Abstract
Differences in genetic background in model organisms can have complex effects on phenotypes of interest. We previously reported a difference in hermaphrodite lifespan between two wild-type lines widely used by C. elegans researchers (N2 hermaphrodite and male stocks). Here, using pathology-based approaches and genome sequencing, we identify the cause of this difference as a nonsense mutation in the filamin gene fln-2 in the male stock, which reduces early mortality caused by pharyngeal infection. We show how fln-2 variation explains previous discrepancies involving effects of sir-2.1 (sirtuin deacetylase) on ageing, and show that in a fln-2(+) background, sir-2.1 over-expression causes an FUDR (DNA synthesis inhibitor)-dependent reduction in pharyngeal infection and increase in lifespan. In addition we show how fln-2 variation confounds effects on lifespan of daf-2 (insulin/IGF-1 signalling), daf-12 (steroid hormone signalling), and eat-2 (putative dietary restriction). These findings underscore the importance of identifying and controlling genetic background variation.
Similar content being viewed by others
Introduction
Research using invertebrate model organisms has revealed much about how genotype specifies many aspects of phenotype, including ageing. Studies on the short-lived nematode Caenorhabditis elegans, in particular, have led to the discovery of signalling pathways that affect lifespan, not only in nematodes but also in higher organisms, including humans1,2. These include the insulin/IGF-like signalling (IIS) and the target of rapamycin (TOR) pathways3,4. In recent years, the importance of genetic variation in C. elegans strain backgrounds has become increasingly clear, partly arising from inadvertent selection acting on the wild type under laboratory conditions and/or random mutation accumulation5,6,7. Such variation may confound the effects of genes of interest.
First isolated more than 60 years ago, the laboratory strain N2 (Bristol) is the standard reference strain for C. elegans research6. N2 is distributed by the Caenorhabditis Genetics Center (CGC) either as a hermaphrodite only stock (which we will refer to here as N2H) or as a male stock (referred to at the CGC as N2 male, which we will refer to as N2M). We previously noted that hermaphrodite lifespan is slightly but consistently longer in N2M than N2H (+11% median lifespan)8. As both stocks have been used as wild type, it is possible that the stock difference may have confounded prior studies of gene effects on lifespan.
Ageing limits animal lifespan by promoting multiple pathologies that increase mortality rate. Using necropsy analysis, we recently identified death with a swollen, infected pharynx (P [big P] death) as one form of mortality in C. elegans9. Under standard laboratory culture conditions10, initial intra-pharyngeal infection occurs in early adulthood during a period of fast pumping and mastication of the bacterial food source, and later accounts for ~40% of N2H deaths, largely those occurring prior to mean lifespan. The remainder of the population die with an uninfected but often atrophied pharynx (p [small p] death). Changes in the shape of survival curves of different C. elegans strains can be understood in terms of altered frequency and/or timing of P and p death (mortality deconvolution)9.
In this study, we use mortality deconvolution to investigate the cause of the difference in lifespan between N2H and N2M, and the question of which of these N2 lines is wild type. We report that the longer lifespan of N2M is attributable to reduced P frequency that results from a single nonsense mutation in the X-linked gene fln-2, indicating that N2H is wild type and N2M mutant. We demonstrate the importance of controlling for fln-2 genotype by illustrating how it can confound investigations, using several examples of genes that have been the focus of many studies of ageing in C. elegans: sir-2.1, daf-2, daf-12 and eat-2.
Results
N2M longevity is due to reduced P death frequency
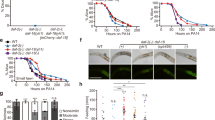
To investigate the causes of the lifespan difference between N2H and N2M, we performed mortality deconvolution on the two lines, comparing timing and frequency of P and p death, using combined mortality and necropsy analysis9. Overall mean lifespan of N2M hermaphrodites was 16.6% longer than N2H (22.1 days vs. 18.7 days, p < 0.0001, log rank test)(Fig. 1a; Supplementary Table 1), consistent with previous findings8. Necropsy analysis revealed that 37% of N2H worms died with P, consistent with previous findings9, but this was the case in only 13% of N2M worms (p = 0.0031, Student’s t test)(Fig. 1b). By contrast, the lifespans of both the P and p subpopulations were similar in the two lines (Fig. 1a; Supplementary Table 1). Thus, the lifespan difference between N2H and N2M is attributable to reduced P death frequency in the latter. This predicts that blocking E. coli proliferation with antibiotics or UV radiation, which completely prevents P death9, should abrogate the difference in lifespan between N2H and N2M, and this proved to be the case (Fig. 1a; Supplementary Fig. 1a; Supplementary Table 1).
