Abstract
Carbon dots (CDs) are a promising nanomaterial for photocatalytic applications. However, the mechanism of the photocatalytic processes remains the subject of a debate due to the complex internal structure of the CDs, comprising crystalline and molecular units embedded in an amorphous matrix, rendering the analysis of the charge and energy transfer pathways between the constituent parts very challenging. Here we propose that the photobasic effect, that is the abstraction of a proton from water upon excitation by light, facilitates the photoexcited electron transfer to the proton. We show that the controlled inclusion in CDs of a model photobase, acridine, resembling the molecular moieties found in photocatalytically active CDs, strongly increases hydrogen generation. Ultrafast spectroscopy measurements reveal proton transfer within 30 ps of the excitation. This way, we use a model system to show that the photobasic effect may be contributing to the photocatalytic H2 generation of carbon nanomaterials and suggest that it may be tuned to achieve further improvements. The study demonstrates the critical role of the understanding the dynamics of the CDs in the design of next generation photocatalysts.
Similar content being viewed by others
Introduction
In photocatalytic hydrogen generation the energy of an incident photon is utilized to drive the energetically up-hill water splitting reaction1,2. The evolved hydrogen can then serve directly as an energy-rich fuel or as a reactant in chemical synthesis of carbon-based fuels, e.g. methane in the Sabatier or Fischer-Tropsch processes3,4. This approach is an appealing alternative to harvest and store abundant solar energy, potentially providing a renewable solution for the global energy supply5. In general, the photocatalytic reaction involves a complex sequence of charge carrier (electron and hole) generation, separation and transfer steps, and typically requires a concurrent proton transfer. The efficiency of such proton-coupled electron transfer (PCET) processes is limited by its slowest component, which could be reactant diffusion6. Consequently, a major challenge in photocatalytic H2 production is to ensure that all participants in the process (photoexcited charge carriers, protons, and possibly other molecules) are delivered to the reaction site at optimal time. Otherwise, the photoexcited carriers may recombine, photodegrade the catalysts or induce unwanted side reactions, in all cases reducing the activity.
Photobases are molecules that have higher proton affinity in the excited state than in the ground state. In other words, the pKa in the excited state is much higher than in the ground state, so that upon photoexcitation the now stronger base abstracts a proton from the environment. Hence, such molecules offer an opportunity to control the proton transfer by light7,8. For instance, quinoline photobases have been shown to form protonated species within tens of picoseconds of the light excitation9. Effectively, the photobase can ensure that the proton promptly and selectively arrives at the reduction site. This result can also be achieved by other means, for example by lowering the pH. Nonetheless, utilizing the excited state acid-base equilibria the same effect can conceivably be realized in a neutral medium, without the potentially corrosive acidic conditions. Therefore, for photocatalytic H2 generation the photobases present an appealing approach, wherein the incident photons not only provide the energy for the water splitting, but also dynamically alter the photocatalyst to enhance the process efficiency10.
Nanostructured photocatalysts, either inorganic, organic, or hybrid, rose to the forefront of the photocatalytic field because their composition, structure, and surface can be controlled to tailor the optoelectronic and morphological properties to the desired function11,12,13. Carbon dots (CDs) are a recent entrant into photocatalysis that has attracted attention due to their versatility, photostability, absence of heavy metals, ease of preparation, and tunable properties14,15. This flexibility derives from their complex internal structure, inherent disorder, and surface functionality. Effectively, small changes in the preparative procedure can result in large differences in the properties. While the application possibilities are impressive, there is still no detailed understanding of their structure and the mechanism of photocatalytic reactions16,17,18. They are generally considered to consist of aromatic, sp2-hybridized domains immersed in a sp3-hybridized amorphous matrix with various functional groups on the surface19,20. In this context, we have shown that the optical properties of CDs prepared from citric acid and ethylenediamine can be reproduced by a simple model of polycyclic hydrocarbons embedded in an amorphous polymer, poly(methyl methacrylate)20. Heterocyclic compounds, including strong molecular fluorophores, form in the presence of nitrogen-containing precursors21,22. Importantly, the position of the nitrogen atom dopant in the heterocyclic aromatic structure, controlled by the synthetic procedure, determines the functionality of the CDs. Whilst the graphitic nitrogen increases the photoluminescence (PL) quantum yield, pyridinic and pyrrolic nitrogen yields much higher photocatalytic H2 generation activity23. Pyridine and its larger analogs, such as quinoline or acridine, are weak bases that can be protonated at the nitrogen atom in the excited state, suggesting that such photocatalytically active CDs contain photobasic moieties in their structure. Interestingly, heptazine (tri-s-triazine), the building block of graphitic carbon nitrides (g-CN), also contains multiple N atoms at the edge sites of the aromatic structure and exhibits photobasic behavior24,25. Recent calculations show that in the excited state heptazine molecules induce proton and electron transfer from hydrogen-bonded water molecules leading to heptazinyl radicals26,27. These species can undergo further photolysis, recovering the heptazine and releasing hydrogen radicals that further react to form molecular H2. In corroborating evidence, Electron Paramagnetic Resonance (EPR) experiments show that a long-lived radical forms in cyanamide-functionalized heptazine-based g-CN upon illumination that can be later, under dark conditions, used to produce H228,29,30. It has been observed that protonation of the g-CN structure in acidic media increases the H2 evolution rate31,32,33. This further supports the argument that the proton transfer rate may be a limiting factor in the photocatalytic H2 generation on g-CN. Overall, these experimental and computational results offer tantalizing hints that the photobasic effect of the constituent units in CDs and in g-CN is highly beneficial for the photocatalytic activity of these nanomaterials and at least partially responsible for their impressive H2 formation rates.
