Abstract
Alkyne metathesis represents a rapidly emerging synthetic method that has shown great potential in small molecule and polymer synthesis. However, its practical use has been impeded by the limited availability of user-friendly catalysts and their generally high moisture/air sensitivity. Herein, we report an alkyne metathesis catalyst system that can operate under open-air conditions with a broad substrate scope and excellent yields. These catalysts are composed of simple multidentate tris(2-hydroxyphenyl)methane ligands, which can be easily prepared in multi-gram scale. The catalyst substituted with electron withdrawing cyano groups exhibits the highest activity at room temperature with excellent functional group tolerance (-OH, -CHO, -NO2, pyridyl). More importantly, the catalyst provides excellent yields (typically >90%) in open air, comparable to those operating under argon. When dispersed in paraffin wax, the active catalyst can be stored on a benchtop under ambient conditions without any decrease in activity for one day (retain 88% after 3 days). This work opens many possibilities for developing highly active user-friendly alkyne metathesis catalysts that can function in open air.
Similar content being viewed by others
Introduction
Alkyne metathesis has been widely applied in the synthesis of natural products1,2,3,4,5, polymers6,7,8,9,10,11,12,13,14,15,16,17,18,19, shape-persistent macrocycles, and molecular cages20,21,22,23,24,25,26,27,28 since its initial discovery in 196829. Within the past two decades, several efficient and well-defined catalysts containing fluorinated alkoxides, silanolates, or amides ligands have been developed (Fig. 1)30,31,32,33,34,35. For example, Tamm and co-workers introduced the tungsten complex I with imidazolin-2-iminato ligands, which is among the first few catalysts operating efficiently at room temperature with a low catalyst loading30,31. Recently, they also reported a series of molecular (MoFn) and silica-supported (MoFn/SiO2‑700) alkyne metathesis catalysts II, which catalyze the cross metathesis of terminal alkynes under mild conditions32,33. Fürstner developed catalyst III, which shows excellent activity, remarkable functional group tolerance (even protic groups) and user-friendliness34,35. Such catalysts have been widely used in natural product synthesis. The precatalyst (IV) is stable in air and becomes metathesis active upon activation35. In 2019, Fürstner and co-workers unveiled an unconventional tris(triarylsilanolate)molybdenum alkylidyne catalyst V, which shows even better functional group tolerance, albeit with slow reaction rates and inertness towards nitro substituted substrates36,37. The most up-to-date report from the Jia Group elucidated a d2Re(V)-based catalyst bearing the PO-chelating ligands and a pyridine ligand, which is air and moisture stable. The catalyst was active for various substrates at high temperature under nitrogen38.
Our group has a long-standing interest in developing highly active alkyne metathesis catalysts. We have successfully developed multidentate ligand-based molybdenum catalysts (VII a-d) that are easily generated in situ by mixing a molybdenum-trisamide alkylidyne precursor (VI) with the multidentate ligands39,40,41,42. Our multidentate catalytic systems exhibit comparable activity and substrate scope to the above mentioned Fürstner’s catalysts. However, similar to all the previous catalysts, they also require inert atmosphere to provide good yields and their reaction rates are relatively slow at room temperature. Alkyne metathesis catalysts that can operate under user-friendly open air conditions with high efficiency and provide high yields for a wide range of substrates are still rare to date43,44,45,46. In this work, we report a class of multidentate catalysts containing simple tris(2-hydroxyphenyl)methane ligands, which can be used in open air, providing similar yields to those obtained under argon and otherwise identical conditions. The catalyst can be stored on a benchtop under ambient conditions when dispersed in paraffin. The catalyst consisting of the cyano-substituted ligands shows the highest activity, catalyzing the reactions in open air with high efficiency (typically <10-30 min, >90% yield) and excellent functional group tolerance.
Results and discussion
Catalyst design, synthesis and characterization
We envisioned that removing the three methylene groups from the ligand VII could provide more rigid ligands, which can minimize the configurational entropic cost during the formation of the podand binding motif with the Mo center. Thus, the ligands 1a–d were prepared from cheap starting materials on multi-gram scales (>5 g) as shown in Fig. 2. The ligands 1a-c have previously been reported by Yasuda and the literature procedure was followed with some modification47,48. The known starting material 2 was prepared in two steps through the addition of aryl lithium to methyl chloroformate followed by p-toluenesulfonic acid-mediated deoxygenation of the alcohol. Deprotection of 2 by BBr3 effectively afforded the desired ligand 1a47. Bromination of 1a in AcOH/CCl4 gave the ligand 1b48. Ligand 1c was obtained by bromination of 2 followed by the deprotection of methoxy groups48. The 1H NMR spectra of 1a-c were consistent with the literature reports47,48. Substitution of 3 with CuCN followed by deprotection yielded the ligand 1d. The ligand 1d was characterized by 1H NMR, 13C NMR, HR-MS, and single crystal X-ray diffraction analysis. Single crystals of compound 1d (Fig. 3) were obtained by slow evaporation of a solution of 1d in acetone/ethyl acetate (1:1) at room temperature over two days. The single crystal X-ray analysis of 1d shows that the three OH groups are “upward”-oriented, which is necessary for the formation of a podand complex. However, it should be noted that the central C-H group is also “upward” oriented, which could cause steric hindrance during the ligand binding with the molybdenum center. The calculation of energy optimized conformers shows that the “upward”-oriented structures, similar to the crystal structure of 1d, are energy-favored for all four ligands 1a–d. Although the difference between Gibbs energies of the “upward”-oriented conformers and “downward”-oriented conformers is small (3.7–5.2 kcal/mol), the necessary conformational change could negatively affect the ligand binding to the molybdenum center and the formation of active species.
