Abstract
An important outstanding question in auditory neuroscience is to identify the mechanisms by which specific motifs within inter-connected neural circuits affect auditory processing and, ultimately, behavior. In the auditory cortex, a combination of large-scale electrophysiological recordings and concurrent optogenetic manipulations are improving our understanding of the role of inhibitory–excitatory interactions. At the same time, computational approaches have grown to incorporate diverse neuronal types and connectivity patterns. However, we are still far from understanding how cortical microcircuits encode and transmit information about complex acoustic scenes. In this review, we focus on recent results identifying the special function of different cortical neurons in the auditory cortex and discuss a computational framework for future work that incorporates ideas from network science and network dynamics toward the coding of complex auditory scenes.
Similar content being viewed by others
Introduction
In auditory processing, a long-standing question has been the function of cortical architecture in specific sensory functions. Auditory cortex (AC) is comprised of neurons of many different types, providing the ability to perform an astonishing number of computations. Even the most basic distinction between neurons into excitatory and inhibitory units markedly expands the computational capacity of a network, and a quest in auditory neuroscience has been to unravel the function of specific microcircuits in sound encoding and plasticity. A particularly interesting aspect of cortical connectivity is the diversity of inhibitory neurons in their morphology and synaptic properties1 (Fig. 1a). Interneurons form reciprocal connections not only with the excitatory neurons, but also with each other (Fig. 1b). Furthermore, the diversity of connections between different types of inhibitory interneurons can affect how information is processed in the network (Fig. 1c).
Simplified views of cortical circuits. (a) Diagram of excitatory–inhibitory circuit with recurrent connections. Theoretical and experimental studies demonstrate that inhibition stabilizes between excitatory and inhibitory neurons in the auditory cortex. (b) Inhibitory–excitatory network can be extended to include several interneuron subtypes. (c) Schematic diagram of connectivity between select neurons in the auditory cortex (note that layer-specific information is omitted here): Exc: Excitatory neurons; PV: parvalbumin-positive interneurons; SOM: somatostatin-positive interneurons; VIP: vasopressin-positive interneurons; TC: Thalamo-cortical projection neurons. All neuron types receive additional inputs from other brain areas, which were omitted from the diagram for simplicity. Open circles: excitatory synapses; closed circles: inhibitory synapses. Solid lines indicate dominant projections; dashed lines indicate occasional connections
Recent advances in optogenetics and imaging2 of the role of cortical circuits comprised of distinct inhibitory neurons in basic auditory functions, such as frequency discrimination and adaptation to temporal stimulus statistics. In combination with computational techniques for inhibitory–excitatory network analysis, these experimental approaches offer promise for unraveling the microcircuits within AC for representing sounds. Here, we discuss progress and limitations in our understanding that emerges from recent investigations of the function of cortical microcircuits in audition.
Role of inhibition in auditory frequency discrimination
Spectral differences between sounds are fundamental cues for identifying a dangerous sound, be it the sound of an approaching predator or screeching brakes; recognizing a familiar speaker; or distinguishing different animal vocalizations3,4,5. Frequency selectivity, originating with spectral decomposition of the acoustic signal by the cochlea, is a strong organizing feature of neuronal responses in the auditory pathway. Neurons in the AC exhibit frequency selectivity6,7,8, responding to a subset of frequencies more strongly than others. This selectivity is thought to support perceptual frequency discrimination acuity9,10,11 (but see refs. 12,13): the greater the difference either in individual or population neuronal responses for tones of neighboring frequencies, the higher frequency discrimination acuity. Either more narrowly tuned neurons, or neurons with higher signal-to-noise ratio in tone-evoked responses would support higher frequency discrimination acuity, as the difference in responses to neighboring tones will be higher in these neurons than in broadly tuned/low signal-to-noise neurons. One of the most extensively tested roles of cortical inhibition in auditory processing has been in shaping frequency selectivity of excitatory neurons. Inhibition may sharpen frequency tuning and increase the signal-to-noise ratio in excitatory tone-evoked responses by suppressing spontaneous excitatory activity; alternatively, either broad or co-tuned inhibitory inputs may sharpen frequency selectivity owing to the rectifying non-linear integration (such as the spiking non-linearity)14,15,16. Differential timing of excitatory and inhibitory co-tuned inputs can further refine frequency tuning of excitatory neurons17. Experimental evidence from pharmacological experiments and intracellular recordings has supported either effect8,14,17,18,19,20. An interesting possibility is that distinct inhibitory neuronal cell types may contribute differentially to shaping frequency selectivity. The development of optogenetic manipulations has promised to disambiguate the effects of different specific neuronal cell types21.
