coli BL21 bacteria transformed with plasmids for RNA synthesis and logic gates were grown at 37 °C in LB with 50 μg/ml kanamycin and 25 μg/ml chloramphenicol, then seeded onto NGM plates supplemented with appropriate inducers as indicated in the supplementary information. After overnight growth of the seeded bacteria on NGM plates, synchronized L1 larvae were added to the plates and day-1 adult worms were used for phenotype assays after 2 days.
To program C. elegans GFP expression using IPTG, plasmids pET24a-T7-dsGFP, pET24a -dsgfp-100bp to pET24a-dsgfp-400bp were transformed into E. coli BL21(DE3) separately and fed to the worms. To induce the RNA synthesis in E. coli, 1 mM of IPTG was added into the NGM plates. To program GFP expression with an AND gate, E. coli BL21 was co- transformed with pTet-Ara-Split-T7-AND-Gate-proD and pET24a-ssgfp-200bp, and pTet-Ara-OR-Gate-proD and pET24a-ssgfp-200bp were co-transformed for OR gate programming. l-arabinose and anhydrotetracycline at 0.2 mg/mL and 0.1 μg/mL, respectively, were used as the external signal for both AND and OR gates.
For twitching phenotype AND gate programming, E. coli BL21 was co-transformed with pTet-Ara-Split-T7-AND-Gate-proD and pET24a-dsunc-22-400 bp. RNA synthesis was induced by 2 μg/mL l-arabinose and/or 0.1 μg/mL anhydrotetracycline. To program twitching phenotype using an OR gate, E. coli BL21 was co-transformed with pTet-Ara-OR-Gate-proD and pET24a-dsunc-22-200bp. RNA expression was induced by 0.2 mg/mL l-arabinose and/or 0.1 μg/mL anhydrotetracycline.
AND gate programming of fat storage in C. elegans was achieved by co-transforming E. coli BL21 with pTet-Ara-Split-T7-AND-Gate-proD and pET24a-dssbp-1-proD-lysS-proD-lacI, and expression was induced by 2 μg/mL l-arabinose and 0.1 μg/mL anhydrotetracycline. OR gate programming of fat storage was done by feeding the worms with E. coli BL21 co-transformed with pTet-Ara-OR-Gate-proD and pET24a-dssbp-1-proD-lysS-proD-lacI, and induced by 0.2 mg/mL l-arabinose and 0.1 μg/mL anhydrotetracycline.
For gfp and sbp-1 RNAi experiments, five worms from each condition were analyzed in one experiment, and each experiment was repeated three times (n = 15 worms from each condition were analyzed in total)31,39. For the twitching phenotype, over 200 worms were analyzed from each condition. In each condition, we observed either twitching in all worms or no twitching at all. RNAi triggered 100% twitching phenotype was also observed in other study32. The number of worms we used for calculation is in the same range as other paper when dealing with GFP quantification, fat storage, and twitching phenotype31,39.
C. elegans GFP fluorescence imaging
Day-1 adult worms were rinsed off the NGM plates using PBS + 0.01% Triton X-100 (PBST) and washed twice with PBST to remove the bacteria. The worms were then transferred onto glass slides with 2% agarose containing 1 mM levamisole. Fluorescence in C. elegans was imaged on an Axiovert 200 M fluorescence microscope using the FTIC filter.
Nile red staining
C. elegans fat storage was characterized by Nile Red staining55. Synchronized L1 stage N2 worms were put onto the bacteria lawns, and allowed to grow for 2 days at room temperature. Young adult worms were washed off the plates by PBST and then fixed by 40% isopropanol (v/v) for 3 min. For lipid staining, a Nile Red stock solution of 0.5 mg/ml in acetone was first prepared. The working solution was then made by adding 6 μL of the stock solution into 1 mL of 40% isopropanol. After the worms were fixed and the supernatant removed, the working solution was added, and the worms were incubated in the working solution in the dark for 2 h. Following the 2-h incubation, the supernatant was again removed, 150 μL PBST was added, and the worms were incubated for another 30 min. The worms were then imaged using an Axiovert 200 M fluorescence microscope, and fluorescence intensity was quantified in ImageJ.
Twitching phenotype recording
After C. elegans were fed with the control and engineered E. coli for 2 days, the worms were washed off the plates using PBST. The worms were then washed once with PBST and resuspended with 20 μL 3 mM levamisole (in water)38. Resuspended worms were immediately transferred onto glass slides for recording under a microscope38.
