Abstract
Hydrogen sulfide radicals in the ground state, SH(X), and hydrogen disulfide molecules, H2S, are both detected in the interstellar medium, but the returned SH(X)/H2S abundance ratios imply a depletion of the former relative to that predicted by current models (which assume that photon absorption by H2S at energies below the ionization limit results in H + SH photoproducts). Here we report that translational spectroscopy measurements of the H atoms and S(1D) atoms formed by photolysis of jet-cooled H2S molecules at many wavelengths in the range 122 ≤ λ ≤155 nm offer a rationale for this apparent depletion; the quantum yield for forming SH(X) products, Γ, decreases from unity (at the longest excitation wavelengths) to zero at short wavelengths. Convoluting the wavelength dependences of Γ, the H2S parent absorption and the interstellar radiation field implies that only ~26% of photoexcitation events result in SH(X) products. The findings suggest a need to revise the relevant astrochemical models.
Similar content being viewed by others
Introduction
Sulfur is one of the more abundant elements in the Universe (S/H ∼ 1.3 × 10−5 in the solar photosphere1, but the abundances of known sulfur-containing molecules in the interstellar medium (ISM) sum to well below this value2. Estimates based on the limited number of S-containing compounds detected in diffuse clouds imply a sulfur abundance in such low-density regions not far from the cosmic value3. However, the abundance of S-containing species in the external layers of the photodissociation region (PDR) in the Horsehead nebula, for example, is deduced to be ~4-fold depleted (cf. the cosmic value)4 and one or more orders of magnitude depletions have been reported in cold molecular clouds5. Such extreme depletions have been reported not only in cold pre-stellar cores (where most of the sulfur may be locked in icy grain mantles)6, but also in hot cores/corinos7,8 where one might expect much of the sulfur to have returned to the gas phase in hot cores and strong shocks—though we note that such conclusions can be very sensitive to assumptions regarding the chemical composition prior to collapse9. Given the high-hydrogen abundances and the mobility of hydrogen in the ice matrix, sulfur atoms impinging on interstellar ice mantles are expected to preferentially form H2S, the chemical- and photo-desorption of which constitutes a source of gas phase H2S10,11.
Commonly used astrochemical models12,13 assume that photon absorption by H2S results in dissociation to H + SH products or, at energies above the first ionization potential (84,432 ± 2 cm−1)14, to ionization with relative probabilities determined by the respective photoabsorption and photoionization cross-sections15. Interstellar SH radicals were first detected in the atmosphere of the S-type star R Andromedae by infrared (IR) spectroscopy16, and subsequently identified by their electronic absorption in the solar atmosphere17 and in translucent interstellar clouds18, but not detected by rotational spectroscopy until 2012, via the lowest energy 2Π3/2; J = 5/2←3/2 transition at 1.383 THz19. Early analyses returned SH/H2S abundance ratios of ~13% that were very much smaller than predicted by standard models for SH and H2S production in turbulent dissipation regions and shocks19. Much of the apparent mismatch was subsequently traced to a computational error, but the sense of the discrepancy was deduced still to be valid 3.
The photodissociation of H2S has been the subject of many prior experimental studies. H2S displays a weak continuous absorption at wavelengths λ < 260 nm and numerous stronger absorption features at λ < 155 nm associated with excitations to Rydberg states20,21,22. Prior photodissociation studies in the long wavelength continuum23 and at λ = 157.6 nm24 revealed prompt S–H bond fission and formation of ground (X2Π) state SH radicals, whereas the SH radicals formed at the Lyman-α photolysis wavelength (λ = 121.6 nm) are exclusively in the excited A2Σ+ state20,25. Equations (1)–(5) show the thermochemical thresholds for various spin-allowed fragmentation processes of interest, derived using literature values for the bond dissociation energies D0°(HS–H)26, D0°(S–H)27, D0°(H–H)28 and the electronic term values T00(SH(A–X)29, and ΔE(S(1D2–3P2))30 detailed in Supplementary Table 1.
In addition, there was an earlier report of isotopic fractionation in solid sulfur films deposited following broadband (~2 nm), non-collision-free photolysis of slowly flowing samples of H2S gas at several VUV wavelengths31.
Herein, we present a photochemical rationale for the deduced paucity of SH radicals in PDRs of the ISM based on the predissociation of most of the primary SH photoproducts. The study employed the intense, pulsed vacuum ultraviolet (VUV)-free electron laser (FEL) at the Dalian coherent light source (DCLS) to measure product state-resolved translational energy spectra of both the H and S(1D) atom photoproducts formed following tuneable VUV photolysis of H2S using, respectively, the H-atom Rydberg tagging and velocity map ion imaging techniques. The experimental results imply that only ~26% of photoexcitation events result in SH(X) products.