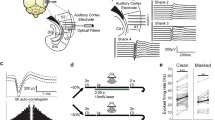
A series of studies that optogenetically manipulated the most common interneuron type, parvalbumin-positive interneurons (PVs) confirmed that inhibitory neurons modulate frequency tuning in AC: activating PVs enhanced feedforward connectivity between excitatory units. The spontaneous activity of excitatory neurons was decreased, and the frequency tuning width was narrower, increasing frequency selectivity22. Consistently, PV activation also increased the strength of tone-evoked responses and improved behavioral frequency discrimination acuity, whereas suppression decreased the strength and tuning width of the tone-evoked responses in putative excitatory neurons, and drove an impairment in behavioral frequency discrimination acuity (Fig. 2a–c)23.
Inhibitory interneurons affect auditory cortical responses and behavior. Activating PVs with ChR2 (a) increases tone-evoked responses and improves behavioral frequency discrimination acuity, whereas suppressing PVs using Arch has the opposite effect (b). (c) Direct activation of excitatory neurons with ChR2 does not change tone-evoked responses or behavioral frequency discrimination acuity on average. (c e) Left: diagram of optogenetic manipulation. Center: mean tone-evoked response magnitude under light-off and light-on conditions based on neuronal recordings. Right: Behavioral response to a shift in frequency under light-off and light-on conditions. Adapted from ref. 23
But are the effects different between distinct interneuron types? There is a range of interneuron classifications available, with at least two major groups of neurons emerging besides PVs, somatostatin (SOMs), and serotonin receptor 5HT3aR, of which a major group are also positive for vassopressin (VIPs)1,24,25. Although each interneuron class includes a number of different cell types and may change with development and experience26, these classes of neurons have received prominent attention, as they approximate a canonical cortical circuit (Fig. 1), and owing to availability of transgenic mouse lines. Optogenetic activation of either PVs or SOMs exerted a similar mix of effects on tone-evoked activity in excitatory cells, with activation providing either multiplicative scaling, as would be expected from co-tuned inhibition, or linear amplification, as would be expected from broad inhibitory inputs27. This variability in combination with spiking threshold non-linearity and strength of suppression across different neurons can both amplify and sharpen tuning properties of excitatory neurons27. On average, suppressing interneurons had differential effects: suppressing SOMs increased the gain of excitatory neurons, whereas suppressing PVs weakened frequency tuning28. Nonetheless, as with activation, in individual neurons inactivation of either type of interneuron showed a range of effects, thus supporting a number of models for interactions between PVs, SOMs and excitatory neurons. Measuring the tuning widths of individual PVs, excitatory neurons and SOMs neurons furthermore did not yield clear distinctions29,30, potentially because these classes of neurons are themselves comprised of multiple cell types. These differences may be exacerbated across studies by the various biases toward specific subclasses by different recording techniques. Indeed, a recent review of SOMs estimated over 100 subtypes of SOM neurons31. Thus, whereas the optogenetic perturbations of PVs and SOMs confirmed their role in shaping frequency tuning in the AC, the results were consistent with the range of results in the pharmacological literature, and a clear distinction between the function of the two interneuron subtypes in frequency tuning has not emerged.
By targeting the electrophysiological recordings to specific cell types, it has begun possible to assess the diversity of neuronal responses in both stimulus selectivity and time course. Interestingly, in the temporal domain, responses of PVs had faster onsets on average than excitatory neurons, whereas SOM neurons exhibited slower response onsets than excitatory neurons29,30. Such differential activation timing may provide an additional mechanism for sharpening of tone-evoked responses in AC32: the differences between inhibitory and excitatory response onsets would drive more precise response onsets over the excitatory population. The timing differences in suppression between PVs and SOMs could support a number of other functional effects, for example when applied to suppression of responses owing to the stimulus history. These observations, facilitated by targeted recordings, provide information about specific neuronal synaptic parameters that should inform the design of computational models. Future studies are required for understanding whether the timing differences in tone-evoked responses of PVs and SOMs results in distinct function of these neurons in frequency discrimination.