N2M is resistant to pharyngeal infection. a N2M (dashed lines) is longer lived than N2H (solid lines) (+16.6%, p < 0.0001, log rank test), yet neither P or p N2M subpopulations are long lived. N2M longevity disappears after treatment with antibiotics. Whole population in black, P death sub-population in red, p death in blue, and antibiotic-treated in green. See Supplementary Table 1 for full statistics, and Supplementary data 1 for raw mortality data. b Necropsy analysis of the two N2 lines. N2M experience less P death than N2H. Error bars represent s.e.m. of six trials. p = 0.0031, Student’s t test. c Antibiotic and phm-2 mutants suppress P at different stages. Pharyngeal invasion and widespread infection were scored using high magnification epifluorescence microscopy in worms fed RFP-expressing bacteria. No detectable RFP within pharyngeal tissue is shown in white, initial invasion with RFP puncta in pink, widespread RFP in red, and worms that have died with swollen pharynxes in maroon. Mean of two trials, n = 18–20 per time point in each trial. d N2M is more resistant to initial bacterial invasion. Colour coding as in (c). Mean of two trials, n = 20–25 animals per time point in each trial. e Reduced P frequency in N2M is not a consequence of parental mating. Error bars represent s.d. of two biological replicates. p = 0.0234 for N2M × N2M mating progeny, Student’s t test. f Resistance to P death following successive rounds of backcrossing is consistent with Mendelian segregation of a single mutant locus. Error bars represent s.d. of two biological replicates. Asterisk indicates p < 0.05, Student’s t test. See Material and Methods for detail. Source data for (b–f) are provided as a Source Data file
To further investigate the difference between N2H and N2M we compared other characteristics of the two lines. No difference in self-fertilised brood size was detected, consistent with previous findings8 (Supplementary Fig. 1b). Mutants with reduced pumping rate often show reduced P frequency9, raising the possibility that pumping rate might be reduced in N2M, but it was not (Supplementary Fig. 1c). Moreover, the difference between N2H and N2M is not attributable to effects of parental mating in the latter: hermaphrodite progeny of mated N2H still show higher frequency of P death than N2M (Supplementary Fig. 1d). Thus, N2M's intrinsic resistance to pharyngeal E. coli infection accounts for its longer lifespan.
We previously reported evidence that pharyngeal infection occurs in two stages: initial bacterial invasion occurs during early adulthood, apparently due to activity-dependent damage to the pharyngeal cuticle, while major infection occurs later in some animals when bacteria escape cyst-like intracellular structures and proliferate9 (for examples of different stages of infection, see Supplementary Fig. 2; Supplementary Movie 1). To test whether each stage can be selectively suppressed, worms were fed with E. coli OP50 expressing a red fluorescent protein (OP50-RFP), which renders initial bacterial invasion visible as red fluorescent puncta within pharyngeal tissue9 (Fig. 1c). Culture on antibiotic-treated E. coli did not suppress initial bacterial invasion of the pharynx (presumably because antibiotics do not suppress grinder-generated mechanical damage and perforation of the pharyngeal cuticle9) but did prevent later bacterial proliferation, while in pumping-defective phm-2 mutants initial invasion was fully suppressed (Fig. 1c), perhaps due to a combination of reduced mechanical damage and bacterial lawn avoidance11. Thus, it is possible to selectively suppress the invasion and proliferation stages.
We then asked: which stage is suppressed in N2M? Initial bacterial invasion was observed in 40% of the population in N2M, compared to 75% in N2H as early as day 4 of adulthood; yet in both strains about half of those showing early signs of invasion die with a swollen, infected pharynx (Fig. 1d). These results suggest that N2M is resistant to pharyngeal cuticle perforation and/or initial bacterial invasion rather than to later bacterial proliferation.
N2M longevity is caused by fln-2(ot611)
Reduced P frequency in N2M likely reflects a different genotype. Analysis of F1 hybrids from a N2H/N2M cross showed that the N2M infrequent P trait is recessive (Fig. 1e). We also noted, during backcrossing with N2M of a strain with high frequency of P, that P frequency dropped in a single round of outcrossing from high to low P frequency (Fig. 1f), suggesting effects of a single gene.
To identify the causative variant, whole genome sequencing was used. An initial comparison between genomic sequences of the two N2 lines revealed 4259 single nucleotide polymorphisms (SNPs) and small insertions and deletions (INDELs). Most of these mutations likely accumulated during separate propagation of N2H and N2M before they were both frozen in 19758. In order to map the mutation affecting P frequency, we performed Variant Discovery Mapping12. For this, N2H and N2M were crossed and the progeny of individual F2 recombinant lines with the N2M phenotype pooled and subjected to genome sequencing (Fig. 2a). Parental allele enrichment peaked near the 10 Mb position on LGX implying the presence of the causal variant in this vicinity (Fig. 2b, c; Supplementary Figs. 3 and 4a). Variants within this region co-segregated with low P frequency in the aforementioned backcrossed strains (Fig. 1f), as established by sequencing (16 strains sequenced). The 3 Mb region near the parental allele enrichment peak contained four genetic differences predicted to alter protein sequence, including three missense and one nonsense mutation (Supplementary Fig. 4b). The nonsense mutation was in fln-2, which encodes a filamin that is localised, among other places, to the pharyngeal cuticle13. We therefore focused on fln-2.
Resistance to P death is caused by a recessive mutation in fln-2. a Design of Variant Discovery Mapping strategy to select the recessive low P phenotype of N2M in F3 progeny. b Frequency of parental (N2M genotype) reads on chromosome X. For results for all chromosomes see Supplementary Fig. 3. c Normalised frequency of pure parental (N2M genotype) reads on chromosome X. d Illustration of the fln-2(ot611) mutation on FLN-2A, the longest isoform of fln-2. e Necropsy analysis and f lifespan of N2H-derived fln-2(syb202) Y800* strain. N2H survival curve in black, N2M in blue, and fln-2 Y800* in purple. Asterisks indicate p < 0.01, Student’s t test. Error bars represent s.e.m. of three trials. See Supplementary Table 2 for full statistics. See Supplementary data 1 for raw mortality data. Source data are provided as a Source Data file
FLN-2 is a distant ortholog of filamin-A (FLNA) in humans, which links actin filaments to membranes and is involved in cytoskeletal remodelling and mechanical force sensing. The variant in fln-2 results in a stop codon at Y800 in N2M, downstream of the N-terminal actin-binding domain in the second immunoglobulin(Ig)-like domain13 (Fig. 2d). Of the 16 isoforms that fln-2 encodes, the premature stop codon affects six, all of which contain the actin-binding domain (Supplementary Fig. 4c). We checked the fln-2 allele in other C. elegans wild isolates available at the Caenorhabditis elegans Natural Diversity Resource (CeNDR)14, and found that none of the wild strains contain the Y800* allele. fln-2 orthologs in a selection of other nematode species also encode the full-length fln-2(+) allele and not the Y800* equivalent (Supplementary Fig. 4d). We therefore conclude that the fln-2 variant in N2M is a nonsense mutation. This mutation was previously observed 6 out of 17 sequenced EMS-mutagenized genomes15 and designated fln-2(ot611) (formerly flnb-1); on the assumption that strains containing the mutant allele were derived from N2M, we will refer to the variant identified here as fln-2(ot611).