The active catalysts were generated in situ by mixing precursor VI with ligand 1a–d in 1:1 ratio. After heating a mixture of 1a and VI at 70 °C for 30 min, we observed that the two singlets at 5.8 ppm (broad) and 6.7 ppm corresponding to the aromatic protons of the amido ligands disappeared, accompanying with the increase of the proton signals (two singlets at 6.4 ppm and 6.5 ppm) of the liberated free aniline ligands (Supplementary Information, Supplementary Fig. 1). We also observed the complete shifts of aromatic protons of ligand 1a. These results indicate the full replacement of the amido ligands with 1a. We also observed a triplet at -0.8 ppm corresponding to the propylidyne CH3 protons, which correlates to a quartet at 1.1 ppm in the COSY spectrum and the carbyne carbon at 317 ppm in the HMBC spectrum (Supplementary Fig. 2 and 3)41. We did not observe any other proton signals that have correlation with carbyne carbons. The 13C signal of the carbyne carbon of the precursor VI shifts downfield from 302 ppm to 317 ppm upon binding of ligand 1a, further supporting the replacement of the amido ligands with 1a. It should be noted that more than one molybdenum propylidyne species were observed oftentimes. For example, although 1H NMR spectrum of the mixture of VI and 1d (VIII-d) shows the presence of only three major proton signals in the aromatic region that clearly belong to the symmetrical ligand 1d, consistent with the structure of the fully bound VIII-d, there are two propylidyne CH3 triplets around −0.6 ppm indicating the presence of two molybdenum propylidyne species. However, the existence of more than one molybdenum propylidyne species did not significantly alter the catalytic activities. Our numerous attempts to obtain the crystal structures of the proposed ligand-Mo-propylidyne complexes (VIII) and unambiguously determine their structures were not successful. The difficulty in isolating the ligand-Mo-propylidyne complex suggests that the VIII structures with all three phenoxide bound to the molybdenum metal center are likely under dynamic equilibrium with other species, e.g., on/off binding of one or two phenolic arms, or formation of molybdenum dimer species.
Catalytic activity
We examined the activity of these multidentate catalysts consisting of ligands 1a–d. Alkyne metathesis of 4-propynylbenzaldehyde, a relatively challenging substrate, was used for the model reaction. We conducted the metathesis reactions in a closed system using 5 Å molecular sieves as the 2-butyne scavenger12. In a typical procedure, the precursor VI and the ligand were mixed in a solvent and heated at 70 °C for 10 min before adding the substrate. The mixture was then reacted at 70 °C for 30 min (3 mol% ligand, 3 mol% precursor, 0.1 mmol 4-propynylbenzaldehyde, 150 mg MS 5 Å, 3 mL CCl4). To our great surprise, the catalyst consisting of the unsubstituted ligand VIII-a was nearly inactive, showing only 5% yield. It is possible that the ligand 1a needs to undergo conformational change (flipping the central C-H group) to bind the molybdenum center, causing the low catalytic activity of VIII-a. In our previous study, the analogous catalyst VIId (R1 = R2 = H) with extra flexible methylene groups in the core of the ligand showed significantly higher activity (60% yield) for the same substrate (4-propynylbenzaldehyde) under similar conditions (3 mol% catalyst loading, 40 °C, 2 h, CCl4). These results appear to contradict with our hypothesis that more rigid ligands can minimize the configurational entropic cost thus would have higher catalytic activity. However, we found the catalyst system VIII with more rigid ligand 1a–d is very sensitive to the electronic effect of the ligand. Although VIII-a has very low activity, there was a significant improvement in the catalytic activity of VIII-b, c, d with the enhancement of electron-withdrawing capability of the ligands. The catalyst VIII-d consisting of ligand 1d substituted with electron-withdrawing cyano groups exhibits the highest activity and can catalyze the reaction at room temperature (3 h, 94%) (Table 1, entry 6). The catalyst VIII-d even outperforms the best performing catalyst VIId (R1 = iPr, R2 = H). The catalyst VIII-d showed higher catalytic activity and faster reaction kinetics compared to VIId (R1 = iPr, R2 = H) in the alkyne metathesis of 1-nitro-4-propynyl benzene (Supplementary Fig. 11a). In addition, VIII-d consistently showed higher activity than VIId (R1 = iPr, R2 = H) in the alkyne metathesis of multiple other substrates in air under otherwise identical conditions (Supplementary Fig. 11b). These results suggest the advantages of the catalyst system consisting of the more rigid ligands. It should be noted that VIII and VIId catalytic systems behave very differently. As we previously reported, the catalyst system VIId is very sensitive to the steric bulk of the ortho substituents on the ligands but does not show obvious trend with the change of the electronic effect of the ligand substituents. For instance, when more electron-withdrawing nitro groups are attached to the ligand, the catalyst VIId (R1 = iPr, R2 = NO2) shows significantly lower activity (two-fold decrease of the conversion) compared to VIId (R1 = iPr, R2 = H) under otherwise identical conditions. On the contrary, the catalyst system VIII is very sensitive to the electronic effect of the ligand. For the VIId system, the catalyst with the isopropyl substituted ligand (R1 = iPr, R2 = H) showed the highest activity, whereas for VIII system, the catalyst with the cyano substituted ligand (R1 = CN, R2 = H) showed the highest activity. Due to the dramatic difference in their responses to the steric and electronic effect of the substituents on the ligands, the two catalyst systems (VIId and VIII) are hard to be directly compared. Therefore, it is unclear how the rigidity of a ligand influences the catalytic activity. Nevertheless, the best catalyst (VIII-d) in the VIII system consisting of the rigid ligand 1d has a higher activity than the best catalyst in the more flexible VIId system. VIII-b and VIII-c consisting of the ligands with weak electron withdrawing bromo substituents show much lower catalytic activity (88% and 62%, respectively) under otherwise identical conditions. Given its superior performance, we used 1d as the catalyst ligand in the following study.