In this paper, we show that the introduction of photobasic units into carbon dots indeed increases the H2 generation rate, on the basis of time-resolved spectroscopy and photocatalytic activity measurements. Due to complexity of nitrogen-containing CDs, it is difficult to find an appropriate blank reference sample which exhibits no nitrogen-related acid-base activity. Therefore, we started with polyethylene glycol (PEG) derived nitrogen-free CDs and introduced acridine, a model photobase, into the photocatalyst. Acridine is an N-heterocyclic aromatic compound, resembling molecules likely present in the CDs20, with pKa = 5.5 in the ground state and pKa* = 10.7 in the excited state34,35,36. This means that at pH 7 it is protonated to a very small extent (3%), but becomes mostly protonated in the excited state (see details in the Supplementary Note 1). Calculations suggest that excitation of acridine in water induces proton-coupled electron transfer leading to acridinyl radical that can split under illumination to release a hydrogen radical37. A common acridine-based derivative photobase, acridine orange, can abstract a proton from alcohols coupled with an electron transfer that can also result in acridinyl-based radicals38,39. This further implied that acridine in its excited protonated state is prone to further reduction reactions (e.g. accepting an electron), making it suitable for studying H2 generation from water10. These studies were focused on molecular interactions of acridine with a potential proton and electron donor (alcohol and water). In our work where acridine is integrated with CDs the process can be even more efficient. This is because the electron can also be readily transferred from the photoexcited CDs, which have a broader absorption range.
In short, we synthesize CDs which comprise a model photobase and we show that the photobasic effect can be a plausible element of the mechanism of photocatalytic hydrogen generation with CDs. The understanding of the mechanism demonstrates that careful design of the CDs enables control by light of the proton transfer rates at the photocatalyst surface to enhance the efficiency of H2 production. In this way, we believe it opens a new avenue for modifying the functionality of CDs to bring further improvements in efficiency.
Results
Preparation and characterization of CDs
The nitrogen-free CDs were prepared in the first step and then combined with a model photobase, acridine (cf. Fig. 1), to demonstrate an increase in photocatalytic activity. The CDs were synthesized in an autoclave from PEG dissolved in ethanol by pyrolysis at 200 °C40. The details of the preparation are provided in the Methods sections. Following the synthesis, ethanol was evaporated and the CDs were dispersed in water. Transmission electron microscopy (TEM, see Fig. 2a and Supplementary Fig. 1) and dynamic light scattering (Supplementary Fig. 2) measurements reveal the formation of ultrasmall monodisperse particles 2 nm in diameter (inset in Fig. 2a). Upon close inspection, most particles exhibit crystal fringes, with lattice plane distances ~2.35 Å, indicating an at least partially crystalline structure (Fig. 2b)23,41.
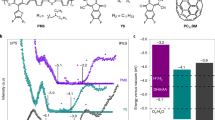
a TEM image of CDs (size distribution in inset); b HR-TEM image of CDs (FFT image in inset). UV-Vis absorption (solid lines) and photoluminescence (dashed lines) spectra of c CDs, d acridine, and e CD-acridine at different pH values. f Time-resolved PL decay of CDs, acridine, and CD-acridine, measured at 450 nm. For the acquisition of PL, in all cases the samples were excited at 350 nm.
In the next step, the optical properties of the CDs were investigated. As the acid-base equilibrium is determined by the pH of the medium, we varied the proton concentration. We focused on three values of pH: 3, 7, and 13, chosen to represent the three relevant populations of acridine: always protonated (pH 3, i.e. acridinium cation, Acr-H+), mostly non-protonated in the ground state, but protonated to a larger extent in the excited state (pH 7), and always non-protonated (pH 13), consistently with the excited state populations reported from fluorescence time-resolved studies42. In effect, these pH values are on either side of acridine pKa and pKa* (5.5 and 10.7, respectively). For reference purposes, the same values were used also for the nitrogen-free CDs. The absorption spectra of the CDs, presented in Fig. 2c, do not exhibit any significant dependence on pH. They are nearly featureless, but contain a long absorption tail extending weakly into the visible part of the spectrum. The CDs are photoluminescent at all pH values, with a single PL peak in every spectrum. However, the Stokes shift is pH-dependent, with the PL maxima at between 344 nm (pH 13) and 375 nm (pH 3). The dependence of PL on proton concentration is in agreement with literature and can be attributed to the presence of hydroxyl groups on the surface of the CDs43. The Fig. 2d shows the absorption and PL spectra of the second component of the photocatalyst, i.e. of acridine. The sharp absorption peaks at 354 nm can be ascribed to transitions to singlet state S2, whose energy is largely unaffected by the changes in pH35,44. This absorption range is very convenient for the excitation during the optical spectroscopy experiments. In contrast to S2, the energy level of S1 state is lower in the protonated state, leading to a pronounced red-shift at pH 3 of the secondary peak at longer wavelengths35. As mentioned before, acridine is mostly non-protonated at pH 7. Hence, the normalized absorption spectrum in neutral conditions is identical to the spectrum acquired at pH 13, with the exception of a small peak at 430 nm due to a small fraction of the protonated species. The PL quantum yield of acridine is strongly dependent on pH, with the yield decreasing with pH35,45. The spectra are also different with the peak of emission of the protonated species (pH 3) at 475 nm and of the non-protonated species (pH 13) at 427 nm. The photobasic behavior of acridine manifests itself in the PL of the sample at pH 7, which comprises the emission peaks of both species. In comparison to absorption, where the spectra of samples at pH 7 and pH 13 are nearly identical, PL emission at pH 7 appears red-shifted. The non-normalized emission spectra (see Supplementary Fig. 3) prove that, despite the higher PL quantum yield of protonated acridine, the small fraction (3%) of the protonated species at pH 7 cannot account for the shift alone. Hence, it is clear that a substantial part of acridine molecules become protonated in the excited state before the radiative charge recombination. This is confirmed by measurements of weakened PL emission of acridine in media of higher pKa (ethanol, 15.6) and increased intensity in media of lower pKa (NH4Cl solution, pKa = 9.2 and boric acid solution, pKa = 9.2), shown in Supplementary Fig. 4.