Results and discussion
Product translational energy distributions
H atom time-of-flight (TOF) spectra were recorded following photolysis of a jet-cooled H2S sample at many wavelengths resonant with its discrete VUV absorption peaks. These spectra were converted to the corresponding H atom translational energy distributions, and momentum conservation arguments were then used to derive the total translational energy distributions, P(ET), where
m is the photofragment mass, d is the distance separating the interaction region and the detector, and t is the H atom TOF measured over this distance. Figure 1 shows P(ET) spectra obtained at λ = (a) 154.53 nm (64712 cm−1), (b) 139.11 nm (71886 cm−1), and (c) 122.95 nm (81334 cm−1). The polarization vector of the photolysis laser radiation (εphot) used to record these spectra was aligned at the magic angle (θ = 54.7°) to the detection axis, thereby ensuring that the spectra are insensitive to the product channel dependent recoil anisotropies revealed by spectra taken with εphot aligned, respectively, parallel (θ = 0°) and perpendicular (θ = 90°) to the detection axis. The inset in Fig. 1b illustrates such recoil anisotropy for the products of photolysis at λ = 139.11 nm. H atom TOF spectra recorded at all three wavelengths with εphot aligned at θ = 0° and 90° are shown in Supplementary Fig. 1, but detailed descriptions of the rovibronic level structure of the SH radical and of the photofragmentation dynamics responsible for the populations and recoil anisotropies of the various SH products formed when exciting H2S at many different wavelengths in the range 122 ≤ λ ≤ 155 nm are reserved for a future publication. Here we choose to highlight just the following features:
-
i.
The SH fragments formed at λ = 154.53 nm are in their ground (X) state, reinforcing the findings of prior photofragmentation studies at λ = 157.6 nm24 and at all longer wavelengths23. The combs festooned above the spectrum show that the SH(X) population is distributed over a wide range of quantum states, spanning rotational (N) levels of most if not all vibrational (v) levels. The SH(X) potential energy curve correlates with H + S(3P) fragments at infinite separation and the small feature at low ET in Fig. 1a is consistent with three-body dissociation to 2 H + S(3P) products, the threshold energy for which (ΔE(3)) lies ~1600 cm−1 below that for two-body dissociation to H + SH(A) products.
-
ii.
λ = 139.11 nm photolysis yields both SH(X) and SH(A) fragments. The latter are revealed by the sharp step at ET = Ephot − ΔE(2), where Ephot is the photolysis photon energy and ΔE(2) is the threshold energy for forming H + SH(A) products. The SH(A) fragments formed at this wavelength are mainly in their v΄ = 0 level but carry high levels of rotational excitation. λ = 149.15 nm was the longest photolysis wavelength at which structure unambiguously attributable to H + SH(A) products was observed.
-
iii.
SH(A) fragments dominate when exciting at λ = 122.95 nm, as at 121.6 nm25. The combs in Fig. 1c show that the SH(A) fragments are formed mostly in low v levels but have rotational state distributions that extend to the highest N΄ levels supported by the SH(A) potential energy function25. Note that these highest N΄ levels have rotational energies well in excess of the bond dissociation energy of the SH(A) state—indicated by the S(1D) + 2 H arrow in Fig. 1c—and that the signal at lowest ET in Fig. 1c must include contributions from direct three-body-dissociation to such products. Population of similar “super-rotor” levels (i.e., rotational levels that are only quasi-bound by virtue of the centrifugal barrier associated with the high-rotational angular momentum) has also been reported recently in the case of OH(X) products arising in the VUV photolysis of H2O32.
-
iv.
SH(A) state radicals predissociate on a nanosecond (or shorter) timescale to yield H + S(3PJ) atom products33. Thus, every primary SH(A) photofragment will dissociate to yield a second H atom during the time that the Rydberg tagging laser radiation is present. Such secondary H atoms must contribute to the measured H atom TOF spectra and the P(ET) spectra derived therefrom. The predissociation of SH(A) radicals favors population of the ground (J = 2) spin-orbit state of the S(3PJ) product33, and a comb indicating the expected ET values of H + S(3P2) products from predissociation of different SH(A, v΄ = 0, N΄) levels formed in the λ = 139.11 nm photolysis of H2S is also included in the inset to Fig. 1b.
P(ET) spectra derived from H atom time-of-flight spectra following photodissociation of H2S at a 154.53 nm, b 139.11 nm, and c 122.95 nm with the detection axis aligned at the magic angle (θ = 54.7°) to the polarization vector of the photolysis laser radiation (εphot). The inset in b shows an expanded view of the low ET part of the corresponding θ = 0° and 90° data. The combs on these spectra show the ET values associated with formation of H atoms in conjunction with selected rovibrational levels of the primary SH(X) and SH(A) fragments and, in b, with H atoms formed by predissociation of primary (SH(A, v΄ = 0, N΄) fragments. The maximum ET values associated with each of channels (1)–(4) are shown by vertical blue, red, navy, and violet arrows, respectively; the navy arrow indicates the Ephot − ΔE(3) value at which we separate the spectrum into primary H + SH(X) and (direct or indirect) three-body product formation. Source data are provided as a Source Data file.