Next, the solvent effect on the catalytic activity of VIII-d was examined using 4-propynylbenzaldehyde as the substrate (Supplementary Information, Supplementary Table 1). The activity of the catalyst varies significantly in different solvents. We obtained the highest yield in carbon tetrachloride (96%). The product yields in chloroform (79%) and chlorobenzene (76%) were good, but in other solvents such as toluene and dichloroethane, the yields were much lower (18% and 4%, respectively). Only about 1% of the dimer products were observed in acetone, THF and CH3CN. Although the catalyst showed the highest activity in CCl4, the toxicity and health safety issues of CCl4 would be a concern for large scale synthesis. Therefore, we also explored the possibility of minimizing the use of CCl4. We found CCl4 is critical for the generation of the active catalyst species but less critical for the metathesis reaction. When the catalyst was generated in a small amount of CCl4 first (10 vol % of the total solvent used in the reaction), then subjected to the metathesis reaction in other solvents, the reaction yields were significantly improved (Supplementary Table 2). Notably, there was only a slight difference between the yields of the reaction in pure carbon tetrachloride (96%) and in toluene-carbon tetrachloride (90%) or chlorobenzene-carbon tetrachloride (91%) co-solvent (solvent/CCl4, v/v, 9/1). Therefore, toluene or chlorobenzene can be used as the main solvent to avoid the use of a large amount of CCl4. The possibility of using other solvents is also beneficial for many substrates with poor solubility in carbon tetrachloride.
The kinetic studies showed that catalyst VIII-d is highly efficient in CCl4 (Fig. 4). The reactions were mostly completed within 30 min at 70 °C even for the electron poor substrates substituted with cyano or nitro groups. The metathesis reaction proceeds significantly faster with the increase of electron density of the substrate alkynyl groups (98% yield within 10 min, Table 2, entry 1, 2, 3). For example, the reaction rate of the even slightly electron-poor boronate substrate is almost 5–6 times faster than the metathesis rate of the cyano-substituted substrate, reaching 90% yield within 5 min. The reaction of 4-methoxy phenylpropyne could be completed within 30 min even at 20 ̊C.
Substrate scope
We then examined the substrate scope of this catalyst system in carbon tetrachloride. The reactions were catalyzed by the in situ generated catalyst from the precursor and ligand 1d (3 mol% loading) in the presence of 5 Å molecular sieves at 70 °C for 10-30 min (at rt for 30–90 min). As shown in Table 2, a broad range of substrates went through the metathesis efficiently. The alkyne metathesis of phenylpropyne substrates with a variety of substituents, such as methoxy, borate ester, chloro groups and even challenging examples containing aldehyde, cyano and nitro functional groups, underwent the metathesis reactions in good to excellent yields (entry 1–6, 89–98% yields). Importantly, the catalyst system achieved 89% conversion of the nitro-substituted phenylpropyne substrate, which is the highest reported to date. The ring-closing alkyne metathesis (RCAM) of diyne to cycloalkyne (entry 10, 11), and the precipitation-driven cyclooligomerization of the carbazole diyne substrate (entry 12) were also successfully carried out. The metathesis of substrates containing potentially problematic substituents such as phenol (entry 9, 98%) also brought excellent results. A low yield was observed with the ortho-propynyl substituted pyridine substrate, whose conversion stopped at 40% (entry 8, and Fig. 4). We found the catalyst is not effective in catalyzing terminal alkynes, propargyl-substituted, or trimethylsilyl-substituted alkynes (3% yield) (Supplementary Table 3).