The combined CD-acridine samples were prepared in two ways. In one method, the CDs were synthesized first, transferred to water, and mixed with an aqueous solution of acridine. This approach was designed to adsorb acridine on the surface of the CDs. In the second method, acridine (same amount as above) was mixed with the precursor of the CDs, PEG, and the synthetic procedure was carried out as before. After the carbonization, acridine could be located within the CDs either as free molecules or incorporated into larger N-hetorocyclic domains, mimicking the proposed composition and structure of N-doped CDs prepared from citric acid and diamines20. Due to different modes of acridine inclusion in this method, complicating the understanding of individual contributions, we used the second method as reference samples only, focusing in the further analysis on the first method that leads to CDs with acridine adsorbed on the outside. The absorption and PL spectra of this CD-acridine sample (see Fig. 2e) show strong resemblance to the free acridine sample. The comparison of non-normalized absorption spectra of CD-acridine with its individual components (see Supplementary Fig. 5) confirms a dominant contribution of acridine in the range 325–400 nm, but a significant contribution of CDs for shorter and longer wavelengths where acridine does not absorb. To investigate the role of the PL emission from the CDs in this combined system, we acquired the PL excitation (PLE) spectrum at 355 nm (see Supplementary Fig. 6). This wavelength was chosen to match the free CDs emission (cf. Fig. 2c). Indeed, the PLE spectrum shows the emission originating at 286 nm, corresponding to the absorption by the aromatic structures in the CDs. Interestingly, in the combined sample, the PL at 350 nm is quenched (cf. Supplementary Fig. 7), suggesting energy or electron transfer from CDs to acridine. The Forster energy transfer pathway appears possible because the 350 nm is very close to the peak acridine absorption. Hence, in a further experiment, we measured the PLE spectra setting the detection wavelength to 450 nm, i.e., at peak acridine emission. As shown in Supplementary Fig. 8, the PLE of the CD-acridine system is higher than of acridine alone in the region 280–320 nm that coincides with strong CD absorption. The emission of CDs at 450 nm is weak, implying that indeed the energy transfer from CDs to acridine can take place. Although the time-correlated single photon counting (TCSPC) measurements at 450 nm (Fig. 2f) show that the PL decay dynamics of the CD-acridine sample is the same as in free acridine, this does not contradict the electron transfer, because the native CD lifetime is shorter than of acridine. To verify the possibility of electron transfer, we performed cyclic voltammetry measurements of CDs and acridine in mildly alkaline conditions to determine the relative positions of the conduction band edge of the CDs versus the LUMO level of acridine. In the voltammograms these levels are manifested as cathodic (reduction) peaks. Supplementary Figure 9 reveals that there are prominent cathodic peaks at around −1.03 V and −0.92 V vs Ag/AgCl for CDs and acridine, respectively. Assuming that no significant reorganization occurs in the excited state of CDs, the reduction potential in the ground state gives a good representation of the oxidation potential in the excited state. Hence, the voltammetry results imply ~100 meV difference in the energy levels that enables electron transfer from excited CDs to the LUMO of non-protonated acridine. Importantly, this difference is even larger in the acidic conditions because the LUMO level of protonated acridine is lower, providing a stronger driving force36,46. Overall, these measurement suggest that both energy and electron transfers can take place. The second important conclusion to be drawn from the PL measurements of the combined system is that the photobasic properties of acridine should be similar in both free acridine and in the CD-acridine sample. This means that free acridine can be treated as a good starting point for the investigation of the charge carrier and proton transfer dynamics.