The term (Ephot − ΔE(3)), henceforth written as ET(3), will prove to be a useful point at which to divide P(ET) spectra such as those shown in Fig. 1. All signal measured at ET > ET(3) is associated with branching into H + SH(X) products, while signal at ET < ET(3) must be associated with three-body dissociation, yielding two H atoms—either directly, or indirectly when the second H atom comes from the predissociation of a primary SH(A) photoproduct. Thus, we can define the following fraction, Γ, for forming SH(X) products following VUV photoexcitation of H2S
where the 0.5 in the second term in the denominator recognizes that, for this fraction of the product yield, the absorption of one photon yields two detectable H atom products. This partitioning would not be perfect if, for example, dissociation at some wavelengths resulted in a (small) yield of SH(X) super-rotor states, but it allows a good estimation of the wavelength dependence of Γ. The implications of the derived Γ(λ) function for interstellar SH number densities are considered later.
Imaging the S(1D) + H2 products
Figure 2a shows a time-sliced velocity map image of the S(1D) atoms formed following photolysis of a jet-cooled H2S sample at λ = 139.11 nm, with εphot aligned in the plane of the image (as indicated by the red arrow) and subsequent resonant ionization by absorption of a single λ = 130.091 nm photon. The latter photons were generated by four wave difference frequency mixing, by overlapping the focussed outputs of two table-top dye lasers operating at λ = 212.556 nm and λ = 580.654 nm in a Kr/Ar gas mixture. Image analysis yields the velocity distribution, and thence the corresponding P(ET) distribution shown in Fig. 2b assuming momentum conservation and that the partner fragment is H2. The image appears isotropic. The P(ET) spectrum is structured and peaks at ET ~ 0, demonstrating preferential population of high v″ and J″ levels of the H2 fragments (henceforth represented as H2#)—as shown by the combs above the spectrum in Fig. 2b. This finding confirms and extends conclusions reached in an early resonance enhanced multiphoton ionization study of the H2 molecules formed via two-photon dissociation of H2S at similar overall excitation energies34.
a Time-sliced velocity map image of the S(1D2) photofragments from photolysis of H2S at λ = 139.11 nm with the polarization vector of the photolysis laser radiation (εphot, shown by the doubled headed red arrow) aligned in the plane of the image. b P(ET) spectrum derived therefrom assuming recoil from an H2 co-fragment. The superposed combs indicate the H2(v″, J″) states responsible for the evident structure with, for simplicity, only the ortho- (odd) J″ states indicated. The red arrow indicates the maximum ET values associated with the S(1D)+2H channel. Source data are provided as a Source Data file.
The H2(X) potential correlates with two ground state H atoms at infinite separation and, recalling Eq. (4), the energetic threshold for the triple fragmentation of H2S to 2H + S(1D) products is ~1960 cm−1 below the energy provided by a 139.11 nm photon. Any such three-body dissociation should thus be revealed by signal appearing with ET < 1960 cm−1 in Fig. 2. This spectrum shows no obvious step or discontinuity at low ET values, but such signal is apparent (as a bright central spot) in S(1D) images recorded at shorter excitation wavelengths. For now, we estimate the possible quantum yield of the 2H + S(1D) three-body dissociation channel as follows: The ratio of the signal appearing with ET values that are, respectively, below and above (Ephot – ΔE(4)) in spectra such as that shown in Fig. 2b provides a relative measure of the branching into channels (4) and (5), i.e., into 2H + S(1D) versus H2 + S(1D) products. Such a partitioning in the case of Fig. 2b returns a ratio of ~0.25, which must be viewed as an upper limit, since it makes no allowance for any primary fragmentations that yield H2 products in super-rotor levels. The slow H atom products from channel (4) must also contribute to the P(ET) spectrum derived from H atom TOF measurements (Fig. 1b and Supplementary Fig. 1b). Very slow H atoms will be under-sampled in the TOF experiment but, recognizing that channel (4) yields two H atoms, our best analyses suggest that this channel accounts for no >3% of the dissociations yielding H atoms at this wavelength—implying a quantum yield for forming H2 + S(1D) products at λ= 139.11 nm ≤ 0.12 and that S–H bond fission is the dominant primary event at this wavelength.
The fragmentation dynamics
As noted previously, detailed discussion of the fragmentation dynamics revealed by the present measurements will be reserved for a future publication, but many aspects of the observed product energy disposal are seen to be qualitatively similar to those found in previous studies of the lighter analog, H2O35. As in H2O, the two key valence absorption continua that support HS–H bond fission arise from excitation of an electron from the highest occupied molecular orbitals (the HOMO, 3px and HOMO–1, 3pz (with b1 and a1 symmetry, respectively, in C2v)) to the 4s (a1) Rydberg orbital. The 4s orbital acquires progressively greater anti-bonding valence σ* character on stretching one S–H bond. The 1B1 and 1A1 states formed by these electron promotions, which we henceforth label \(\widetilde A\) and \(\widetilde B\) to emphasize the parallels with H2O, are a Renner–Teller pair and degenerate at linear geometries. Both correlate with ground state H + SH(X) products at linear geometry (and the ground \(\widetilde B\)1A1 state of H2S correlates with excited H + SH(A) products). However, the \(\widetilde A\) and \(\widetilde B\) states split apart on bending. The \(\widetilde A\) state potential energy surface (PES) is relatively flat with respect to changing the interbond angle, ∠HSH, but the \(\widetilde B\) state potential rises in energy and its crossings with the \(\widetilde X\) state PES at extended HS–H (and H–HS) bond lengths develop into conical intersections (CIs) upon bending away from linearity. At all non-linear geometries, therefore, the \(\widetilde B\) state PES correlates with electronically excited SH(A) products.