Alkyne metathesis in open air
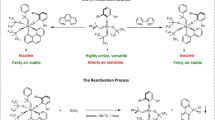
As discussed before, the sensitivity of the catalysts to air/moisture represents a key limiting factor, impeding the wide practical applications of alkyne metathesis. Given the excellent performance of this class of multidentate catalysts, subsequently we explored the possibility of running alkyne metathesis under open-air conditions as shown in Fig. 5. We used CCl4 in the open-air experiments, which is the best performing solvent and also highly immiscible with water (0.13 g H2O/kg CCl4, 130 ppm). After mixing the precursor and the ligand 1d (ratio = 1:1 for activation) in CCl4 for 10 min under argon, the catalyst mixture was taken out of the glove box and transferred to a rotavapor. The solvent was removed by rotary evaporation and a solution of the substrate (0.1 mmol) in CCl4 (3 mL) was added. The mixture was heated at 70 °C in an open flask for 10–30 min without adding 5 Å molecular sieves. The butyne was removed as a vapor from the open reaction bottle. For all the tested substrates, the yields of the reactions in air were nearly the same as those conducted under argon (Table 2, the last column), indicating the high stability and activity of VIII-d catalyst system in open air conditions.
a Open-air alkyne metathesis using catalyst VIII-d. The alkyne metathesis was performed in an open flask at 70 °C in the absence of molecular sieves. The activated catalyst was concentrated to dryness using a rotavap. The whole process took approximately 40 min. b Comparison of the catalytic activities of VIII-d and III in air and argon. Conditions for VIII-d in open air: 0.1 mmol substrate, 0.003 mmol catalyst (3 mol%), 3 mL CCl4, 70 °C, 30 min; Conditions for VIII-d in argon: 0.1 mmol substrate, 0.003 mmol catalyst (3 mol%), 3 mL CCl4, 150 mg MS 5 Å, 70 °C, 30 min. Conditions for III in open air: 0.1 mmol substrate, 0.002 mmol catalyst (2 mol%), 3 mL toluene, 70 °C, 30 min; Conditions for III in argon: 0.1 mmol substrate, 0.002 mmol catalyst (2 mol%), 3 mL toluene, 100 mg MS 5 Å, 70 °C, 30 min. c Stability of the catalyst dispersed in paraffin wax and stored on a bench top under ambient conditions. 4-Methoxylpropynylbenzene was used as the substrate and the reaction conversion was monitored at different time intervals in air.
For a few substrates, we also tested Fürstner’s Mo(VI) triphenylsilanolate catalyst III (Ar = Ph) in metathesis reactions and compared the activity with VIII-d. As shown in Fig. 5b, the alkyne metathesis of chloro-substituted and methoxy-substituted substrates provided similar yields when catalyzed by either III or VIII-d, under identical conditions (70 °C, 30 min, in the glovebox filled with argon). However, the metathesis of the pinacol boronate (BPin) substituted substrate catalyzed by III showed a significantly (more than ten-fold) lower yield (9%) than that (96%) catalyzed by VIII-d. The catalyst III was inert for the substrates substituted with electron withdrawing nitro and aldehyde groups at room temperature under argon, which is in great contrast to the high yields (96% and 89% respectively) of these reactions under the catalysis of VIII-d. In open air, the catalyst III was completely inactive for all the five substrates tested. The significantly higher activity of VIII-d compared to III for multiple substrates with various substituents (electron donating or withdrawing) in both open air and argon can be attributed to the use of multidentate ligand 1d, which generally has higher cooperative chelating effect compared to the monodentate ligand used in III.
To gain a better understanding of the high activity and stability of the catalyst VIII-d, we also examined the catalytic activity of the best performing catalyst from our previous flexible trisbenzylmethine system, VIId (R1 = iPr, R2 = H), also consisting of a multidentate ligand. The catalyst VIId showed a similar activity (~96% yield) to VIII-d under argon for the three tested substrates, methoxy, aldehyde, or pinacol boronate-substituted propynyl benzene. However, significantly decreased yields were observed when the metathesis reactions were performed in open air (Supplementary Fig. 11). The yields of the metathesis reaction under the catalysis of VIId (R1 = iPr, R2 = H) in open air were decreased to 93%, 83%, and 63%, respectively for methoxy, pinacol boronate, and aldehyde substituted propynyl benzenes. The yield of the metathesis of 4-propynylbenzaldehyde in open air under the catalysis of VIII-d was 1.5 times (96% vs 63%) higher than that of the reaction conducted using VIId (R1 = iPr, R2 = H) under otherwise identical conditions. Although the catalytic activity of VIId in open air is much lower than that of VIII-d, it is still significantly higher than the activity of III consisting of the monodentate ligands. These results indicate the multidentate rigid ligands are indeed advantageous for improving the stability and activity of the catalyst. Our result represents the rare experimental demonstration of a practical strategy for storing and use of the alkyne metathesis active complex in air, thus making a great stride in developing user-friendly alkyne metathesis catalysts.