Proton transfer rate
Transient absorption spectroscopy was then used to determine the rate of proton transfer in the excited state of acridine-based systems. To this end, the acridine solutions in water at different pH values were excited with 100 fs pump pulses at 350 nm and the differential absorption spectra were acquired with a broad band pulse after a time delay up to 2.0 ns (see Fig. 3). The spectrum at pH 3 contains two excited state absorption (ESA) maxima, at 455 nm and 512 nm, respectively. The intersystem crossing rate is considered to be very low in the acridinium cation9, therefore the peaks are attributed to excitations from S1 to Sn for the protonated and non-protonated species, respectively47. The singlet spin multiplicity of the molecules is corroborated by the presence of a negative ΔOD region at 530–570 nm. There is no absorption of the protonated species in this region, but it overlaps with the low-energy tail of the PL emission. Therefore, it can be ascribed to stimulated emission of the singlet Acr-H+. The presence of the minor ESA peak at 512 nm indicates a presence of a small fraction of non-protonated acridine even at pH 3. This assignment can be inferred from the position of the corresponding peak observed at 518 nm at pH 7 and 13 (Fig. 3c, e), with the shift caused by the overlap with the strong emission of Acr-H+ in acidic conditions. To ascertain the claim, we performed an analogous experiment at lower pH, where the non-protonated acridine would not be expected at all. Indeed, as shown in Supplementary Fig. 10, the peak vanishes completely at pH 1 and the broad stimulated emission has an onset already at 520 nm, confirming the assignment. Meanwhile, at alkaline conditions (Fig. 3e), the PL is much weaker and no stimulated emission regions can be seen. This is in agreement with a faster intersystem crossing and low PL quantum yield of the S1 state expected in the non-protonated species35,36,44. The resulting higher triplet state then relaxes to lower lying T1 state36. The two ESA peaks at 518 nm and 440 nm are then attributed to the excitation from the S1 to Sn and from T1 to Tn, respectively (see Supplementary Fig. 11)47. No traces of protonation are observed, as expected at this pH. This was also confirmed by taking the measurements in ethanol which is harder to deprotonate than water (see Supplementary Fig. 12). The differential spectrum in ethanol contains the same ESA peaks with a very clear conversion between then, consistent with a transition from S1 to T1. The same process, albeit on a smaller scale, is also visible in water. The differential spectra at pH 7 (see Fig. 3c) are broadly similar to the ones acquired at pH 13, which is in agreement with the mostly deprotonated state of acridine at these conditions.
To inspect the dynamics of system more closely, the transient absorption traces of the acridine were plotted in Fig. 3 for several relevant wavelengths. The aim was to detect the formation of the protonated species, expected ~450 nm (cf. Fig. 3a). However, at 440 nm the traces are essentially the same at pH 7 and pH 13, in contrast to pH 3. This is because the signal is then dominated by the rise, after ~65 ps, of the ESA peak corresponding to T1 to Tn transition of non-protonated acridine. This rise is absent in acidic conditions. To shift away from this ESA peak, in Fig. 3d we plotted the traces recorded at 465 nm. Here it becomes clear, that the signal at pH 7 follows the dynamics of the non-protonated acridine until 30 ps (cf. Supplementary Table 2), when the two traces start to diverge. There is no rise of the T1 to Tn peak, suggesting that acridine became protonated. From this time, the dynamics follows that of the pH 3 sample. The time scale of 30 ps is in remarkable agreement with the protonation time scale reported earlier for quinolone (28 ps), a lower molecular mass analog of acridine7,9. A similar time scale can be extracted from the dynamics monitored at 550 nm (Fig. 3f), where the stimulated emission is observed at pH 3 in the protonated sample, seen as a nearly constant negative ΔOD signal. At pH 7 the signal is positive with dynamics initially similar to pH 13. However, an additional decay component arises ~30 ps resulting in a faster decrease of the ΔOD signal. Such change is consistent with the protonation of acridine and the ensuing increase in PL emission by the acridinium cation. Importantly, while the analysis of the transient absorption measurements of the CD-acridine sample is complicated by the contributions of the CDs themselves, a very similar protonation rate emerges from the transient trace plotted at 465 nm (see Supplementary Fig. 13). Therefore, the spectroscopic analysis provides evidence that the protonation time of acridine (free or adsorbed at the surface of CDs) at pH 7 is only ~30 ps. Remarkably, this time is comparable with electron transfer to co-catalyst in many inorganic semiconductor-based photocatalysts and faster than photogenerated hole transfer in such systems48,49. Consequently, owing to the photobasic effect, the proton transfer could no longer be a rate-limiting step in photocatalytic H2 generation.