Role of inhibition in adaptation to stimulus statistics
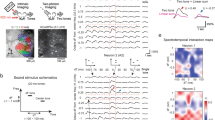
Time processing is particularly important for auditory perception. Perception is formed as much by the present stimulus as by the history of preceding stimuli; it is an interaction between the representation of new events and memory of past events. Sensory cortex constantly reshapes responses to present stimuli dependent on the context. Responses of nearly all neurons in the AC (95%) change with stimulus temporal history, exhibiting stimulus-specific adaptation (SSA)33,34. Typically, the firing rate is reduced in response to repeated stimuli through a process that involves integration of stimulus statistics over time33,34. Inhibitory–excitatory networks support this transformation, and PVs and SOMs were found to play a differential role in cortical adaptation. SSA is quantified as the index of change in the firing rate in response to a rare ‘deviant’ tone presented as part of a sequence with another common ‘standard’ tone, at varying presentation probabilities (for example, 10%, deviant vs. 90% standard). SSA is thought to be supported by a combination of thalamo-cortical depression and intra-cortical inhibitory–excitatory circuit effects35. Indeed, suppressing either PVs or SOMs optogenetically increased the strength of adaptation in excitatory neurons34 (Fig. 3a, b). However, PVs and SOMs differed in their contribution to adaptation: suppression of SOMs evoked a stimulus-selective increase in excitatory responses to the standard, but not the deviant tones, whereas suppressing PVs led to a non-specific response increase. When examining the effect of SOM suppression on excitatory responses to repeated presentations of the standard tones following a deviant tone, disinhibition of the excitatory response increased with repeated presentations (Fig. 2a–c), further confirming that SOMs provided a selective inhibitory contribution to SSA. PV and SOM interneurons themselves both exhibit SSA34,36, so that the unique contribution of SOMs in stimulus-specific inhibition of excitatory responses was possible through selective suppression of this cell type.
Specific inhibitory neuron type mediates auditory adaptation. (a) Top: The effect of SOM and PV inactivation on stimulus-specific adaptation to frequent tones was tested using an oddball stimulus, with two tones at 10–90 ratio, light every 5th tone. Bottom: The mean firing rate (FR) during repeated tones adapted with successive presentations of the standard tone. (b) SOMs provide stimulus-specific inhibition, as the effect of SOM suppression increased with repeated standard tones. PVs provided constant inhibition regardless of adaptation. a, b adapted from ref. 34. (c) Passive exposure to a tone stimulus lead to a decrease in excitatory and an increase in inhibitory activity over 5 days. Left: calcium activity was imaged using two-photon microscopy in populations of identified inhibitory and excitatory neurons before and after subjecting the mouse to prolonged exposure to tones. Neuronal activity was measured as spike counts inferred from the imaged fluorescence signal. Right: change index of the mean activity in response to the tone to which the mouse was exposed, averaged over populations of excitatory (red) or inhibitory (blue) neurons, over days since prolonged tone exposure onset. Mean excitatory activity decreased with exposure, whereas mean inhibitory activity increased. (d) Among the inhibitory neurons, the activity of SOMs increased following passive tone exposure, whereas the activity of PVs decreased. Mean z-scored time course of Calcium activity of SOMs or PVs in response to a tone at day 1 (black traces) and day 5 (blue traces). c, d adapted from ref. 37
Further work revealed that stimulus-specific inhibition mediated by SOMs persisted over a longer time scale, in habituation following passive exposure to sounds over several days37 (Fig. 3c, d). Whereas the excitatory and PV neuronal responses to habituated sounds were reduced over several weeks of exposure, SOM responses increased, as did inhibition from SOMs. Thus, in temporal domain, the function of SOMs is consistent with regulation of the gain of cortical responses to sounds based on their behavioral prominence or relevance. SOMs thus contribute to adaptation and habituation, acting on several time scales to control the gain in response to commonly presented acoustic stimuli, exerting a more specific modulation than PVs. Such modulation of excitatory activity may contribute to the more general context-specific gain modulation and adaptation observed within AC38. Indeed, SOMs similarly play a specialized role in driving more general adaptation to temporally repeated tones39, and exert a differential effect on excitatory neuronal responses than PVs depending on the preceding stimulus40. Thus, the temporal history of the stimulus is important for the differential function of interneuron modulation. This functional dissociation likely underlies other temporally differentiated functions, such as integration of stimulus sequences, or more general computation of spectro-temporal statistical regularities in sound sequences. A promising direction for future studies would be to continue the exploration of the function not just over the instantaneous responses to tones, but in understanding how, over a range of time scales, inhibition may modulate dynamic changes in sound response properties.