To further improve the user friendliness in handling and storage of the catalyst VIII-d and make it easy to operate in air, we also explored benchtop storage of the catalyst in paraffin49. The activated catalyst powder was dispersed in hot liquid paraffin wax and the mixture was solidified with stirring at room temperature in the glove box. Then the catalyst wax was transferred into air, weighed (~5 mol %), and dissolved in the solvent CCl4 (3 mL) before adding 4-methoxypropynylbenzene (0.1 mmol) substrate. The mixture was heated at 70 ̊C in air for 10 min without the addition of 5 Å molecular sieves. The yield (97%) was similar to that of the reaction strictly conducted under argon (98%). The catalyst wax exhibits high stability in air: its catalytic activity remained the same after 24 h benchtop storage under ambient conditions (Fig. 5c, 96% yield). To our delight, the catalyst provided 53% yield even after being stored in air for one month. When we doubled the catalyst loading (10 mol%), 94% yield was obtained using the one-month aged catalyst.
Computer modeling
Computer modeling and calculations were also conducted to gain some theoretical understanding of the superior catalytic activity of such triphenylmethane based multidentate catalyst (Fig. 6). Computations were performed by the Gaussian09 suites of programs in this work. Molecular geometries were optimized at the B3LYP level of density functional theory. The SDD basis set was used for Mo, and the 6-31 G** basis set was used for C, N, O and H atoms. The relative values of the atomic charges yielded by population analysis can be used as a parameter to understand the chemical reactivity50,51. The Mulliken charge distribution within the Mo-based catalysts was performed at the same level of theory. We also calculated the energy gap between the HOMO of the substrate (1-chloro-4-propynylbenzene) and the LUMO of the catalyst. The calculation showed the advantages of the catalyst with the electron-withdrawing substituents. More electrophilic metal centers with higher Mulliken charge values of +1.023, 1.004, and +1.013 were observed for VIII-b, c, d, compared to the catalyst VIII-a (+0.993) (Fig. 6). The higher electrophilicity of molybdenum center would greatly facilitate the initial Mo-alkyne association step. The electrophilicity order (VIII-b > VIII-d > VIII-c > VIII-a) of the metal center of the catalyst is roughly consistent with the order of their activity (VIII-d > VIII-b > VIII-c > VIII-a). In addition, the electron withdrawing groups of the ligands reduce the LUMO energy of the catalyst, resulting in the lower energy gap between the HOMO of the substrate and the LUMO of the catalyst. The catalyst VIII-d shows the lowest HOMO-LUMO energy gap, which is consistent with its highest catalytic activity. The order of the HOMO-LUMO energy gap (VIII-d < VIII-b < VIII-c < VIII-a) of the catalysts matches well with the order of their catalytic activity (VIII-d > VIII-b > VIII-c > VIII-a). Our previous catalyst VIId (R1 = R2 = H) showed similar HOMO-LUMO energy gap and the electrophilicity of the molybdenum metal center to those of VIII-a, which has the lowest activity among the VIII catalyst system. Our calculation results demonstrate the effect of the ligand structure and substituents on the Mulliken charge distribution and the energy gap between the substrate HOMO and the catalyst LUMO on the efficiency of metathesis reactions, which provides useful insights in correlating the catalysts’ structure-activity relationship.
Energy minimized structures of the catalytically active species (VIId, VIII-a, VIII-b, VIII-c, and VIII-d) were optimized at the B3LYP level of density functional theory. The energy gap (HOMO-LUMO gap) between the LUMO energy level of the catalyst and HOMO energy level of the model substrate 1-chloro-4-propynylbenzene was calculated for each catalyst. The Mulliken charge values of the Mo and carbyne carbon (pointed by blue arrows) of each catalyst are shown.
In summary, we developed a class of highly active and robust catalysts, consisting of precursor VI and rigid multidentate ligands 1a–d, for alkyne metathesis reactions. The catalyst VIII-d is even active at room temperature and can operate under user-friendly open-air conditions with high efficiency and comparable product yields to those conducted under argon. The catalyst dispersed in paraffin wax shows high stability in air and remains fairly active even after one-month storage in air. The catalysts are tolerant of many organic functional groups, such as phenol, aldehyde, nitro, cyano, and heterocycles, and exhibit high activity. Our study brings chemists a step closer to developing highly active robust user-friendly catalyst system for alkyne metathesis.
Methods
Synthesis of tris(2-methoxyphenyl)methanol
The literature procedure was followed with some modification47. To a 250 mL Schlenk tube were added anisole (10.7 g, 99.0 mmol), N,N,N’,N’- tetramethylethylenediamine (2.31 g, 19.8 mmol), and THF (50 mL). The anisole solution was cooled to 0 °C and a solution of nBuLi in hexane (99.0 mmol, 39.6 mL, 2.5 M) was introduced slowly in the Schlenk tube. After stirring for 9 h at room temperature, the Schlenk tube was cooled to 0 °C again and methyl chloroformate (2.85 g, 30.0 mmol) was added slowly. After stirring the reaction mixture overnight at rt, H2O (1 L) was added to quench the reaction and the white precipitate was collected by filtration. The crude product (7.68 g, 73%) was used for the next step without further purification.