Photocatalytic H2 evolution
In order to verify that the inclusion of a photobasic moiety affects the H2 generation rate, we illuminated the samples with a Xenon lamp and measured the evolved hydrogen (see Methods section for details). Methanol was used as a hole scavenger. As shown in Fig. 4, the rate of H2 generation at pH 7 is indeed much faster for the combined CD-acridine sample (0.032 μmol h−1) than for CDs only (0.08 μmol h−1) or acridine itself (0.08 μmol h−1). The increase in the rate is also obvious when the amount of H2 is normalized by the mass of either the CDs or of acridine (Supplementary Fig. 14). The increase is observed irrespective of the choice of the preparation method of the CD-acridine. In fact, it is even stronger (10-fold) for the sample where acridine is partially embedded inside the CDs (Supplementary Fig. 15). This implies that the stronger interaction between CDs and acridine, expected in this architecture, is indeed beneficial for the enhancement of H2 production. Furthermore, the increase is the strongest at neutral conditions where the photobase effect is expected to manifest itself (cf. Fig. 4c). In the alkaline conditions the amount of evolved H2 is the same as for the components individually. At low pH the increase can also be observed but is much less pronounced. This is to be expected, as at high proton concentration, its transport is unlikely to be a limiting factor. The fact that the rate of H2 generation is highest at pH 3 also indicates that proton transport could be limiting the process at its lower concentrations. In a control experiment, CD-acridine sample was illuminated through a 400-nm long-pass filter which cuts off the acridine absorption and leaves only the tail of CD absorption. The very small observed H2 production confirms that the absorption by acridine is needed for an improvement. Two further control experiments were performed to check whether just the adsorption of an acridine-like compound has an effect. To this end, we have added two structurally similar molecules to CDs which, however, do not exhibit photobasic behavior at pH 7: anthracene and 9-aminoacridine. The former is not a photobase at all, the latter has pKa = 10 and even higher pKa*, so it cannot be protonated at pH 750. In neither of these cases any increase in the H2 generation rate is observed (see Fig. 4d). This confirms that the photobasic property is critical to the enhancement. Finally, it is also important to establish the source of the evolved hydrogen. To this end, we performed a photocatalytic experiment in D2O instead of H2O. We noted a decrease in hydrogen generation rate by a factor of 1.43 (cf. Supplementary Fig. 16). This result implies a strong kinetic isotope effect and is consistent with water, rather than CDs or acridine, as the source of protons for the produced hydrogen51.
a Hydrogen evolution and b the average hydrogen production rate for CDs, acridine, and the mixture under Xe lamp irradiation at pH 7; c the total amount of evolved hydrogen over 6 h of illumination. d Comparison of hydrogen generation of mixtures of CDs with acridine, a non-photobase (anthracene) and a photobase (9-aminoacridine) with pKa 10.
Discussion
The rate of proton transfer determined by the time-resolved absorption measurements is consistent with the literature results obtained for smaller N-heterocyclic photobases (e.g. quinoline)7,9 and for acridine derivatives (e.g. acridine orange)39. Several studies on photobase – proton donor (water and alcohols) systems also report that the proton transfer can be accompanied or quickly followed by electron transfer from the same molecule39,52,53. The concurrent electron transfer might also explain why the protonation rate is faster than reported earlier for acridine42,54. It may facilitate a charge transfer complex pathway which is usually involved in picoscond timescale protonation processes52,55. Eventually, the resulting radical (e.g. Acr-H•) can undergo light-driven dissociation and lead to molecular hydrogen via Heyrovsky mechanism56,57. Acridine can only attract one proton, but in larger polycyclic structures with multiple photobasic moieties potentially in vicinity, Tafel reaction to form H2 may also be possible58. Here, in the CD-acridine system, there is second feasible source of the electron, i.e., the photoexcited CDs. The cyclic voltammetry measurements provide evidence that the band alignment is appropriate for the electron transfer from CD to acridine. It is anticipated that the electron transfer would be even more efficient for a photobasic moiety integrated (or embedded) in the aromatic domains of the CDs, that was postulated to occur in N-doped CDs23, and concurred by our experiments on the efficiency of the CDs with embedded acridine. The system comprising a CD and a photobase can thus transfer the two participating particles, a proton and an electron, to the reaction site at approximately the same time from two different sources, as depicted in Fig. 1. Under such scenario, any of the components would not have to wait for another, as they are delivered when needed, upon photoexcitation. This reduces the chances of parasitic charge recombination that inhibits photocatalytic activity. The second advantage of the CD-photobase system over a direct electron transfer from the proton donor is a generation of oxidized form of the latter (e.g. hydroxyl or alcoxyl radicals) in the vicinity of the reduction site. These reactive species could induce a back reaction with the H atom, reversing the benefit of the process. In a combined CD-photobase system, the photogenerated hole in the CD can be taken away by the scavenger at a more distant site, elsewhere on the dot. In such circumstances, the back reaction would be less likely. Moreover, it is worth noting that, while the experiments in the paper have focused on H2 generation, the proton transfer is an integral part also of the CO2 photocatalytic reduction. Thus, it can be surmised that the ability to induce the proton transfer via an increased basicity in the excited state could also be exploited in this process for increasing efficiency or selectivity of the CO2 reduction pathways.
In summary, we have shown that the proton transport in photocatalytic systems can be controlled by light. In the excited state photobase molecules can abstract a proton from the water within tens of picoseconds. This enables very fast - concerted or in quick succession - proton-coupled electron transfer and thereby substantially increases the rate of the overall photocatalytic process. We demonstrate this effect by fabricating nitrogen-free CDs and then including a model photobase, acridine, in the photocatalyst. This increases 4–10-fold the H2 generation rate of the sample in the neutral conditions. As photobasic moieties are building blocks of many photocatalytically active nitrogen-containing CDs and of carbon nitrides, we propose that the photobasic effect may be an important part of the mechanism of the process on these materials and may partially account for their success in the photocatalytic H2 production. We believe that the uncovering of this mechanism could contribute to an improved design of the carbon nanomaterial-based photocatalysts and lead to further enhancements in activity.