To test whether fln-2(ot611) causes the N2M infrequent P trait, we first tested effects of the mutation fln-2(ok1305), which contains a deletion in the N-terminal region. Although it showed infrequent P death (Supplementary Fig. 5a), the strain RB1240 also contained ot611, making this result difficult to interpret. Another fln-2 mutant allele, fln-2(tm4687), which does not contain ot611, has a deletion in the C-terminal region affecting all fln-2 isoforms. This also caused low P frequency (Supplementary Fig. 5b), demonstrating that variation at fln-2 can affect P incidence. Additionally, we introduced the Y800* mutation into N2H using CRISPR/Cas9. The resulting fln-2(syb202) Y800* allele led to recapitulation of the N2M phenotype, reducing P frequency from 45 to 19%, with little effect on p lifespan (Fig. 2e, f; Supplementary Table 2). Taken together, these results indicate that fln-2(ot611) is the cause of the reduced P frequency of N2M. Tests on four C. elegans wild isolates showed a P death incidence similar to that of N2H (Supplementary Fig. 5c), suggesting that this relatively high P frequency is a species-typical (i.e. wild-type) characteristic of C. elegans.
Although N2H is predominantly used as the wild-type strain in C. elegans research (requested from the CGC 3,633 times compared to 173 times for N2M [2006–2016]), N2M is sometimes used for strain construction or backcrossing. As a limited test of the presence of fln-2(ot611) among C. elegans strains, we genotyped the fln-2 allele in a selection of strains present in our strain collection. This revealed the presence of fln-2(ot611) in 23/50 of cases, including some strains generated by the C. elegans Gene Knockout Project (gk and ok alleles)(ot611 present: VC prefix strains, 6/10; RB prefix strains, 6/6) and the C. elegans Expression Project (s alleles)(ot611 present: 9/9)16,17 (Supplementary Table 3).
Discrepant effect of sir-2.1 attributable to fln-2 variation
Given the effect of the fln-2(ot611) allele on pharyngeal pathology and lifespan, and the fact that the Gems lab routinely used N2M as the wild-type for backcrossing8, we set out to revisit some earlier, puzzling results. In budding yeast (Saccharomyces cerevisiae) over-expression of the NAD-dependent histone deacetylase SIR2 (sirtuin) increases replicative lifespan18. There have been conflicting reports about whether or not increasing sirtuin levels in C. elegans increases lifespan. In C. elegans, independent transgenic lines with sir-2.1 over-expression (oe) generated in two studies were long lived19,20. In the case of one high copy number sir-2.1 over-expresser (transgene array geIs3, hereafter referred to as sir-2.1(oe))19 it was subsequently reported that longevity of the original strain (LG100) was attributable to a second site dyf gene mutation, and that sir-2.1(oe) no longer increased lifespan after backcrossing with N2M21. However, in another study that confirmed the presence and action of the second site mutation, a residual, smaller lifespan-increasing effect of sir-2.1(oe) was seen after outcrossing with N2 (line not specified)22.
We asked: could this discrepancy be attributable to effects of N2H vs. N2M background differences? Genotyping of the strains used by Viswanathan and Guarente (2011)22 and Burnett et al. 21 confirmed that the former were fln-2(+), and the latter fln-2(ot611), presumably reflecting the different N2 lines used in backcrossing of the original sir-2.1(oe) line. Consistent with both previous reports, sir-2.1(oe) resulted in increased lifespan in a fln-2(+) background (+27% mean lifespan, p < 0.0001, log rank test), but not a fln-2(ot611) background (p = 0.96, log rank test) (Fig. 3a; Supplementary Fig. 6a; Supplementary Table 4) under culture conditions as previously described, in which the thymidylate synthase inhibitor 5-fluorodeoxyuridine (FUDR) is added to block progeny development (see Methods and below).