Synthesis of compound 2
The literature procedure was followed with some modification47. To a solution of compound tris(2-methoxyphenyl)methanol (3.51 g, 10.0 mmol) in acetonitrile (20 mL) and THF (30 mL) was added TsOH·H2O (2.10 g, 11.0 mmol) at 0 °C. After stirring at room temperature for 12 h, H2O (200 mL) was added. The solution was extracted with Et2O (3 × 50 mL). The combined organic layer was dried (Na2SO4) and concentrated to give the compound 2 as a yellow solid (3.07 g, 92%).
Synthesis of ligand 1a
The literature procedure was followed with some modification47. To a solution of compound 2 (3.34 g, 10.0 mmol) in dichloromethane (20 mL) was added BBr3 (3.12 mL, 33.0 mmol) at -78 °C. After stirring at rt for 12 h, water (10 mL) was added to the mixture at 0 °C and the white precipitate was collected by filtration. The crude product was recrystallized (hexane/ethyl acetate, v/v = 6/1) to give the pure product 1a (2.81 g, 96%).
Synthesis of ligand 1b
The literature procedure was followed with some modification48. To a solution of ligand 1a (1.17 g, 4.00 mmol) in CCl4 (40 mL) was added glacial acetic acid (32 mL) and bromine (5.00 g, 32.0 mmol). The mixture was stirred at rt for 21 h and the white precipitate was collected by filtration. The crude product was recrystallized from acetone/hexane mixture, affording the product as a white solid (2.94 g, 96%).
Synthesis of tris(5-bromo-2-methoxyphenyl)- methane
The literature procedure was followed with some modification48. To a solution of compound 2 (3.35 g, 10.0 mmol) in CCl4 (80 mL) was added glacial acetic acid (64 mL) and bromine (12.8 g, 80.0 mmol). The mixture was stirred at room temperature for 20 h and the white precipitate was collected by filtration. The precipitate was dissolved in CH2Cl2 (200 mL), washed with H2O (50 mL), dried over Na2SO4 and concentrated in vacuo. The crude product (4.58 g, 80%) was used for the next step without further purification.
Synthesis of ligand 1c
The literature procedure was followed with some modification48. To the solution of compound 3 (5.71 g, 10.0 mmol) in dichloromethane (50 mL) was added BBr3 (3.20 mL, 33.0 mmol) at −78 °C. After stirring at room temperature for 48 h, the mixture was poured into 100 mL of ice/water. The mixture was stirred for 1 h and the white precipitate was collected by filtration. The crude product was purified by column chromatography (hexane/ethyl acetate = 50:50) to give the product (4.77 g, 90%) as a white solid.
Synthesis of 3,3’,3”-methanetriyltris(4- methoxybenzonitrile)
To the solution of compound 3 (5.71 g, 10.0 mmol) in DMF (70 mL) was added CuCN (4.50 g, 50.0 mmol). After stirring at 156 °C for 12 h, FeCl3 solution (20 mL in 1 mol/L HCl) was added to the mixture at 60 °C and stirred for 1 h. The mixture was poured into water (600 mL) and the white precipitate was collected by filtration. The crude product (3.77 g, 92%) was used for the next step without further purification.
Synthesis of ligand 1d
Compound 4 (2.05 g, 5.0 mmol) and LiI (2.50 g, 150 mmol) were added to quinoline (30 mL) and the mixture was heated at 170 °C for 1 h. 2 N HCl (300 mL) was added to precipitate out the product. The product was redissolved in 0.1 M NaOH and washed with dichloromethane. Neutralization of the aqueous layer with 2 N HCl precipitated out the product as a white solid (1.65 g, 90%).
Data availability
Experimental procedure and characterization data of new compounds are available in Supplementary Information. The X-ray crystallographic data for ligand 1d reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 1974825. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors upon reasonable request.
References
Fürstner, A. Alkyne metathesis on the rise. Angew. Chem. Int. Ed 52, 2794–2819 (2013).
Ehrhorn, H. & Tamm, M. Well-defined alkyne metathesis catalysts: developments and recent applications. Chem. Eur. J. 25, 3190–3208 (2019).
Zhang, W. & Moore, J. S. Alkyne metathesis: catalysts and synthetic applications. Adv. Synth. Catal. 349, 93–120 (2007).
Benson, S. et al. Second-generation total synthesis of spirastrellolide F methyl ester: the alkyne route. Angew. Chem. Int. Ed 50, 8739–8744 (2011).
Valot, G. et al. Total synthesis of amphidinolide F. Angew. Chem. Int. Ed 52, 9534–9538 (2013).
Fischer, F. R. & Nuckolls, C. Design of living ring-opening alkyne metathesis. Angew. Chem. Int. Ed. 49, 7257–7260 (2010).
Paley, D. W. et al. Alcohol-promoted ring-opening alkyne metathesis polymerization. Angew. Chem. Int. Ed. 52, 4591–4594 (2013).
Sedbrook, D. F., Paley, D. W., Steigerwald, M. L., Nuckolls, C. & Fischer, F. R. Bidentate phenoxides as ideal activating ligands for living ring-opening alkyne metathesis polymerization. Macromoleculars 45, 5040–5044 (2012).