Methods
Materials
Polyethylene glycol (PEG, average Mn 400 and 600, ACS reagent ≥99.5%), ethanol, methanol, acridine, anthracene, 9-aminoacridine, and deuterium oxide (99.9% atom D) were purchased from Sigma Aldrich and used without further purification. Distilled water (ultrapure) was used for the synthesis and for diluting samples for characterization. Methanol was used as a hole scavenger in the photocatalysis experiments.
Synthesis of CDs
The nitrogen-free CDs were synthesized by a straightforward pyrolysis method. Briefly, 318 mg of waxy solid PEG600 was dissolved in 10 mL ethanol. Then the transparent solution was transferred into a 15-mL poly(para-phenol)-lined stainless-steel autoclave and heated to 200 °C for 2 h. Afterwards, it was cooled down naturally to room temperature. The obtained solution was purified by centrifugation at 5000 rpm for 5 min with only the supernatant taken for further characterization. To correlate the optical properties of CDs with photocatalytic activity in water, ethanol was removed by evaporation in N2 flow and the sample was re-dispersed in the same amount of distilled water.
CDs-Acridine samples were prepared by two methods. In the first method, the aqueous solution of CDs was mixed with the saturated acridine solution in water in 1:3 (v/v) ratio, and then stirred with a magnetic bar to obtain a homogeneous solution. In the second method, acridine (same amount as in the first method) was mixed with PEG 400 in ethanol. This mixture was subsequently used as a reactant instead if pure PEG in the procedure that was the same as in CDs synthesis, described above. Ethanol in the sample was also removed and replaced with same amount of distilled water.
Morphological and optical characterizations
For electron microscopy measurements, the samples were first centrifuged at 5000 rot min−1 for 5 min. Then an aliquot of the supernatant was drop-cast on a carbon coated copper grid and allowed to dry in the ambient environment. The transmission electron microscopy (TEM) and high-resolution transmission electron microscopic (HR-TEM) images were taken by a Titan Themis at 120 kV accelerating voltage. UV-vis absorption measurements were carried out with a Varian Cary 5000 UV-Vis-IR spectrometer. A Horiba Jobin Yvon Fluorolog-3 FL3-22 spectrometer with a 450W Xe lamp was used to acquire the photoluminescence spectra. Its double monochromators for both excitation and emission were mounted at 90° angle with a water-cooled photomultiplier tube. The resulting spectra were calibrated with excitation intensities and corrected. To perform time-resolved fluorescence measurements, a home-made device with a white light laser (NKT SuperK Extreme EXR-20), extended with an EXTEND-UV box, was used. The diluted samples were excited with 350 nm laser with a repetition rate of 5.56 MHz. The signal was detected with a Princeton Instruments monochromator fiber connected to a low-noise Avalanche Photodiode from Excelitas.
Femtosecond transient absorption spectroscopy
Ultrafast transient absorption measurements in the present investigation has been conducted in a custom-built transient absorption setup from Newport. A multi-pass Ti: Sapphire femtosecond regenerative amplifier laser system (Libra HE+ from Coherent Inc.) produces ~100 fs laser pulse having 800-nm central wavelength and at 1 kHz repetition rate. A fraction of the 800-nm beam was passed through a spectrally tunable optical parametric amplifier (OPA) to produce the pump pulses at 350 nm. The near UV-visible probe pulses (300–750 nm) were generated by focusing another part of the initial 800-nm beam through a 2-mm-thick motorized CaF2 crystal. Further, the near UV-visible beam is split to two beams, probe and reference, to achieve better signal-to-noise ratios. The pump pulses were overlapped spatially and temporally on the sample with the probe pulses after passing through a 0.5-kHz optical chopper to read out the changes in probe transmission for each pump-probe cycle. To provide a time delay between the two pulses, a motorized optical delay line was positioned in the probe arm. MS260i spectrograph by Newport, Inc. acquired the transmitted signal used for further analysis of the data. Finally, the changes in the absorptions (ΔA) of the excited state were computed by subtracting the absorptions of excited and unexcited samples.
Photocatalytic hydrogen generation
The experiments were conducted in a custom-built metal-free quartz cuvette. The 4.4 ml cuvette was filled with 2 ml aqueous solution containing 10% (v/v) methanol and placed 35 cm from the illumination source. A 450W Xe lamp was used for broad-band UV-Vis illumination without any filter, but as shown in the emission spectrum (Supplementary Fig. 17), the intensity decreases significantly for wavelengths shorter than 350 nm (10–15 mW intensity measured with 320 nm fluorescence filter). Prior to illumination, the cuvette was purged with argon for 1 min to remove air completely. The calculated illumination area was 1 cm2. The generated hydrogen was detected with a Shimadzu GC 2014 gas chromatograph by taking 10 μL aliquots of the headspace from the cuvette at regular time intervals and injecting into the GC. Argon was used as a carrier gas in the chromatograph.
Data availability
Data is available from the corresponding author upon reasonable request.
References
Chen, X., Shen, S., Guo, L. & Mao, S. S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 (2010).
Stolarczyk, J. K., Bhattacharyya, S., Polavarapu, L. & Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 8, 3602–3635 (2018).
Ozin, G. A. Throwing new light on the reduction of CO2. Adv. Mater. 27, 1957–1963 (2015).