Reduction of P death by fln-2(ot611) masks effects of high copy number sir-2.1 over-expression (oe) on lifespan. a Lifespan and b necropsy analysis of sir-2.1(oe) and control strains used in previous studies. GA strains are indicated by solid lines: GA468 geIs3 sir-2.1(oe); fln-2(ot611) (blue) and GA707 wuEx166; fln-2(ot611) control (black). LG strains are indicated by dashed lines: LG394 geIs3 sir-2.1(oe) (blue) and LG398 geIs101 control (black). sir-2.1(oe) reduced P death and extended lifespan in LG strains but not GA strains, consistent with both previous, conflicting studies21,22. Error bar represents s.e.m. of three trials. See Supplementary Table 4 for full statistics. See Supplementary data 1 for raw mortality data. Source data are provided as a Source Data file. c Lifespan and d necropsy analysis of sir-2.1(oe) and control strains after backcrossing four times with N2H. GA outcrossed in solid lines: GA1909 geIs3 sir-2.1(oe) (blue) and GA1907 wuEx166 control (black); LG outcrossed in dashed lines: GA1913 geIs3 sir-2.1(oe) (blue) and GA1915 geIs101 control (black). sir-2.1(oe) strains (blue) from both labs showed a reduced frequency of P death compared to control strains (black) in fln-2(+) background. See Supplementary Table 5 for full statistics. a–d Trials conducted in the presence of 10 μM FUDR. See Supplementary data 1 for raw mortality data. e Lifespan and f necropsy analysis of backcrossed sir-2.1(oe) and control strains, with FUDR excluded. Colour coding as in (c, d). See Supplementary Table 7 for full statistics. See Supplementary data 1 for raw mortality data. g sir-2.1(oe) and 10 μM FUDR interact to protect against progression of bacteria infection. Pharyngeal invasion and widespread infection were scored in worms fed RFP-expressing bacteria. Colour coding as in Fig. 1c. Mean of two trials, n = 16–20 per time point in each trial
Mortality deconvolution revealed that there was no significant difference in the frequency of P death in a fln-2(ot611) background (GA strains: GA468 geIs3 [sir-2.1(+) rol-6]; fln-2(ot611) compared to GA707 wuEx166 [rol-6]; fln-2(ot611)), but that sir-2.1(oe) significantly reduced P death frequency in a fln-2(+) background (LG strains: LG394 geIs3 [sir-2.1(+) rol-6] compared to LG398 geIs101 [rol-6]) (Fig. 3b; Supplementary Fig. 6b). All four strains were then backcrossed with N2H, which for the GA strains replaced fln-2(ot611) with fln-2(+), after which sir-2.1(oe) reduced P death frequency and increased lifespan in both sets of strains (+19% and +32% mean lifespan, respectively) (Fig. 3c, d; Supplementary Table 5). In a fln-2(+) background, the increase in lifespan resulting from sir-2.1(oe) was the result not only of reduced P death frequency, but also of a small but statistically significant increase in p lifespan (+14%, +13% mean lifespan in GA strains and LG strains, p = 0.0016 and 0.0002, respectively, log rank test) (Supplementary Fig. 6c; Supplementary Table 5).
In the previous studies, FUDR was used to block progeny development: 1.6 mM in the 2001 study19, and 10 to 50 μM21 or an unspecified concentration22 in the 2011 studies. Therefore in the trails described above FUDR was included (10 μM in Fig. 3a–d, and 50 μM in Supplementary Fig. 6a, b). Since impacts of many interventions on lifespan are affected by the presence of FUDR23,24,25,26, and since fluoropyrimidines have complex effects both on E. coli and C. elegans27, we also tested effects of sir-2.1(oe) on P frequency and lifespan (overall and P or p alone) in the absence of FUDR. Here no effect was detected in either fln-2 background, in either the original strains or the outcrossed strains (2 trials each) (Fig. 3e, f; Supplementary Fig. 6d, e; Supplementary Tables 6 and 7).
We further tested for interaction between FUDR and sir-2.1 over-expression using a previously described low copy number transgene array pkIs164220. It was previously reported that longevity resulting from pkIs1642 sir-2.1(oe) was abrogated by outcrossing21. Here both original and outcrossed strains were fln-2(+). Nonetheless, we observed that the longevity of the non-outcrossed pkIs1642 strain was, again, FUDR dependent (Supplementary Fig. 7; Supplementary Table 8). However, here longevity resulted from increased p lifespan rather than reduced P frequency. For more details, see Supplementary Fig. 6.
We also asked whether loss of sir-2.1 increases P frequency, using the sir-2.1(ok434) null allele that harbours a deletion that disrupts the sirtuin catalytic domain28,29. After backcrossing into a fln-2(+) background, sir-2.1(ok434) reduced overall lifespan independently of FUDR (−8% and −9% mean lifespan, p = 0.0043 and 0.0425, with and without FUDR respectively, log rank test), consistent with previous reports20,28. However, the manner in which sir-2.1(ok434) shortened lifespan was FUDR dependent: with FUDR it increased P death frequency, but without FUDR it reduced p lifespan (Supplementary Fig. 8a, b; Supplementary Table 9). Thus, again, the impact of sir-2.1 on worm ageing is FUDR dependent.
The FUDR dependence of the reduced P death caused by sir-2.1(oe) suggests that SIR-2.1 and FUDR act synergistically to protect against bacterial infection of the pharynx. To explore the mechanism of this protection we compared the progression of pharyngeal infection in OP50-RFP-fed worms with either sir-2.1(oe), FUDR, or both. Without FUDR, as expected, sir-2.1(oe) had little effect on either the initiation or subsequent spread of E. coli infection; with FUDR, the appearance of intracellular RFP puncta was unaffected by sir-2.1(oe), but the progression of infections in older animals was suppressed, with fewer animals showing widespread RFP (Fig. 3g). Notably, FUDR alone delayed the appearance of RFP puncta, though it had little effect on the frequency of P (Fig. 3g). Thus, FUDR and sir-2.1(oe) affect pharyngeal infection by different mechanisms: FUDR delays initial invasion, while SIR-2.1 suppresses later spread of infection. This suggests that FUDR-induced resistence to bacterial pathogenicity enables SIR-2.1 to reduce later E. coli proliferation, leading to synergy between the two interventions. Consistent with this, pre-treatment of C. elegans with FUDR increases resistance to the Gram-negative bacterial pathogen Pseudomonas aeruginosa30.
fln-2 variation confounds effects of daf-12 on lifespan
The daf-12 gene encodes a steroid hormone receptor, and has been shown to have complex effects on different daf-2 insulin/IGF-1 receptor mutants31,32,33. For example, daf-2(m41) longevity (the Age phenotype) is partially suppressed by daf-12(m20) under certain conditions31. We noted that daf-12(m20) shortens daf-2(m41) lifespan by increasing early mortality31, and that the daf-12 and fln-2 loci are closely linked on LGX (at X:2.36 ± 0.007 cM and X:1.18 ± 0.015 cM, respectively). This raised the possibility that some observed effects of daf-12 on daf-2 Age might be attributable to the differences in P frequency due to variation at the fln-2 locus.