The ground state and the Rydberg (R) states of current interest all have bent equilibrium geometries (∠HSH ~ 92°). The R states are diabatically bound with respect to HS–H bond extension but, as with H2O molecules excited to the \(\widetilde C\) 1B1 state36,37,38, H2S molecules excited to such Rydberg states can predissociate by coupling to the \(\widetilde A\) and/or \(\widetilde B\) state continua. Given the relative energetics and the topographies of the respective PESs39,40,41, H2S(R) molecules prepared at longer excitation wavelengths would be expected to dissociate via the former, lower energy pathway to yield H + SH(X) products in a range of v levels but with relatively modest rotational excitation—in accord with experimental observation at λ = 154.53 nm, for example.
H2S(R) molecules prepared by excitation at shorter wavelengths can couple to the \(\widetilde B\) state PES also, which supports much richer fragmentation dynamics. The \(\widetilde B\) state PES (illustrated as a contour plot in Fig. 3 as functions of ∠HSH and one S–H bond length, RS–H, with the other held fixed at its ground state equilibrium value of 2.5 bohr) encourages both HS–H bond extension and opening of ∠HSH towards linearity. Molecules that reach linear geometries at HS–H bond lengths less than or equal to the RS–H value of the \(\widetilde B\)/\(\widetilde X\) CI that we henceforth term CI1 (labeled type I trajectories in Fig. 3) can transition to the \(\widetilde A\) state PES (by \(\widetilde B\)/\(\widetilde A\) Renner–Teller coupling) or the \(\widetilde X\) state PES (by non-adiabatic coupling at CI1) and dissociate to H atoms, together with highly rotationally excited SH(X) products. Other H2S(R) molecules that couple to the \(\widetilde B\) state PES will fail to attain linearity before reaching this critical RS–H distance, however. These molecules remain on the \(\widetilde B\) state PES and can dissociate to H + (highly rotationally excited) SH(A) products via type II trajectories—as observed following excitation at λ = 139.11 and 122.95 nm. In other cases, the separating H atom may “sling-shot” around the heavy S atom and explore the second \(\widetilde B\)/\(\widetilde X\) CI at linear S–H–H geometries (henceforth termed CI2) having followed a type III trajectory. Analogy with H2O42,43,44 suggests that such type III trajectories are likely to offer another route to highly vibrationally and/or rotationally excited SH(X) fragments and to S(1D) + H2 products.
Potential energy surface of the \(\widetilde B\) state of H2S plotted as a function of RS–H (the distance of the departing H atom from the S atom, with the other S–H bond held fixed at the ground state equilibrium bond length (2.5 bohr)) and the interbond angle ∠HSH. The contour labels are given in units of 103 cm−1, with the energy zero calculated at RS–H = 100 bohr (i.e., the H + SH(X) dissociation limit). The two regions of conical intersection with the \(\widetilde X\) state PES at linear H–S–H and S–H–H geometries (CI1 and CI2, respectively) are highlighted in red. The green curves show illustrative type I, II and III trajectories originating from the region sampled by vertical Franck–Condon excitation at the ground state equilibrium geometry. Source data are provided as a Source Data file.
These various fragmentation pathways are all illustrated schematically in Fig. 3 for the case of H2S excitation with a VUV photon of intermediate energy, e.g., 139.11 nm. The topography of the \(\widetilde B\) state PES encourages high-rotational excitation in the SH(A) products and rovibrational excitation in the H2# products from the respective two-body fragmentations—outcomes that are accentuated further by tuning to shorter wavelengths. The present discussion suggests that three-body dissociation to 2H + S(1D) products at shorter photolysis wavelengths can arise via two distinct mechanisms involving, respectively, initial distortions towards H----SH(A) products in the vicinity of CI1 and towards S----H2# products around CI2.
Implications for SH densities in the interstellar medium
The present imaging studies show that the S(1D) + H2 product channel is active at λ = 139.11 nm (and at neighboring wavelengths), but with a rather small quantum yield, thus confirming that S–H bond fission is the dominant primary event following photoexcitation of H2S at VUV wavelengths. However, S–H bond fission can result in several final outcomes (channels (1)–(4)), only one of which yields stable SH(X) products. The H-atom Rydberg tagging studies show Γ decreasing from 1 (at λ ≥ 157.6 nm) to 0 (by λ ≤ 121.6 nm). Figure 4 collects together the wavelength dependences of: Γ determined in the present study; the general interstellar radiation field (ISRF, from Draine45 and extended according to van Dishoeck and Black46; and the total photoabsorption20 and photoionization cross-sections15 of the parent H2S molecule (σtot and σion, respectively) down to the wavelength corresponding to the ionization threshold of atomic H (λ = 91.2 nm)30. Note, the very short lived nature of the excited states populated at all wavelengths implies that the photodissociation and photoabsorption cross-sections are identical at all λ > 118.4 nm.