RT-qPCR
Synthesized single-stranded gfp RNA was also evaluated by RT-qPCR. Indicated bacteria was first allowed to grow overnight at 37 °C with 250 rpm shaking in LB medium with appropriate antibiotics. The overnight culture was then diluted 100 times the next day with supplementation of the appropriate inducers and antibiotics. The total RNA was extracted with RNeasy Mini Kit (Qiagen) when the O.D. reached 0.6–0.8. A total amount of 20 ng of the extracted RNA was then used for RT-qPCR by qScript 1-Step SYBR Green qRT-PCR Kit (Quantabio) on a LightCycler 96 (Roche). Each sample was measured with three biological replicates, and each biological replicate had three technical replicates on the 96-well plate. The primers used to quantify gfp RNA synthesis levels were gfp-F: 5′-CGGAGAAGAACTTTTCACTGG-3′, and gfp-R: 5′-GGTAAGTTTTCCGTATGTTGC-3′. Synthesized gfp RNA levels were normalized to the expression of cysG56 with the primers cysG-F: 5′-GATCGCGACTGTCTGATTG-3′, and cysG-R: 5′-CGGTGAACTGTGGAATAAACG-3′.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper. The datasets generated and analyzed during the current study are available from the corresponding author upon request. Source data are provided with this paper.
References
Siuti, P., Yazbek, J. & Lu, T. K. Synthetic circuits integrating logic and memory in living cells. Nat. Biotechnol. 31, 448–452 (2013).
Fernandez-Rodriguez, J., Moser, F., Song, M. & Voigt, C. A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 13, 706–708 (2017).
Nielsen, A. A. et al. Genetic circuit design automation. Science 352, aac7341 (2016).
Huang, Z. et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci. Adv. 6, eaay9209 (2020).
Donahue, P. S. et al. The COMET toolkit for composing customizable genetic programs in mammalian cells. Nat. Commun. 11, 779 (2020).
Auslander, S., Auslander, D., Muller, M., Wieland, M. & Fussenegger, M. Programmable single-cell mammalian biocomputers. Nature 487, 123–127 (2012).
Gaber, R. et al. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat. Chem. Biol. 10, 203–208 (2014).
Groves, B. et al. Computing in mammalian cells with nucleic acid strand exchange. Nat. Nanotechnol. 11, 287–294 (2016).
Lino, C. A., Harper, J. C., Carney, J. P. & Timlin, J. A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 25, 1234–1257 (2018).
Mansouri, M. & Berger, P. Multigene delivery in mammalian cells: Recent advances and applications. Biotechnol. Adv. 36, 871–879 (2018).
Guan, S. & Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 24, 133–143 (2017).
Tasic, B. et al. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl Acad. Sci. USA 108, 7902 (2011).
Tesson, L. et al. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 29, 695–696 (2011).
Wingender, G. et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 418–428 (2012).
Ezenwa, V. O., Gerardo, N. M., Inouye, D. W., Medina, M. & Xavier, J. B. Animal behavior and the microbiome. Science 338, 198 (2012).
Atkinson, M. A. & Chervonsky, A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia 55, 2868–2877 (2012).
Pasquaretta, C., Gomez-Moracho, T., Heeb, P. & Lihoreau, M. Exploring interactions between the gut microbiota and social behavior through nutrition. Genes https://doi.org/10.3390/genes9110534 (2018).
Cagliari, D. et al. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 10, 1319 (2019).
Bento, F. M. M. et al. Gene silencing by RNAi via oral delivery of dsRNA by bacteria in the South American tomato pinworm, Tuta absoluta. Pest Manag. Sci. 76, 287–295 (2020).
Yang, L. et al. Promotion of plant growth and in situ degradation of phenol by an engineered Pseudomonas fluorescens strain in different contaminated environments. Soil Biol. Biochem. 43, 915–922 (2011).
Daeffler, K. N. M. et al. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol. Syst. Biol. 13, 923 (2017).
Riglar, D. T. et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat. Biotechnol. 35, 653–658 (2017).
Mimee, M. et al. Programming a human commensal bacterium, bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst. 1, 62–71 (2015).
Chowdhury, S. et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 25, 1057–1063 (2019).