We genotyped daf-2(m41) and daf-2(m41); daf-12(m20) double mutant strains used in earlier studies31,32, and found that the daf-2(m41) strain, but not the daf-2; daf-12 strain, contained fln-2(ot611). Consistent with previous findings31, daf-2; daf-12 lifespan was shorter than daf-2; fln-2; this was due mainly to a 9-fold higher P frequency in daf-2; daf-12 (Fig. 4a, b). Comparison of daf-2; daf-12 and daf-2 after removal of fln-2(ot611) by outcrossing showed a much smaller but statistically significant 60% increase in P frequency (Fig. 4b). Notably, daf-12 had little effect on daf-2(m41) p lifespan (Fig. 4c); moreover, fln-2(ot611) significantly reduced daf-2(m41) p lifespan (Fig. 4c; Supplementary Table 10). We therefore conclude that while daf-12 does partially suppress the daf-2(m41) Age, it acts solely by increasing P frequency, an effect that appeared greater due to the presence of fln-2(ot611) in the daf-2 mutant. The fact that the lifespans of daf-2 and daf-2; fln-2 nematodes were similar was due to antagonistic effects of fln-2 on P frequency (increasing lifespan) and p survival (reducing lifespan) in this condition. These results further illustrate how fln-2 variation can confound studies of lifespan genetics.
Effect of daf-12 on daf-2 at 25 °C is partially affected by fln-2(ot611) variation. a Lifespan and b necropsy analysis of daf-2(m41); daf-12(m20) and daf-2(m41) mutants in fln-2(+) or fln-2(ot611) backgrounds. daf-2 fln-2 in green, daf-2 in blue, and daf-2 daf-12 in purple. Asterisks indicate significant differences, p < 0.0001 and 0.01, respectively. Error bars represent s.d. of two trials. See Supplementary Table 10 for full statistics, and Supplementary data 1 for raw mortality data. Source data are provided as a Source Data file. c Lack of lifespan effect by daf-12 in daf-2 p sub-population lifespan in fln-2(+) background. See Supplementary data 1 for raw mortality data
fln-2(ot611) masks eat-2 Age effect
Dietary restriction (DR) increases healthspan and lifespan in many model organisms34. In C. elegans, some Eat mutants with reduced feeding rate (e.g. eat-2) show increased lifespan, which has been interpreted as resulting from DR35. In previous work in this lab with eat-2 alleles crossed into an N2M background, we often saw little effect of eat-2 on lifespan (A. Benedetto, M. Keaney, J.M.A. Tullet, and D. Gems, unpublished). More recently we showed that many Eat mutants show reduced P frequency, which may reflect reduced mechanical damage to the pharynx otherwise caused by high pumping rate in early life9. Mortality deconvolution of eat-2 populations showed that the lifespan increase is largely attributable to reduced P death frequency. This predicts that eat-2 should increase lifespan more in a N2H background than a N2M background, due to the higher P frequency in the former. To test this, we performed mortality deconvolution on eat-2(ad1116) mutants in fln-2(+) and fln-2(ot611) backgrounds. As expected, P death frequency was reduced by either fln-2 or eat-2 (Fig. 5a). Notably, eat-2 increased lifespan more in a fln-2(+) background than in a fln-2(ot611) background (mean lifespan +27% and +9%, p < 0.0001, p = 0.0043, respectively, log rank test) (Fig. 5b; Supplementary Table 11), while the effect on p lifespan was modest in both backgrounds (+8% and +5%, p = 0.0183 and 0.0149, respectively, log rank test) (Fig. 5c). When P was eliminated using antibiotics, effects of fln-2 genetic background disappeared, as expected (Fig. 5d).
Reduction of P death by fln-2(ot611) masks lifespan effects of eat-2. a Necropsy analysis and b lifespan of eat-2(ad1116) mutants in fln-2(+) or fln-2(ot611) backgrounds. N2H survival curve indicated by solid black line, N2M by dashed black line, eat-2 by solid purple line, and eat-2 fln-2 by dashed purple line. Error bars represent s.e.m. of three trials. See Supplementary Table 11 for full statistics, and Supplementary data 1 for raw mortality data. Source data are provided as a Source Data file. c eat-2(ad1116) causes a small but significant increase in p sub-population lifespan in both fln-2(+) and fln-2(ot611) backgrounds. See Supplementary data 1 for raw mortality data. d Effects of fln-2(ot611) on lifespan in eat-2(ad1116) mutants disappears after treatment with antibiotics (200 μg/ml carbenicillin). See Supplementary data 1 for raw mortality data
Discussion
The findings reported here reveal a major confounding variable in studies of ageing in C. elegans: variation at the fln-2 locus between two widely used N2 lines. We demonstrate how this has actually confounded research on C. elegans ageing, distorting experimental results, and causing confusion and controversy, issues that can now be readily resolved. These results also provide further evidence of the power of mortality deconvolution and, more broadly, pathology-centred approaches to solve complex problems in biogerontology.
The advance of science often requires a process of over-coming limitations within a field, both conceptual and technical. The latter includes identification and control of confounding variables, which, over time, can lead to accumulation of unsolved questions and conflicting results. This is a particular problem with model organism biogerontology, since lifespan as a trait is particularly sensitive to uncontrolled variables36,37. For C. elegans, these include variables affecting genetic background, such as that between N2H and N2M, and effects of variation at fln-2 as described here. It also includes a range of environmental variables; since the introduction of a standardised C. elegans plate culture system10, several modifications have been introduced, including the use of FUDR to block progeny production, and antibiotics or UV irradiation to block E. coli proliferation. It is becoming increasingly clear that the effects of many interventions that alter C. elegans lifespan reflect, at least partially, changed interactions between C. elegans and E. coli9,38,39. Thus, variables affecting C. elegans-E. coli interactions, including fln-2 allele variation and use of different culture conditions, either introduce confounding variables, or make it difficult to relate results from different research groups to one another.