Plot showing the wavelength dependences of a the general interstellar radiation field (ISRF, the black line) and the total absorption (σtot, the blue line) and photoionization (σion, the blue dotted line) cross-sections of H2S and b the quantum yield for forming SH(X) photoproducts, Γ (Eq. (7)), determined in the present work (the red dots). The sigmoidal function (the black line) through the latter data is used to derive the reported overall SH(X) product quantum yield. The error bars represent the standard deviation of three independent measurements. Source data are provided as a Source Data file.
The peak of the ISRF matches with the region of strong parent absorption, within which Γ is changing rapidly. Reference to Fig. 4 shows that absorption at wavelengths below the ionization limit (λ ≤ 118.4 nm) will increasingly result in ionization, but any dissociative ionization at λ > 91.2 nm will yield S+ or SH+ products (i.e., no neutral SH(X) products)15. It is thus reasonable to assume Γ = 0 at all wavelengths in Fig. 4 shorter than those studied in the present work, and to estimate the fraction ϕ of all photoexcitation events that result in SH(X) fragments by convoluting Γ with the standard model of the ISRF and with σtot. The uncertainty in Γ can be quantified (12.5%, defined as the normalized (by range) root-mean-square error from the fitted sigmoidal function), but to estimate an uncertainty on ϕ also requires knowledge of the λ-dependent uncertainties associated with the ISRF and with σtot. These are not available, but are likely dominated by the uncertainty in the form of the ISRF, which is quoted as being “within about 50%”47. From all the foregoing, ϕ is definitely «1, with a best estimate of ϕ ~26% based on the wavelength dependent functions displayed in Fig. 4. The largest source of uncertainty in ɸ is likely to be that associated with the ISRF, which is necessarily high for it to represent the galactic average. However, the remaining quantities are invariant for H2S molecules anywhere in the universe and a more precise value of ɸ can be determined for comparison with a particular observation by employing a better representation of the radiation field specific to the region of space under investigation. The Supplementary Information includes a directory containing a Python script and the necessary data files to determine ɸ for any supplied ISRF. Note, any such analysis is still likely to return an upper limit on the branching into SH(X) fragments, as it makes no allowance for the (small but non-zero) branching into H2 + S(1D) products.
The SH(X) radicals detected in the ISM are in low N levels of the ground (v = 0) state. Such products dominate when H2S is photolyzed in the weak long wavelength continuum (e.g., at λ > 193 nm)23, but the SH(X) fragments formed at shorter photolysis wavelengths span a wide range of higher v, N levels (as shown in Fig. 1a, b. Given the low pressures (and collision frequencies) prevailing in the ISM, such internally excited photoproducts will relax radiatively, with rates that can be found in the recently published ExoMol list of SH rotational, rovibrational and rovibronic term values and transitions48. Supplementary Fig. 2, which shows the calculated energy level dependent radiative lifetimes, implies ~0.1 s lifetimes for the range of SH(X, v, N) levels populated by shorter wavelength photolysis of H2S. The decay of such population to the v = 0, low N levels identified in the ISM will involve radiative cascade via tens (or more) of transitions but, in total, should be complete within minutes (Supplementary Note 1). Measurements of interstellar SH radicals using the GREAT instrument on SOFIA19 led to initial SH/H2S abundance ratio estimates of ~13%—much smaller than that predicted by standard astrochemical models. The present study clearly identifies competition from hitherto under-appreciated three-body dissociation as an inevitable source of SH(X) radical depletion (cf. H2S) in the ISM. The findings may need to be added into the related astrochemical models.
Methods
Vacuum ultraviolet free electron laser (VUV-FEL) radiation
The experiment employs a recently constructed apparatus for molecular photochemistry centered on a VUV-FEL facility49 operating in the high gain harmonic generation (HGHG) mode47, wherein a seed laser is injected to interact with the electron beam in the modulator. The seed laser pulse (in the range 240 < λseed < 360 nm) is generated from a picosecond (ps) Ti:sapphire laser. The electron beam is generated from a photocathode radio frequency gun and accelerated to a beam energy of ~300 MeV by 7 S-band accelerator structures, with a bunch charge of 500 pC. The micro-bunched beam is sent through the radiator which is tuned to the nth harmonic of the seed wavelength and coherent FEL radiation with wavelength λseed/n is emitted. Optimizing the linear accelerator results in a high-quality beam with emittance ~1.5 mm·mrad, a projected energy spread of ~1‰, and pulse duration of ~1.5 ps. The VUV-FEL currently operates at 10 Hz, the maximum pulse energy is ~500 μJ/pulse and with a specified continuous tunability range of 50 < λ < 150 nm and a typical spectral bandwidth of ~50 cm−1. However, as the present study shows, the stated long wavelength limit can be slightly exceeded.