Inhibitory cascades within the AC
Although the above studies mainly considered the effects of PVs and SOMs on excitatory neurons, understanding how PVs and SOMs act within a cortical microcircuit is of particular importance given the modulatory roles these interneurons play when targeted by feedback from other brain regions and different neuromodulatory projections. In a circuit that supports reduction of auditory responses during locomotion, a subset of secondary motor cortex neurons, which are active during movement, suppresses excitatory tone-evoked responses in AC by activating PVs, which in turn suppress excitatory neurons41,42. In addition, PVs are involved in inter-hemispheric information integration, as callossal projections terminate on PVs, which suppress cortico-cortical excitatory neurons43. PVs and SOMs can be activated by oxytocin, which likely supports the sharpening of responses to pup calls observed in mothers44. PVs also shape cortical responses to tones coupled with aversive stimuli45, as part of circuit, which includes projections from the amygdala to layer-1 neurons in AC. Thus, projections from cognitive and emotional brain centers likely preferentially target inhibitory interneurons, and may affect behavioral and emotional processing.
Other inhibitory neuronal subtypes further contribute to inhibitory cascades in the cortex. For example, there is extensive evidence that PVs and SOMs are regulated by VIPs. Cortical VIP interneurons are recruited by projections from other brain regions46,47 and can be modulated by cholinergic projections48,49,50,51 allowing for external control of these local microcircuits. In the AC, engagement in an auditory task enhanced the activity of PVs, SOMs, and VIPs, attributed to cholinergic modulation51. Activation of VIP interneurons disinhibited excitatory responses52, consistent with an additional inhibitory synapse between VIPs, another inhibitory neuron and excitatory neuron. Indeed, in vitro activation of VIPs suppresses PV and SOM activity, thereby providing a mechanism for delayed activation of excitatory tone-evoked responses. Interestingly, VIP neurons were driven by sounds at much lower intensities than either PV or SOM neurons53. The increased excitatory neuronal activity due to VIP activation may contribute to increased gain of sensory inputs, although it remains to be determined whether the relative timing of disinhibition may provide an increase in gain, rather than non-specific elevation in cortical activity. VIP neurons have also been implicated in integration of cross-modal activity, as responses of VIPs in the visual cortex are suppressed by sound54. This wide range of effects of VIP activation suggests that the connections between VIPs and inhibitory and excitatory neurons are likely modulated in a task- and modality-specific fashion, and therefore the interpretation of their function should inherently be studied in a specific statistical and behavioral context. To understand whether and how these circuits integrate with each other and what biophysical constraints are required for their function, the results of these studies need to be incorporated in a circuit-level model that include interactions between the different circuits.