Variation at fln-2 can confound studies of the genetics of lifespan, as illustrated here using three cases, involving sir-2.1, daf-2 and daf-12, and eat-2. It is therefore possible that variation at fln-2 has also confounded other studies of C. elegans ageing.
Our results resolve a controversy surrounding effects of sir-2.1 over-expression in C. elegans lifespan21,22,40. This provides a useful lesson in how science can be impeded by confounding variables, in this case three of them. The first was the presence of a dyf mutation which increased lifespan in a sir-2.1(oe) strain21,22. The second was the presence of fln-2(ot611) in strains backcrossed with N2M, which suppressed the effect of sir-2.1(oe) on lifespan21. The third was the use of FUDR, which as we show here is required for sir-2.1(oe) to increase lifespan; it is notable that FUDR-dependent induction of increased lifespan by hypertonic stress is partially sirtuin dependent24. Does this FUDR-dependence invalidate the conclusion that sir-2.1(oe) protects against ageing? This question raises important issues about the use of C. elegans as a model for understanding human ageing. We have argued elsewhere41,42 for a reconceptualization of the relationship between ageing and lifespan, as follows. Lifespan is not a read out of one process, i.e. ageing, but rather a function of some (but not all) senescent pathologies that arise as animals age. Importantly, the identity of the pathologies that limit lifespan will vary not only between species, but within species depending upon genotype and environment41,42. According to this view, the key objective for understanding ageing is to understand the etiologies of any pathology, whether they affect lifespan or not. In terms of sir-2.1(oe), FUDR and lifespan, if the use of FUDR enables studies that yield useful insights about senescent pathology, then this is a good model to study.
We showed here that under standard culture conditions (proliferating E. coli, no FUDR), previously reported suppression of daf-2(m41) Age (25 ˚C) by daf-12(m20) is likely to have been partially attributable to variation at fln-2 (Fig. 4a–c). There is other evidence that signalling via the DAF-9/DAF-12 pathway can promote daf-2 Age. For example, at 22.5˚C daf-9(rh50) shortens daf-2(e1368) lifespan43, and daf-2(e1368) Age can be enhanced by treatment with exogenous DAF-12 ligand (Δ4-dafachronic acid)44. However, our results imply that clearer understanding of how the IIS and DAF-12 steroid signalling pathways interact to affect ageing could be achieved by enhanced control of confounding variables, including fln-2 and culture condition variables (as listed above).
These results reveal that the greater lifespan of N2M is attributable to fln-2(ot611). The presence of the fln-2(+) allele in N2H and all C. elegans wild isolates examined demonstrates that N2H is the wild type and N2M a long-lived mutant, in contrast to a previous suggestion8. This is an argument for discontinuing the use of N2M as wild type for most purposes. The mechanisms by which fln-2(ot611) protects against pharyngeal infection remain to be explored. Comparison between the two N2 strains raised on fluorescent E. coli suggests that fln-2(ot611) is resistant to cuticle perforation and/or bacteria invasion. The presence of FLN-2 protein in the pharyngeal cuticle13 is consistent with a role as a direct, cell autonomous determinant of susceptibility to mechanical damage and/or bacterial invasion.
Methods
Culture methods and strains
C. elegans maintenance was performed using standard protocols10. Strains were grown at 20˚C on NGM agar plates seeded with E. coli OP50. C. elegans strains used: N2 and N2 male stocks recently obtained from the CGC (designated N2H and N2M, respectively), CF1038 daf-16(mu86), RB1240 fln-2(ok1305ot611), FX4687 fln-2(tm4687), UN1066 xbEx0816 [fln-2c::gfp], GA1947 fln-2(syb202), GA468 fln-2(ot611) geIs3 [sir-2.1(+)+rol-6(su1006)], GA707 fln-2(ot611) wuEx166 [rol-6(su1006)], LG394 geIs3 [sir-2.1(+)+rol-6(su1006)], LG398 geIs101 [rol-6(su1006)], NL3908 unc-119(ed3) pkIs1641(unc-119[+]), NL3909 unc-119(ed3) pkIs1642(unc-119[+] sir-2.1[+]), GA1934 sir-2.1(ok434), GA1928 daf-2(e1370), DR1296 daf-2(e1370) daf-12(m20), GA1945 daf-2(m41), DR1547 daf-2(m41) daf-12(m20), DA1116 eat-2(ad1116), GA66 eat-2(ad1116) fln-2(ot611). Strains genotyped with respect to fln-2 allele are listed in Supplementary Table 3.
Lifespan assays and necropsy analysis
Unless otherwise specified, lifespan assays were conducted at 20 ˚C and without FUDR, and animals were transferred daily during the egg laying period, and every 3-7 days thereafter. Animals lost due to causes other than senescence (e.g. desiccation on the side of the plate, internal hatching, extensive rupture through the vulva) were censored. For lifespans performed at 25 °C (daf-2 daf-12 experiments), worms were raised at 20 °C, and transferred to 25 °C at L4 stage. For trials performed in the presence of FUDR, L4 stage animals were transferred to seeded NGM plates containing the specified concentration of FUDR, and animals were transferred every 3–7 days to avoid hypertonic stress24. For antibiotic treatment, 200 μg/ml carbenicillin was added to two-day old lawns of OP50, and nematodes were transferred to antibiotic plates as L4 larvae. Mortality and pharyngeal status of corpses were scored every 2–3 days. For lifespan analysis on UV-irradiated E. coli OP50, bacteria were inoculated onto NGM plates and allowed to grow overnight at room temperature before exposure to UV light (Stratagene UV Stratalinker 2400) for 5 min, 2 days prior to use (to detect and exclude plates with bacterial growth). Bacteria-free (alkaline hypochlorite-treated) C. elegans eggs were then plated on irradiated E. coli lawns. Lifespan assays were repeated at least twice, and raw mortality data are provided in the Ziehm tables (Supplementary Data 1). Survival plots were generated in GraphPad Prism using combined lifespan data from multiple trials, with L4 as day 0 on the time scale.