Aresta, M., Dibenedetto, A. & Angelini, A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 114, 1709–1742 (2014).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Pannwitz, A. & Wenger, O. S. Proton-coupled multi-electron transfer and its relevance for artificial photosynthesis and photoredox catalysis. Chem. Commun. 55, 4004–4014 (2019).
Hunt, J. R. & Dawlaty, J. M. Photodriven deprotonation of alcohols by a quinoline photobase. J. Phys. Chem. A 122, 7931–7940 (2018).
Hunt, J. R., Tseng, C. & Dawlaty, J. M. Donor–acceptor preassociation, excited state solvation threshold, and optical energy cost as challenges in chemical applications of photobases. Faraday Discuss. 216, 252–268 (2019).
Driscoll, E. W., Hunt, J. R. & Dawlaty, J. M. Proton capture dynamics in quinoline photobases: substituent effect and involvement of triplet states. J. Phys. Chem. A 121, 7099–7107 (2017).
Lennox, J. C., Kurtz, D. A., Huang, T. & Dempsey, J. L. Excited-state proton-coupled electron transfer: different avenues for promoting proton/electron movement with solar photons. ACS Energy Lett. 2, 1246–1256 (2017).
Costi, R., Saunders, A. E. & Banin, U. Colloidal hybrid nanostructures: a new type of functional materials. Angew. Chem. Int. Ed. 49, 4878–4897 (2010).
Osterloh, F. E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 42, 2294–2320 (2013).
Wilker, M. B., Schnitzenbaumer, K. J. & Dukovic, G. Recent progress in photocatalysis mediated by colloidal II-VI nanocrystals. Isr. J. Chem. 52, 1002–1015 (2012).
Fernando, K. A. S. et al. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 7, 8363–8376 (2015).
Arcudi, F., Đorđević, L. & Prato, M. Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc. Chem. Res. 52, 2070–2079 (2019).
Cao, L. et al. Carbon dots for energy conversion applications. J. Appl. Phys. 125, 220903 (2019).
Cadranel, A., Margraf, J. T., Strauss, V., Clark, T. & Guldi, D. M. Carbon nanodots for charge-transfer processes. Acc. Chem. Res. 52, 955–963 (2019).
LeCroy, G. E. et al. Functionalized carbon nanoparticles: syntheses and applications in optical bioimaging and energy conversion. Coord. Chem. Rev. 320-321, 66–81 (2016).
Lim, S. Y., Shen, W. & Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Fu, M. et al. Carbon dots: a unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 15, 6030–6035 (2015).
Schneider, J. et al. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 121, 2014–2022 (2017).
Ehrat, F. et al. Tracking the source of carbon dot photoluminescence: aromatic domains versus molecular fluorophores. Nano Lett. 17, 7710–7716 (2017).
Bhattacharyya, S. et al. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 8, 1401 (2017).
Rabe, E. J., Corp, K. L., Sobolewski, A. L., Domcke, W. & Schlenker, C. W. Proton-coupled electron transfer from water to a model heptazine-based molecular photocatalyst. J. Phys. Chem. Lett. 9, 6257–6261 (2018).
Wang, Y. et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 4, 746–760 (2019).
Ehrmaier, J., Karsili, T. N. V., Sobolewski, A. L. & Domcke, W. Mechanism of photocatalytic water splitting with graphitic carbon nitride: photochemistry of the heptazine–water complex. J. Phys. Chem. A 121, 4754–4764 (2017).
Schlomberg, H. et al. Structural insights into poly(heptazine imides): a light-storing carbon nitride material for dark photocatalysis. Chem. Mater. 31, 7478–7486 (2019).
Lau, V. W.-h et al. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 7, 12165 (2016).
Lau, V. W.-h et al. Dark photocatalysis: storage of solar energy in carbon nitride for time-delayed hydrogen generation. Angew. Chem. Int. Ed. 56, 510–514 (2017).
Dvoranová, D. et al. EPR investigations of polymeric and H2O2-modified C3N4-based photocatalysts. J. Photochem. Photobiol. A 375, 100–113 (2019).
Xu, J., Zhang, L., Shi, R. & Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 1, 14766–14772 (2013).
Zhang, Y., Thomas, A., Antonietti, M. & Wang, X. Activation of carbon nitride solids by protonation: morphology changes, enhanced ionic conductivity, and photoconduction experiments. J. Am. Chem. Soc. 131, 50–51 (2009).
Ong, W.-J. et al. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: a combined experimental and first-principles DFT study. Nano Res. 10, 1673–1696 (2017).
Perrin, D. D. Dissociation Constants Of Organic Bases In Aqueous Solution. (Butterworths, London, 1965).
Artyukhov, V. Y. et al. A combined theoretical and experimental study on molecular photonics. Russ. Phys. J. 51, 1097–1111 (2008).
Rubio-Pons, Ò., Serrano-Andrés, L. & Merchán, M. A theoretical insight into the photophysics of acridine. J. Phys. Chem. A 105, 9664–9673 (2001).
Liu, X., Karsili, T. N. V., Sobolewski, A. L. & Domcke, W. Photocatalytic water splitting with the acridine chromophore: a computational study. J. Phys. Chem. B 119, 10664–10672 (2015).
Liu, X., Karsili, T. N. V., Sobolewski, A. L. & Domcke, W. Photocatalytic water splitting with acridine dyes: Guidelines from computational chemistry. Chem. Phys. 464, 78–85 (2016).