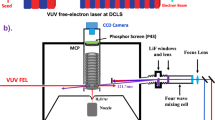
The H-atom Rydberg tagging TOF technique, pioneered by Welge and coworkers50, employs a sequential two-step excitation of the H atom on, first, the n = 2←n = 1 transition by absorption of a 121.6 nm photon, then by UV laser excitation (365 nm) from the n = 2 level to a high-n Rydberg state. The neutral Rydberg atoms fly a distance d ~ 280 mm to a rotatable microchannel plate (MCP) detector where they are field ionized, giving a TOF temporal resolution of <0.5%. The sample beam was generated by expanding a mixture of 1% H2S and Ar at a stagnation pressure of 600 Torr through a 0.5 mm-diameter pulsed nozzle. The molecular beam crosses the VUV-FEL beam at right angles. The polarization of the VUV-FEL pulse is fixed in the horizontal plane, so “parallel” and “perpendicular” TOF spectra are obtained by rotating the MCP detector about the VUV-FEL propagation axis. The 121.57 nm tagging laser beam generates a background H atom spectrum also, which is measured by recording spectra with the VUV-FEL beam on and off and subtracted accordingly.
The apparatus and most of the procedures for the velocity map imaging (VMI) experiments have been described in the Supplementary Fig. 3 and Supplementary note 251,52. The pulsed supersonic beam was again generated by expanding a mixture of 1% H2S and Ar into the source chamber where it was skimmed before entering (through a 2 mm hole in the first electrode), and propagating along the center axis of, the ion optics assembly mounted in the same differentially pumped reaction chamber. The molecular beam was intersected at right angles by the photolysis and probe laser beams between the second and the third plates of the ion optics assembly. The photolysis photons were provided by the FEL with, again, εphot fixed in the horizontal plane and thus parallel to the front face of the detector. The S(1D2) photoproducts are probed by one photon excitation at λ = 130.091 nm, which populates the autoionizing 3p3(2Do)5 s; 1D2o level. These latter photons were generated by four wave difference frequency mixing the frequency doubled output from one dye laser (at λ = 212.556 nm) with the fundamental output of a second dye laser (at λ = 580.654 nm) in a Kr/Ar gas mixture. The S+ (1D2o) ions are accelerated through the remaining ion optics and a 740 mm long field-free region before impacting on a 70 mm-diameter chevron double MCP detector coupled with a P43 phosphor screen. Transient images on the phosphor screen were recorded by a charge-coupled device (CCD) camera, using a 30 ns gate pulse voltage in order to acquire time-sliced images. Further details of the FEL-VMI experiment are provided in the Supplementary Information.
References
Asplund, M., Grevesse, N., Sauval, A. J. & Scott, P. The chemical composition of the Sun. Ann. Rev. Astron. Astrophys. 47, 481–522 (2009).
Rivière-Marichalar, P. et al. Abundances of sulphur molecules in the Horsehead nebula. First NS+ detection in a photodissociation region. Astron. Astrophys. 628, A16 (2019).
Neufeld, D. A. et al. Sulphur-bearing molecules in diffuse molecular clouds: new results from SOFIA/GREAT and the IRAM 30m telescope. Astron. Astrophys. 577, A49 (2015).
Goicoechea, J. R. et al. Low sulphur depletion in the Horsehead PDR. Astron Astrophys. 456, A565–A580 (2006).
Vastel, C. et al. Sulphur chemistry in the L1544 pre-stellar core. Mon. Not. R. Astron. Soc. 478, 5519–5537 (2018).
Laas, J. C. & Caselli, P. Modelling sulfur depletion in interstellar clouds. Astron. Astrophys. 624, A108 (2019).
Wakelam, V. et al. Sulphur-bearing species in the star forming region L1689N. Astron. Astrophys. 413, 609–622 (2004).
Esplugues, G. B., Viti, S., Goicoechea, J. R. & Cernicharo, J. Modelling the sulphur chemistry evolution in Orion KL. Astron. Astrophys. 567, A95 (2014).
Vidal, T. H. G. & Wakelam, V. A new look at sulphur chemistry in hot cores and corinos. Mon. Not. R. Astron. Soc. 474, 5575–5587 (2018).
Vidal, T. H. G. et al. On the reservoir of sulphur in dark clouds: chemistry and elemental abundance reconciled. Mon. Not. R. Astron. Soc. 469, 435–447 (2017).
Oba, Y., Tomaru, T., Lamberts, T., Kouchi, A. & Watanabe, N. An infrared measurement of chemical desorption from interstellar ice analogues. Nat. Astron. 2, 228–232 (2018).
Heays, A. N., Bosman, A. D. & van Dishoeck, E. F. Photodissociation and photoionisation of atoms and molecules of astrophysical interest. Astron. Astrophys. 602, A105 (2017).
van Dishoeck, E. F. et al. Photoprocesses in protoplanetary disks. Faraday Discuss. 113, 231–243 (2006).
Fischer, I. et al. The nonresonant two-photon zero kinetic energy photoelectron spectrum from the electronic ground state of H2S. J. Chem. Phys. 98, 3592–3599 (1993).