Bellone, D. E., Bours, J., Menke, E. H. & Fischer, F. R. Highly selective molybdenum ono pincer complex initiates the living ring-opening metathesis polymerization of strained alkynes with exceptionally low polydispersity indices. J. Am. Chem. Soc. 137, 850–856 (2015).
von Kugelgen, S., Bellone, D. E., Cloke, R. R., Perkins, W. S. & Fischer, F. R. Initiator control of conjugated polymer topology in ring-opening alkyne metathesis polymerization. J. Am. Chem. Soc. 138, 6234–6239 (2016).
von Kugelgen, S. et al. Templated synthesis of end-functionalized graphene nanoribbons through living ring-opening alkyne metathesis polymerization. J. Am. Chem. Soc. 141, 11050–11058 (2019).
Kloppenburg, L., Song, D. & Bunz, U. H. F. Alkyne metathesis with simple catalyst systems: poly(p-phenyleneethynylene)s. J. Am. Chem. Soc. 120, 7973–7974 (1998).
Ge, P. H. et al. Structural characterization of a cyclohexameric meta-phenyleneethynylene made by alkyne metathesis with in situ catalysts. Angew. Chem. Int. Ed. 39, 3607–3610 (2000).
Hu, K., Yang, H., Zhang, W. & Qin, Y. Solution processable polydiacetylenes (PDAs) through acyclic enediyne metathesis polymerization. Chem. Sci. 4, 3649–3653 (2013).
Yang, H., Jin, Y., Du, Y. & Zhang, W. Application of alkyne metathesis in polymer synthesis. J. Mater. Chem. A. 2, 5986–5993 (2014).
Lu, G. et al. Synthesis of a conjugated porous Co (II) porphyrinylene- ethynylene framework through alkyne metathesis and its catalytic activity study. J. Mater. Chem. A. 3, 4954–4959 (2015).
Zhu, Y., Yang, H., Jin, Y. & Zhang, W. Porous poly(aryleneethynylene) networks through alkyne metathesis. Chem. Mater. 25, 3718–3723 (2013).
Roland, C. D., Li, H., Abboud, K. A., Wagener, K. B. & Veige, A. S. Cyclic polymers from alkynes. Nat. Chem. 8, 791–796 (2016).
Nadif, S. S. et al. Introducing “Ynene” metathesis: ring-expansion metathesis polymerization leads to highly cis and syndiotactic cyclic polymers of norbornene. J. Am. Chem. Soc. 138, 6408–6411 (2016).
Zhang, C. X., Wang, Q., Long, H. & Zhang, W. A highly C70 selective shape-persistent rectangular prism constructed through one-step alkyne metathesis. J. Am. Chem. Soc. 133, 20995–21001 (2011).
Wang, Q. et al. A tetrameric cage with D2h symmetry through alkyne metathesis. Angew. Chem. Int. Ed. 53, 10663–10667 (2014).
Wang, Q. et al. Dynamic covalent synthesis of aryleneethynylene cages through alkyne metathesis: dimer, tetramer, or interlocked complex? Chem. Sci. 7, 3370–3376 (2016).
Wang, Q. et al. Solution-phase dynamic assembly of permanently interlocked aryleneethynylene cages through alkyne metathesis. Angew. Chem. Int. Ed. 54, 7550–7554 (2015).
Zhang, C. X., Long, H. & Zhang, W. A C84 selective porphyrin macrocycle with an adaptable cavity constructed through alkyne metathesis. Chem. Commun. 48, 6172–6174 (2012).
Lee, S., Yang, A., Moneypenny, T. P. & Moore, J. S. Kinetically trapped tetrahedral cages via alkyne metathesis. J. Am. Chem. Soc. 138, 2182–2185 (2016).
Moneypenny, T. P. et al. Product distribution from precursor bite angle variation in multitopic alkyne metathesis: evidence for a putative kinetic bottleneck. J. Am. Chem. Soc. 140, 5825–5833 (2018).
Lee, S., Chénard, E., Gray, D. L. & Moore, J. S. Synthesis of cycloparaphenyleneacetylene via alkyne metathesis: C70 complexation and copper-free triple click reaction. J. Am. Chem. Soc. 138, 13814–13817 (2016).
Pattillo, C. C. & Moore, J. S. A tetrahedral molecular cage with a responsive vertex. Chem. Sci. 10, 7043–7048 (2019).
Pennella, F., Banks, R. & Bailey, G. Disproportionation of alkynes. Chem. Comm. (London) 1548-1549 (1968).
Beer, S. et al. Efficient room-temperature alkyne metathesis with well-defined imidazolin-2-iminato tungsten alkylidyne complexes. Angew. Chem. Int. Ed. 46, 8890–8894 (2007).
Beer, S. et al. Experimental and theoretical investigations of catalytic alkyne cross-metathesis with imidazolin-2-iminato tungsten alkylidyne complexes. Organometallics 28, 1534–1545 (2009).
Estes, D. P. et al. Alkyne metathesis with silica-supported and molecular catalysts at parts-per-million loadings. Angew. Chem. Int. Ed. 55, 13960–13964 (2016).