Caveats in optogenetic result interpretation
In interpreting these rich and varied experimental results, it is important to account for limitations and potentially confounding factors: An important caveat to grouping the interneurons into classes based on molecular markers is that each of these classes are comprised of multiple interneuron subtypes, and those subtypes are distributed differentially across the different cortical laminae. For instance, PV interneurons are comprised of not only two already diverse large groups of neurons, basket and chandelier cells, but also of a number of other neurons1. SOM interneurons include Martinotti cells, whose axons target the distal dendrites of pyramidal neurons, as well as at least two other classes of layer 2/3 targeting neurons55. A recent review estimated between 4 and 100 subtypes of SOMs depending on classification method, such as differential labeling and projection patterns31. Incorporating some specific aspects of neuronal morphology, such as by building multi-compartment neuronal models and accounting for expression of different molecules involved in neuronal communication, may prove essential for differentiating between the effects of dendrite-targeting SOMs and cell body targeting interneurons. Indeed, the excitatory–inhibitory circuit composition likely differs between cortical layers with some neuronal types being overrepresented and targeting different parts of the excitatory cell body, leading to differences in integration and non-linearity56,57,58. Different recording techniques, such as extracellular recordings of activity of optically tagged neurons vs. two-photon guided patch-clamp recordings might be biased toward different subclasses within the optogenetically identified groups and different cortical sublayers, potentially reporting conflicting results on the response properties of different cell types29,30. By measuring a number of essential connectivity parameters, the strength and time constant of synapses between different neuronal types within different layers, it should be possible to further develop models to extrapolate the results across different cortical connectivity patterns. In turn, electrophysiological experiments, including multiple-neuron intracellular recordings, could be used to establish the specific parameters for connectivity between different neuronal cell types59. The computational approaches to implement such detailed biophysical models have been developed, and, as detailed in the next section, a small number of studies started using these computational frameworks to build models incorporating multiple neuronal subtypes in a variety of circuit motifs.
Computational models for excitatory–inhibitory interactions
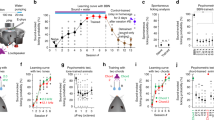
The approaches for incorporating inhibition into modeling sensory cortical function have ranged from implementing massive inhibition, forming inhibition-stabilized networks60,61,62 to fine-tuning parameters of individual neuronal feedback circuits63. Indeed, the large range of response profiles to activation of interneurons in inter-connected networks was extensively studied in the context of inhibition-stabilized networks that interpret the function of inhibition as supporting “stability” in neuronal pattern discharges across neuronal population activity62,64,65. Stabilization of excitatory neuron activity by recurrent inhibition can be explained by analyzing the dynamics of firing rates of excitatory and inhibitory populations over time, including feedback propagation of activity (Fig. 4a). This relatively simple model also explained heterogeneous findings from experiments testing the effect of increasing inhibition on network activity23,27,28 as providing input to inhibitory neurons resulting in increased activity of excitatory neurons in a model of an excitatory–inhibitory network60. Additional studies of inhibition-stabilized circuits focused on the role of inhibition in improving tuning of neurons for specific sensory features, such as orientation selectivity in the visual cortex using more abstract supralinear networks with feedback inhibition65, and the ability to tightly track the stimulus fluctuations in a balanced excitatory–inhibitory regime32. Extending the inhibitory–excitatory network model to incorporate the connectivity patterns between different types of interneurons, including PVs, SOMs, and VIPs, required a different set of constraints for explaining cortical tuning properties than when only one inhibitory subtype was used66 (Fig. 1b). Moreover, the resulting network did not require the massive inhibitory feedback consistent with inhibition-stabilized networks to model the observed effects for tuning properties. This was particularly important for understanding the tuning properties of neurons in layers 2/3 of the visual cortex, where only weak inhibition was identified experimentally.