Feng, R., Cooper, G. & Brion, C. E. Absolute oscillator strengths for hydrogen sulphide II. Ionic fragmentation and photoionization in the valence shell continuum regions (10-60eV). Chem. Phys. 249, 223–236 (1999).
Yamamura, I., Kawaguchi, K. & Ridgway, S. T. Identification of SH Δv = 1 ro-vibrational lines in R Andromedae. Astrophys. J. 528, L33–L36 (2000).
Berdyugina, S. V. & Livingston, W. C. Detection of the mercapto radical SH in the solar atmosphere. Astron. Astrophys. 387, L6–L9 (2002).
Zhao, D., Galazutdinov, G. A., Linnartz, H. & Krelowski, J. Mercapto radical (SH) in translucent interstellar clouds. Astron. Astrophys. 579, L1 (2015).
Neufeld, D. A. et al. Discovery of interstellar mercapto radicals (SH) with the GREAT instrument on SOFIA. Astron. Astrophys. 542, L6 (2012).
Masuko, H., Morioka, Y., Nakamura, M., Ishiguro, E. & Sasanuma, M. Absorption spectrum of the H2S molecule in the vacuum ultraviolet region. Can. J. Phys. 57, 745–760 (1979).
Lee, L. C., Wang, X. & Suto, M. Quantitative photoabsorption and fluorescence spectroscopy of H2S and D2S at 49-240nm. J. Chem. Phys. 86, 4353–4361 (1987).
Mayhew, C. A. et al. High-resolution studies of the electronic spectra of H2S and D2S. J. Chem. Soc. Faraday Trans. 83, 417–434 (1987). 2.
Wilson, S. H. S., Howe, J. D. & Ashfold, M. N. R. On the near ultraviolet photodissociation of hydrogen sulphide. Mol. Phys. 88, 841–848 (1996) (and references therein).
Liu, X. et al. Photodissociation of hydrogen sulfide at 157.6nm: Observation of the SH bimodal rotational distribution. J. Chem. Phys. 111, 3940–3945 (1999).
Cook, P. A., Langford, S. R., Dixon, R. N. & Ashfold, M. N. R. An experimental and ab initio reinvestigation of the Lyman-α photodissociation of H2S and D2S. J. Chem. Phys. 114, 1672–1684 (2001).
Shiell, R. C., Hu, X. K., Hu, Q. J. & Hepburn, J. W. A determination of the bond dissociation energy (D 0(H–SH)): threshold ion-pair production spectroscopy (TIPPS) of a triatomic molecule. J. Phys. Chem. A 104, 4339–4342 (2000).
Zhou, W. D., Yuan, Y., Chen, S. P. & Zhang, J. S. Ultraviolet photodissociation dynamics of the SH radical. J. Chem. Phys. 123, 054330 (2005).
Komasa, J. et al. Quantum electrodynamics effects in rovibrational spectra of molecular hydrogen. J. Chem. Theor. Comput. 7, 3105–3115 (2011).
Linstrom, P. J. & Mallard, W. G. (eds.), NIST Chemistry WebBook, NIST Standard Reference Database Number 69. (National Institute of Standards and Technology, Gaithersburg MD, 20899) (retrieved 29 October, 2019).
Kramida, A., Ralchenko, Y., Reader, J. & NIST ASD Team. NIST Atomic Spectra Database (version 5.7.1), [Online]. Available: https://physics.nist.gov/asd [Tue 29 Oct, 2019]. (National Institute of Standards and Technology, Gaithersburg, MD).
Chakraborty, S., Jackson, T. L., Ahmed, M. & Thiemens, M. H. Sulfur isotopic fractionation in vacuum UV photodissociation of hydrogen sulphide and its potential relevance to meteorite analysis. Proc. Natl Acad. Sci. USA 110, 17650–17655 (2013).
Chang, Y. et al. Hydroxyl super rotors from vacuum ultraviolet photodissociation of water. Nat. Commun. 10, 1250 (2019).
Rose, R. A. et al. Photodissociation dynamics of the A2Σ+ state of SH and SD radicals. J. Chem. Phys. 130, 034307 (2009).
Steadman, J. & Baer, T. The production and characterization by resonance enhanced multiphoton ionization of H2(v = 10-14) from photodissociation of H2S. J. Chem. Phys. 91, 6113–6119 (1989).
Ashfold, M. N. R., King, G. A., Murdock, D., Nix, M. G. D. & Oliver, T. A. A. πσ* excited states in molecular photochemistry. Phys. Chem. Chem. Phys. 12, 1218–1238 (2010).
Ashfold, M. N. R., Bayley, J. M. & Dixon, R. N. Molecular predissociation dynamics revealed through multiphoton ionization spectroscopy. I. The C1B1 states of H2O and D2O. Chem. Phys. 84, 35–50 (1984).
Hodgson, A., Simons, J. P., Ashfold, M. N. R., Bayley, J. M. & Dixon, R. N. Quantum state-selected photodissociation dynamics in H2O and D2O. Mol. Phys. 54, 351–368 (1985).
Yuan, K. J. et al. Nonadiabatic dissociation dynamics in H2O: Competition between rotationally and non-rotationally mediated pathways. Proc. Natl Acad. Sci. USA 105, 19148–19153 (2008).