Eisenhart, T. T. & Dempsey, J. L. Photo-induced proton-coupled electron transfer reactions of acridine orange: comprehensive spectral and kinetics analysis. J. Am. Chem. Soc. 136, 12221–12224 (2014).
Fan, R.-J., Sun, Q., Zhang, L., Zhang, Y. & Lu, A.-H. Photoluminescent carbon dots directly derived from polyethylene glycol and their application for cellular imaging. Carbon 71, 87–93 (2014).
Baker, S. N. & Baker, G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 49, 6726–6744 (2010).
Pines, E., Huppert, D., Gutman, M., Nachliel, N. & Fishman, M. The pOH jump: determination of deprotonation rates of water by 6-methoxyquinoline and acridine. J. Phys. Chem. 90, 6366–6370 (1986).
Yang, L. et al. One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 7, 6104–6113 (2015).
Kasama, K. et al. Relaxation mechanism of excited acridine in nonreactive solvents. J. Phys. Chem. 85, 1291–1296 (1981).
Ryan, E. T., Xiang, T., Johnston, K. P. & Fox, M. A. Absorption and fluorescence studies of acridine in subcritical and supercritical water. J. Phys. Chem. A 101, 1827–1835 (1997).
Rak, J., Blazejowski, J. & Zauhar, R. J. Theoretical studies on the prototropic tautomerism, structure, and features of acridine and 9-acridinamine free bases and their protonated forms. J. Org. Chem. 57, 3720–3725 (1992).
Hirata, Y. & Tanaka, I. Build-up of T—T absorption of acridine following singlet excitation with a picosecond pulse. Chem. Phys. Lett. 41, 336–338 (1976).
Simon, T., Carlson, M. T., Stolarczyk, J. K. & Feldmann, J. Electron transfer rate vs recombination losses in photocatalytic H2 generation on Pt-decorated CdS nanorods. ACS Energy Lett. 1, 1137–1142 (2016).
Wu, K. et al. Hole removal rate limits photodriven H2 generation efficiency in CdS-Pt and CdSe/CdS-Pt semiconductor nanorod–metal tip heterostructures. J. Am. Chem. Soc. 136, 7708–7716 (2014).
Casadio, R. & Melandri, B. A. The behavior of 9-aminoacridine as an indicator of transmembrane pH difference in liposomes of natural bacterial phospholipids. J. Bioenerg. Biomembr. 9, 17–29 (1977).
Hisatomi, T., Takanabe, K. & Domen, K. Photocatalytic water-splitting reaction from catalytic and kinetic perspectives. Catal. Lett. 145, 95–108 (2015).
Hammes-Schiffer, S. & Stuchebrukhov, A. A. Theory of coupled electron and proton transfer reactions. Chem. Rev. 110, 6939–6960 (2010).
Rahman, M. Z. & Mullins, C. B. Understanding charge transport in carbon nitride for enhanced photocatalytic solar fuel production. Acc. Chem. Res. 52, 248–257 (2019).
Munitz, N., Avital, Y., Pines, D., Nibbering, E. T. J. & Pines, E. Cation-enhanced deprotonation of water by a strong photobase. Isr. J. Chem. 49, 261–272 (2009).
Mohammed, O. F., Pines, D., Nibbering, E. T. J. & Pines, E. Base-induced solvent switches in acid–base reactions. Angew. Chem. Int. Ed. 46, 1458–1461 (2007).
de Chialvo, M. R. G. & Chialvo, A. C. Hydrogen evolution reaction: Analysis of the Volmer-Heyrovsky-Tafel mechanism with a generalized adsorption model. J. Electroanal. Chem. 372, 209–223 (1994).
Kou, T. et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat. Commun. 11, 590 (2020).
Machado, S. A. S. & Avaca, L. A. The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 39, 1385–1391 (1994).
Acknowledgements
This work was supported by the Bavarian State Ministry of Science, Research, and Arts through the grant “Solar Technologies go Hybrid (SolTech)”, the EU ERANETMED program (project Hydrosol, Grant No. ENERG-11-132), the China Scholarship Council (J. Fang), and the Alexander von Humboldt-Stiftung (T.D. and S.B.). We thank local research clusters and centers such as the Center of Nanoscience (CeNS) and e-conversion for providing communicative networking structures.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.D. performed the HR-TEM measurements; J.Fang performed all other experiments, in part together with T.D. and S.B. for the time-resolved spectroscopic experiments and sample preparation, respectively. J.K.S. wrote the manuscript with the inputs and comments from all other authors. J.K.S. and J.Feldmann supervised the work. All authors contributed to the design of the experiments, the interpretation of the results, and a discussion of the outline of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Pradip Pachfule and other, anonymous, reviewer(s) for their contributions to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, J., Debnath, T., Bhattacharyya, S. et al. Photobase effect for just-in-time delivery in photocatalytic hydrogen generation. Nat Commun 11, 5179 (2020). https://doi.org/10.1038/s41467-020-18583-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-18583-6
This article is cited by
-
In situ constructing atomic interface in ruthenium-based amorphous hybrid-structure towards solar hydrogen evolution
Nature Communications (2023)
-
A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications
Nature Nanotechnology (2022)
-
Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.