Progressively complex view of cortical dynamics. (a) Diagram of the time course of inhibition-stabilized recurrent dynamics. Adapted from ref. 62. (b) Reduced model of mutually coupled Excitatory (Exc)—PV—SOM network. Exc rate is a non-linear—linear function of excitatory synaptic inputs (filled circles) evoked by the tone, as well as inhibitory inputs (open circles) from PVs and SOMs. PV and SOM firing rate is a non-linear—linear function of excitatory synaptic inputs from excitatory cells and tone-evoked excitatory inputs. Optogenetic manipulation by Arch is modeled as an inhibitory synaptic input. Adapted from ref. 34. (c) Left: network modules identified based on the correlation network structure. Adapted from ref. 95. Right: diagram of the time course of transformation in brain network structure with learning: Nodes belonging to the same module are colored in the same color. Black lines refer to the edges of the network. Note that with learning, the connectivity within and between modules is transformed. Adapted from ref. 101
In the AC, simpler models have already proved useful for understanding the heterogeneity of the effects of optogenetic manipulations. A static model illustrated the origin of the heterogeneous effects of optogenetic perturbations of PVs and SOMs on excitatory neuronal activity by shifting the threshold as well as the strength of inhibitory–excitatory conductances27. This model demonstrated plausibility that linear scaling and additive effects are produced by the same underlying mechanism—addition followed by rectification. A mutually coupled excitatory–inhibitory firing rate model reproduced the differential effects of manipulating PVs and excitatory neurons on the baseline and tone-evoked responses by adjusting the non-linearity at the inhibitory–excitatory synapse23. Differential synaptic strength of connections between the excitatory and inhibitory neurons could account for the differences between SOM and PV effects on adaptation in excitatory neurons, including flat and saturating non-linearities for synapses between PVs or SOMs and excitatory neurons, respectively (Fig. 4b). The principles outlined by the simplified models illustrate that by manipulating a specific aspect of input integration, the same wiring pattern can produce the heterogeneous results observed experimentally.
Several additional studies have used similar approaches to explore the role of distinct interneurons in cortical processing across sensory modalities. Using a combination of anatomical and optogenetic data in the somatosensory cortex67, a model could identify correlations in connection strengths between different neuronal subtypes68. Furthermore, a model of mutually coupled, fast-spiking, and non-fast-spiking interneurons, revealed the limitations in the role of fast-spiking neurons in cortical oscillations69. Beyond the sensory cortex, model circuits incorporating several inhibitory interneuron subtypes exhibited recurrent memory across a number of biophysically plausible configurations70,71. Indeed, recurrence in neuronal circuits increased the network’s capacity to efficiently store and recall memories72, as originally proposed73. Measuring whether and how inhibitory neuronal populations control and contribute to recurrent activity using recently developed methods for efficient model training63 should prove a fruitful way forward for understanding not only the key cell types that are affected by optogenetic perturbations, but also the time scales of their modulatory effects.
The temporal impact of optogenetic manipulations on auditory activity might differ between opsins, and thus have variable behavioral effects74. Expanding current models to account for the effects of inhibition at a circuit level will clarify how inhibition shapes trajectories of neuronal population dynamics75. In a complex network, where stimulus-evoked activity and neuronal connectivity patterns are highly heterogeneous23,76,77, computational models incorporating mutually coupled excitatory–inhibitory cells can reveal network features that may otherwise be obscured by results of electrophysiological and imaging experiments, which are biased toward stronger connections78. Recurrent circuit dynamics may be the dominating feature of cortical circuits, and the interpretation of results based on optogenetic perturbations need to incorporate feedback dynamics in their design79,80,81.
As the number of simultaneously observed neurons has increased with recent advances in functional imaging and dense electrophysiological recording techniques, there is a growing need to efficiently represent how units in a large population relate to one another and how these relations change over time82. To meet these demands, current computational approaches address the dynamics of neuronal populations that exhibit non-random dynamics and form higher-degree connectivity59,83,84. The study of the structure of excitatory–inhibitory connectivity can be combined with synaptic organizational principles to understand the basis for cortical dynamics80,85.
One such approach is to apply principles from network science to tracking the dynamics of neuronal cortical populations (Fig. 4c). Network science has been extensively used to characterize large-scale brain networks, revealing modular, hierarchical or random organization86,87. There is extensive evidence that neuronal populations exhibit stereotyped, temporally precise tone-activated patterns of activity in AC88, which are repeated during spontaneous firing, reflecting stereotypical population activity organization89. Such population activity patterns may differ between the synchronized and the desynchronized state of the cortical network90, and identifying inter-connected modules using network science methods can reveal whether and how the modules are transformed between the different brain states. Shared variability in neuronal populations can potentially be explained by distinct patterns of connections between neurons91, whereas diverse response patterns may correspond to different coupling patterns between single neurons and neuronal populations92—network analysis of activity in cortical slices already identifies functional modules, that exhibit similar organization across sensory cortex93,94 (Fig. 4c). Two-photon imaging of calcium activity in large networks of neurons identified similar population activity modes in the AC95. Such modes correlated with behavioral responses96. Analyzing neuronal activity in terms of population firing rate or activity variability discounts the complex temporal structure of these cellular networks, and thus might underestimate the information contained in neuronal responses90,97. Network analysis can furthermore reveal whether and how the neurons within functional modules reorganize with adaptation and learning, and whether specific inhibitory neurons assume specialized roles within networks.