Heumann, B., Weide, K., Duren, R. & Schinke, R. Non-adiabatic effects in the photodissociation of H2S in the 1st absorption band – an ab initio study. J. Chem. Phys. 98, 5508–5525 (1993).
Flouquet, F. Ab initio study of potential energy surface of lowest A1 symmetry excited state of H2S. Chem. Phys. 13, 257–263 (1976).
Simah, D., Hartke, B. & Werner, H. -J. Photodissociation dynamics of H2S on new coupled ab initio potential energy surfaces. J. Chem. Phys. 111, 4523–4534 (1999).
Dixon, R. N. et al. Chemical ‘double slits’: Dynamical interference of photodissociation pathways in water. Science 285, 1249–1253 (1999).
Harich, S. et al. Photodissociation of H2O at 121.6 nm: A state-to-state dynamical picture. J. Chem. Phys. 113, 10073–10090 (2000).
van Harrevelt, R., van Hemert, M. C. & Schatz, G. C. A comparative classical-quantum study of the photodissociation of water in the B band. J. Phys. Chem. A 105, 11480–11487 (2001).
Draine, B. T. Photo-electric heating of inter-stellar gas. Astrophys. J. Suppl. Ser. 36, 595–619 (1978).
van Dishoeck, E. F. & Black, J. H. The excitation of inter-stellar C2. Astrophys. J. 258, 533–547 (1982).
Yu, L. H. et al. High-gain harmonic-generation free-electron laser. Science 289, 932–934 (2000).
Gorman, M. N., Yurchenko, S. N. & Tennyson, J. ExoMol molecular line lists XXXVI: X2Π−X2Π and A2Σ+−X2Π transitions of SH. Mon. Not. R. Astron. Soc 490, 1652–1665 (2019).
Chang, Y. et al. Tunable VUV photochemistry using vacuum ultraviolet free electron Laser combined with H-atom Rydberg tagging time-of-flight spectroscopy. Rev. Sci. Instrum. 89, 063113 (2018).
Schnieder, L., Meier, W., Welge, K. H., Ashfold, M. N. R. & Western, C. M. Photodissociation dynamics of H2S at 121.6 nm and a determination of the potential energy function of SH(A2Σ+). J. Chem. Phys. 92, 7027–7037 (1990).
Eppink, A. T. J. B. & Parker, D. H. Velocity map imaging of ions and electrons using electrostatic lenses: application in photoelectron and photofragment ion imaging of molecular oxygen. Rev. Sci. Instrum. 68, 3477–3484 (1997).
Chen, W. T. et al. Observation of the carbon elimination channel in vacuum ultraviolet photodissociation of OCS. J. Phys. Chem. Lett. 10, 4783–4787 (2019).
Acknowledgements
The experimental work is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB17000000), the Chemical Dynamics Research Center (Grant No. 21688102), the National Natural Science Foundation of China (NSFC Nos. 21873099, 21673232, 21673234, 21922306), and the international partnership program of Chinese Academy of Sciences (No. 121421KYSB20170012). C.S.H. acknowledges receipt of an Australian Research Council Discovery Early Career Award (DE200100549). M.N.R.A. gratefully acknowledges funding from the Engineering and Physical Sciences Research Council (EPSRC, EP/L005913) and both C.S.H. and M.N.R.A. are grateful to the NSFC Center for Chemical Dynamics for the award of Visiting Fellowships. The authors thank the Dalian Coherent Light Source for experimental technique supports.
Author information
Authors and Affiliations
Contributions
K.J.Y., M.N.R.A., and X.M.Y. designed the experiments. J.M.Z., Y.R.Z., C.S.H., Y.C., J.Y.Y., Y.Y., G.K.C., Z.C.C., and Z.G.H. performed the experiments. K.J.Y., M.N.R.A., C.M.W., J.M.Z., Y.R.Z., and C.S.H. analyzed the data and C.S.H. prepared the Python scripts included in the Supplementary Information. K.J.Y., M.N.R.A., C.S.H., Z.C.C., S.R.Y., H.B.D., W.Q.Z., G.R.W., D.X.D., and X.M.Y. discussed the experimental results. K.J.Y., M.N.R.A., C.S.H., and X.M.Y. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, J., Zhao, Y., Hansen, C.S. et al. Ultraviolet photolysis of H2S and its implications for SH radical production in the interstellar medium. Nat Commun 11, 1547 (2020). https://doi.org/10.1038/s41467-020-15343-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-15343-4
This article is cited by
-
Advanced oxidation/reduction processes (AO/RPs) for wastewater treatment, current challenges, and future perspectives: a review
Environmental Science and Pollution Research (2023)
-
State selective fragmentation of doubly ionized sulphur dioxide
Scientific Reports (2021)
-
Rotational and nuclear-spin level dependent photodissociation dynamics of H2S
Nature Communications (2021)
-
Vibrationally excited molecular hydrogen production from the water photochemistry
Nature Communications (2021)
-
Sulfur Ice Astrochemistry: A Review of Laboratory Studies
Space Science Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.