OP50-RFP infection analysis
An overnight culture of OP50-RFP in LB supplemented with tetracycline was washed three times in M9 to remove tetracycline, and seeded onto NGM plates two days before use. L4 worms cultured on OP50 bacteria were transferred to OP50-RFP plates, and transferred daily during the reproductive period. Before imaging, live worms were mounted on 2% agar pads and anesthetised using 0.2% levamisole. Fluorescence within pharyngeal tissue were scored at 630x magnification using a Zeiss AxioImager Z2 microscope with a rhodamine filter. A different cohort of worms was imaged for each time point so as to avoid recovering worms from levamisole.
Test cross to confirm single-locus association
Strain GR1307 daf-16(mgDf50) was backcrossed with either N2H or N2M males, and F1s allowed to self. Single F2 animals were picked at random and the daf-16 allele genotyped, and F3 populations of single daf-16 homozygous animals were scored in necropsy analysis and used for further backcrossing. The same analyses were performed for a single F4, F6, and F8 animal of each F2 line. All progeny of the backcrossed F2, F4, F6, and F8 animals used in lifespan were frozen and subsequently genotyped with respect to the fln-2 allele.
Whole genome sequencing
Genomic DNA was extracted using Gentra Puregene Tissue Kit (Qiagen), and sequenced with an average coverage of 100× (Center for Applied Genomics, The Hospital for Sick Children, Toronto, Canada). N2M genome sequence is available on ENA with the accession number PRJEB34655 [https://www.ebi.ac.uk/ena/browser/view/PRJEB34655], and will be incorporated in the N2 genome sequence on CeNDR14. For variant discovery mapping, N2H hermaphrodites and N2M males were allowed to mate overnight, after which males were removed. F1 hermaphrodites were allowed to self-fertilise, and a total of 250 F2 animals were singled and allowed to self-fertilise. From each plate, 50–60 F3 progeny were transferred to plates containing 10 μM FUDR, allowed to age, and then scored for pharyngeal phenotype. 50 of the populations displaying lowest frequency of P death were re-analysed and 25 populations that showed less than 20% P death in both tests were selected. F3 and F4 genomic DNA from selected populations were then extracted and pooled to be sequenced. The sequencing data were processed in CloudMap using the variant discover mapping method, which mapped the causal variant to the vicinity of the 10 Mb position on chromosome X.
Genotyping fln-2 alleles
The following primers were used to amplify the genomic region flanking the fln-2 mutation site: forward 5′-GGTGTTCGATTCTGGTCTGG-3′; reverse 5′-ACATCGACGAGAAGACAACAC-3′. PCR product was sequenced using primer 5′-TGTACCCAGAAATTGACAAGATAC-3′. Allele-specific PCR can be used to discriminate between the genotypes, using the following primers: wild-type-specific forward 5′-taccattccgagcttattgattgttacctGGACGGCGCTGGTCCATAC-3′; ot611-specific forward 5′-GGACGGCGCTGGTCTATAA-3′; ASP reverse 5′- ATCGCATGAACCATAAATGATG-3′; the wild-type PCR product is 30nt longer than the mutant product.
fln-2(syb202) Y800* allele
The allele harbouring the Y800* mutation was generated using co-CRISPR method in N2H (Sunybiotech). sgRNAs TCATTCACTCCGGACGGCGCTGG and CCACGTTCTTTTTAACAGAATGG were used, and synonymous mutations were made in the sgRNA/PAM site. The final mutated sequence is tcattcactccggacggAgctggtcaatAAaaaatAcaTgttctttttaacagaatgg.
Statistics
Survival analyses of whole populations (i.e. P and p deaths) were conducted using GraphPad Prism, including right censoring of animals lost due to causes other than senescence. To test for statistically significant differences between survival curves, the non-parametric log rank test was used. In the case of P or p subpopulations, due to the absence of information about corpse type, deaths due to causes other than senescence were not censored but rather excluded altogether from statistical analysis. Sample sizes and statistics for each experiment are provided in the lifespan tables in the Supplementary file. An unpaired Student's t test was used to compare frequencies of P death between different strains and/or conditions.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All statistics relating to survival analysis are provided in the Supplementary tables. Raw mortality data are provided in Ziehm tables9 in Supplementary Data 1. Source data relating to mortality analysis are provided as a Source Data file. N2M genome sequence is available on ENA with the accession number PRJEB34655.
References
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Bitto, A., Wang, A., Bennett, C. & Kaeberlein, M. Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb. Perspect. Med. 5, a025114 (2015).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A. C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Vellai, T. et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003).
Weber, K. P. et al. Whole genome sequencing highlights genetic changes associated with laboratory domestication of C. elegans. PloS one 5, e13922 (2010).
Sterken, M. G., Snoek, L. B., Kammenga, J. E. & Andersen, E. C. The laboratory domestication of Caenorhabditis elegans. Trends Genet.: TIG 31, 224–231 (2015).
Large, E. E. et al. Selection on a subunit of the NURF chromatin remodeler modifies life history traits in a domesticated strain of Caenorhabditis elegans. PLoS Genet. 12, e1006219 (2016).
Gems, D. & Riddle, D. L. Defining wild-type life span in Caenorhabditis elegans. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 55, B215–B219 (2000).