Zhang, R. & Hou, A. Host-microbe interactions in Caenorhabditis elegans. ISRN Microbiol. 2013, 356451 (2013).
Gerbaba, T. K., Green-Harrison, L. & Buret, A. G. Modeling Host-Microbiome Interactions in Caenorhabditis elegans. J. Nematol. 49, 348–356 (2017).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Zeng, J. et al. Cross-kingdom small RNAs among animals, plants and microbes. Cells https://doi.org/10.3390/cells8040371 (2019).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395, 854–854 (1998).
Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G. & Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2, RESEARCH0002–RESEARCH0002 (2001).
Lezzerini, M., van de Ven, K., Veerman, M., Brul, S. & Budovskaya, Y. V. Specific RNA interference in Caenorhabditis elegans by ingested dsRNA expressed in Bacillus subtilis. PLoS ONE 10, e0124508 (2015).
Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001).
Xiao, R. et al. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 11, 1123–1133 (2015).
Li, W., Koutmou, K. S., Leahy, D. J. & Li, M. Systemic RNA interference deficiency-1 (SID-1) extracellular domain selectively binds long double-stranded rna and is required for RNA transport by SID-1. J. Biol. Chem. 290, 18904–18913 (2015).
Winston, W. M., Molodowitch, C. & Hunter, C. P. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295, 2456 (2002).
Calixto, A., Chelur, D., Topalidou, I., Chen, X. & Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7, 554–559 (2010).
Shis, D. L. & Bennett, M. R. Library of synthetic transcriptional AND gates built with split T7 RNA polymerase mutants. Proc. Natl Acad. Sci. USA 110, 5028 (2013).
Jose, A. M., Smith, J. J. & Hunter, C. P. Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proc. Natl Acad. Sci. USA 106, 2283–2288 (2009).
Nomura, T., Horikawa, M., Shimamura, S., Hashimoto, T. & Sakamoto, K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 5, 17–27 (2010).
Moser, F. et al. Dynamic control of endogenous metabolism with combinatorial logic circuits. Mol. Syst. Biol. 14, e8605 (2018).
Leveau, J. H. J. & Lindow, S. E. Appetite of an epiphyte: Quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl Acad. Sci. USA 98, 3446 (2001).
Jones, M. L., Chen, H., Ouyang, W., Metz, T. & Prakash, S. Microencapsulated genetically engineered lactobacillus plantarum 80 (pCBH1) for bile acid deconjugation and its implication in lowering cholesterol. J. Biomed. Biotechnol. 2004, 610768 (2004).
Ma, C. et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Long, S. L., Gahan, C. G. M. & Joyce, S. A. Interactions between gut bacteria and bile in health and disease. Mol. Asp. Med. 56, 54–65 (2017).
Rivière, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7, 979 (2016).
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41 (2017).
Bui, T. P. N. et al. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 6, 10062 (2015).
Han, B. et al. Microbial genetic composition tunes host longevity. Cell 169, 1249–1262 (2017).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, eaau7975 (2019).
Sobko, T. et al. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 41, 985–991 (2006).
Tiso, M. & Schechter, A. N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 10, e0119712 (2015).
Stiernagle, T. WormBook: The Online Review of C. elegans Biology (Oxford University Press, 2006).
Studier, F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J. Mol. Biol. 219, 37–44 (1991).
Moffatt, B. A. & Studier, F. W. T7 lysozyme inhibits transcription by T7 RNA polymerase. Cell 49, 221–227 (1987).
Escorcia, W., Ruter, D. L., Nhan, J. & Curran, S. P. Quantification of lipid abundance and evaluation of lipid distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. https://doi.org/10.3791/57352 (2018).
Zhou, K. et al. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol. Biol. 12, 18–18 (2011).
Acknowledgements
Lei Chen created Figs. 1, 2a, and 4a. This work is supported by Texas A&M University Experiment Station and Chemical Engineering Department Start-up funds.
Author information
Authors and Affiliations
Contributions
Q.S. conceived the study. B.G. and Q.S. designed the experiments. B.G. constructed plasmids and strains, and conducted all the worm-related experiments. Q.S. and B.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Christian Frøkjær-Jensen, Caroline Kurtz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, B., Sun, Q. Programming gene expression in multicellular organisms for physiology modulation through engineered bacteria. Nat Commun 12, 2689 (2021). https://doi.org/10.1038/s41467-021-22894-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-22894-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.