Estes, D. P. et al. Molecular and silica-supported molybdenum alkyne metathesis catalysts: influence of electronics and dynamics on activity revealed by kinetics, solid-state NMR, and chemical shift analysis. J. Am. Chem. Soc. 139, 17597–17607 (2017).
Bindl, M. et al. Molybdenum nitride complexes with Ph3SiO ligands are exceedingly practical and tolerant precatalysts for alkyne metathesis and efficient nitrogen transfer agents. J. Am. Chem. Soc 131, 9468–9470 (2009).
Heppekausen, J., Stade, R., Goddard, R. & Fürstner, A. Practical new silyloxy-based alkyne metathesis catalysts with optimized activity and selectivity profiles. J. Am. Chem. Soc 132, 11045–11057 (2010).
Hillenbrand, J., Leutzsch, M. & Fürstner, A. Molybdenum alkylidyne complexes with tripodal silanolate ligands: the next generation of alkyne metathesis. Catalysts. Angew. Chem. Int. Ed. 58, 15690–15696 (2019).
Thompson, R. R. et al. Siloxide podand ligand as a scaffold for molybdenum-catalyzed alkyne metathesis and isolation of a dynamic metallatetrahedrane intermediate. Organometallics 38, 4054–4059 (2019).
Cui, M., Bai, W., Sung, H. H., Williams, I. D. & Jia, G. Robust alkyne metathesis catalyzed by air stable d2 Re (V) alkylidyne complexes. J. Am. Chem. Soc. 142, 13339–13344 (2020).
Jyothish, K. & Zhang, W. Introducing a podand motif to alkyne metathesis catalyst design: a highly active multidentate molybdenum(VI) catalyst that resists alkyne polymerization. Angew. Chem. Int. Ed. 50, 3435–3438 (2011).
Jyothish, K., Wang, Q. & Zhang, W. Highly active multidentate alkyne metathesis catalysts: ligand-activity relationship and their applications in efficient synthesis of porphyrin-based aryleneethynylene polymers. Adv. Synth. Catal. 354, 2073–2078 (2012).
Yang, H., Liu, Z. & Zhang, W. Multidentate triphenolsilane-based alkyne metathesis catalysts. Adv. Synth. Catal. 355, 885–890 (2013).
Du, Y. et al. Highly active multidentate ligand-based alkyne metathesis catalysts. Chem. Eur. J. 22, 7959–7963 (2016).
Grela, K. & Ignatowska, J. An improved catalyst for ring-closing alkyne metathesis based on molybdenum hexacarbonyl/2-fluorophenol. Org. Lett. 4, 3747–3749 (2002).
Kloppenburg, L., Song, D. & Bunz, U. H. F. Alkyne metathesis with simple catalyst systems: poly(p-phenyleneethynylene)s. J. Am. Chem. Soc. 120, 7973–7974 (1998).
Pschirer, N. G. & Bunz, U. H. F. Alkyne metathesis with simple catalyst systems: high yield dimerization of propynylated aromatics; scope and limitations. Tetrahedron Lett 40, 2481–2484 (1999).
Haberlag, B., Freytag, M., Jones, P. G. & Tamm, M. Tungsten and molybdenum 2,4,6- trimethylbenzylidyne complexes as robust pre-catalysts for alkyne metathesis. Adv. Synth. Catal. 356, 1255–1265 (2014).
Yasuda, M. et al. Cage-shaped borate esters with enhanced lewis acidity and catalytic activity. Org. Lett. 8, 761–764 (2006).
Yasuda, M. et al. Cage-shaped borate esters with tris(2-oxyphenyl)methane or -silane system frameworks bearing multiple tuning factors: geometric and substituent effects on their lewis acid properties. Chem. Eur. J. 17, 3856–3867 (2011).
Sather, A. C., Lee, H. G., Colombe, J. R., Zhang, A. & Buchwald, S. L. Dosage delivery of sensitive reagents enables glove-box-free synthesis. Nature 524, 208–211 (2015).
Zhang, P., Bao, P. & Gao, J. Dipole preserving and polarization consistent charges. J. Comput. Chem. 32, 2127–2139 (2011).
He, P. et al. Interaction of elemental mercury with defective carbonaceous cluster. J. Hazard.Mater. 300, 289–297 (2015).
Acknowledgements
This work was supported by University of Colorado Boulder, K. C. Wong Education Foundation, the Science Foundation of Shandong Province for Excellent Young Scholars (ZR2017JL015), The Innovative Research Programs of Higher Education of Shandong Province (2019KJC009), and Academic Promotion Programme of Shandong First Medical University (2019QL008).
Author information
Authors and Affiliations
Contributions
Y.G. performed the experiments. S.H., Y.H., S.K., M.O. helped with the synthesis and characterization of some compounds. L.Z. and L.H. conducted the computational study. Y.G., Y.J., and W.Z. conducted the data analysis and wrote the paper. W. Zhang conceived and directed the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Matthias Tamm and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ge, Y., Huang, S., Hu, Y. et al. Highly active alkyne metathesis catalysts operating under open air condition. Nat Commun 12, 1136 (2021). https://doi.org/10.1038/s41467-021-21364-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-21364-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.