Zhao, Y. et al. Two forms of death in ageing Caenorhabditis elegans. Nat. Commun. 8, 15458 (2017).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Kumar, S. et al. Lifespan extension in C. elegans caused by bacterial colonization of the intestine and subsequent activation of an innate immune response. Dev. Cell 49, 100–117.e106 (2019).
Minevich, G., Park, D. S., Blankenberg, D., Poole, R. J. & Hobert, O. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192, 1249–1269 (2012).
DeMaso, C. R., Kovacevic, I., Uzun, A. & Cram, E. J. Structural and functional evaluation of C. elegans filamins FLN-1 and FLN-2. PloS one 6, e22428 (2011).
Cook, D., Zdraljevic, S., Roberts, J. & Andersen, E. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 45, D650–D657 (2017).
Sarin, S. et al. Analysis of multiple ethyl methanesulfonate-mutagenized Caenorhabditis elegans strains by whole-genome sequencing. Genetics 185, 417–430 (2010).
C. elegans Deletion Mutant Consortium. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 (Bethesda, Md.) 2, 1415–1425 (2012).
Hunt-Newbury, R. et al. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 5, e237 (2007).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Viswanathan, M., Kim, S. K., Berdichevsky, A. & Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Developmental cell 9, 605–615 (2005).
Burnett, C. et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485 (2011).
Viswanathan, M. & Guarente, L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 477, E1–E2 (2011).
Aitlhadj, L. & Sturzenbaum, S. R. The use of FUdR can cause prolonged longevity in mutant nematodes. Mechanisms Ageing Dev. 131, 364–365 (2010).
Anderson, E. N. et al. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mechanisms ageing Dev. 154, 30–42 (2016).
Burnaevskiy, N. et al. Reactivation of RNA metabolism underlies somatic restoration after adult reproductive diapause in C. elegans. eLife 7, https://doi.org/10.7554/eLife.36194 (2018).
Van Raamsdonk, J. M. & Hekimi, S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mechanisms ageing Dev. 132, 519–521 (2011).
Scott, T. et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell 169, 442–456 (2017).
Wang, Y. & Tissenbaum, H. A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mechanisms ageing Dev. 127, 48–56 (2006).
Greiss, S., Hall, J., Ahmed, S. & Gartner, A. C. elegans SIR-2.1 translocation is linked to a proapoptotic pathway parallel to cep-1/p53 during DNA damage-induced apoptosis. Genes Dev. 22, 2831–2842 (2008).
Miyata, S., Begun, J., Troemel, E. & Ausubel, F. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178, 903–918 (2008).
Larsen, P. L., Albert, P. S. & Riddle, D. L. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567–1583 (1995).
Gems, D. et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150, 129–155 (1998).
Dumas, K., Guo, C., Shih, H.-J. & Hu, P. Influence of steroid hormone signaling on life span control by Caenorhabditis elegans insulin-like signaling. G3 3, 841–850 (2013).
Kapahi, P., Kaeberlein, M. & Hansen, M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev., https://doi.org/10.1016/j.arr.2016.12.005 (2016).
Lakowski, B. & Hekimi, S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 95, 13091–13096 (1998).
Partridge, L. & Gems, D. Benchmarks for ageing studies. Nature 450, 165–167 (2007).
Lucanic, M. et al. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat. Commun. 8, 14256 (2017).
Podshivalova, K., Kerr, R. & Kenyon, C. How a mutation that slows aging can also disproportionately extend end-of-life decrepitude. Cell Rep. 19, 441–450 (2017).
Cabreiro, F. et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239 (2013).
Couzin-Frankel, J. Genetics. Aging genes: the sirtuin story unravels. Sci. (New Y., N. Y.) 334, 1194–1198 (2011).
Ezcurra, M. et al. C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Curr. Biol.: CB 28, 2544–2556 (2018).
Gems, D. The aging-disease false dichotomy: understanding senescence as pathology. Front. Genet. 6, 212 (2015).
Gerisch, B., Weitzel, C., Kober-Eisermann, C., Rottiers, V. & Antebi, A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Developmental cell 1, 841–851 (2001).
Gerisch, B. et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc. Natl Acad. Sci. USA 104, 5014–5019 (2007).
Acknowledgements
The authors thank the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for strains used in this study, and A. Daul at the CGC for information. We thank the Mitani Lab National BioResource Project for strains. The authors thank E. Cram, H. Richly and B. Tursun for strains and reagents. This work was supported by a Wellcome Trust Strategic Award to D. Gems (098565/Z/12/Z) and a Wellcome Trust Research Career Development Fellowship to R.J. Poole (095722/Z/11/Z). The authors would also like to thank A. Benedetto for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
D.G. and Y.Z. conceived the study. H.W. and Y.Z. performed the experiments, analysed and interpreted the data. R.P. performed the Variant Discovery Mapping. D.G. and Y.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Alexander Mendenhall and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Wang, H., Poole, R.J. et al. A fln-2 mutation affects lethal pathology and lifespan in C. elegans. Nat Commun 10, 5087 (2019). https://doi.org/10.1038/s41467-019-13062-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-13062-z
This article is cited by
-
Colony level fitness analysis identifies a trade-off between population growth rate and dauer yield in Caenorhabditis elegans
BMC Ecology and Evolution (2024)
-
The chromatin factors SET-26 and HCF-1 oppose the histone deacetylase HDA-1 in longevity and gene regulation in C. elegans
Nature Communications (2024)
-
The C. elegans Myc-family of transcription factors coordinate a dynamic adaptive response to dietary restriction
GeroScience (2024)
-
C. elegans ageing is accelerated by a self-destructive reproductive programme
Nature Communications (2023)
-
Filamin A cooperates with the androgen receptor in preventing skeletal muscle senescence
Cell Death Discovery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.