Outlook/future directions
The diversity of these computational approaches for modeling the function of excitatory–inhibitory circuits and population neuronal activity in cortical sensory processing provides for the basic framework for moving forward in identifying the cortical circuits involved in auditory scene analysis. The complex effects of network interactions can only partially be understood in the context of natural audition when tested with a limited set of isolated stimuli. To the extent that we can view audition through the lens of temporal processing, recurrent inhibitory–excitatory networks can yield unique capabilities for storing and recalling complex temporal sequences, detecting unexpected events, and recognizing patterns of activation characteristic of more complex stimuli79,80,81, such as speech/vocalizations/music. The models should be fitted on data from experiments that would incorporate progressively complex acoustic stimuli; constructed either by varying spectro-temporal complexity, based on the scale-invariant statistical structure of environmental sounds98,99, or using statistical methods for shaping random signals to match different sound textures100. Taking advantage of the full computational toolset provided by inhibitory–excitatory network modeling, recurrent network dynamics and network science will allow us to tackle the complex richness of cortical circuits, and generalize results across sensory modalities and behavioral paradigms.
The use of dynamic analysis tools to explore sets of possible neuronal activity regimes makes inhibitory–excitatory networks a powerful framework for testing hypotheses on population responses in the cortex. The expansion of these models to include different cell types and wiring schemes in combination with analysis of the network structure dynamics is required for understanding the functions and sources of variability within specific neuronal populations. To fully understand cortical network function, our computational models must take into account the field’s wealth of data concerning neuronal subtypes, how they are connected locally and to other areas, how they respond to stimuli, and how optogenetic manipulation perturbs them in order to make testable predictions about how these networks behave. Thus, modeling studies need to be combined with a range of experimental techniques that would allow measurement of the strength of synaptic connections between neurons within specific layers and more precisely defined cellular classes.
References
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Roux, L., Stark, E., Sjulson, L. & Buzsaki, G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr. Opin. Neurobiol. 26, 88–95 (2014).
Bregman, A. S. Auditory scene analysis: the perceptual organization of sound (MIT Press, 1990).
Feng, A. S. & Ratnam, R. Neural basis of hearing in real-world situations. Annu. Rev. Psychol. 51, 699–725 (2000).
Aizenberg, M. & Geffen, M. N. Bidirectional effects of auditory aversive learning on sensory acuity are mediated by the auditory cortex. Nat. Neurosci. 16, 994–996 (2013).
Abeles, M. & Goldstein, M. H. Jr. Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J. Neurophysiol. 33, 172–187 (1970).
Shamma, S. A., Fleshman, J. W., Wiser, P. R. & Versnel, H. Organization of response areas in ferret primary auditory cortex. J. Neurophysiol. 69, 367–383 (1993).
Wehr, M. & Zador, A. M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003).
Talwar, S. K. & Gerstein, G. L. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J. Neurophysiol. 86, 1555–1572 (2001).
Tramo, M. J., Shah, G. D. & Braida, L. D. Functional role of auditory cortex in frequency processing and pitch perception. J. Neurophysiol. 87, 122–139 (2002).
Dykstra, A. R., Koh, C. K., Braida, L. D. & Tramo, M. J. Dissociation of detection and discrimination of pure tones following bilateral lesions of auditory cortex. PLoS ONE 7, e44602 (2012).
Ohl, F. W., Wetzel, W., Wagner, T., Rech, A. & Scheich, H. Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated tones but not of pure tones. Learn. Mem. 6, 347–362 (1999).
Gimenez, T. L., Lorenc, M. & Jaramillo, S. Adaptive categorization of sound frequency does not require the auditory cortex in rats. J. Neurophysiol. 114, 1137–1145 (2015).
Wu, G. K., Arbuckle, R., Liu, B. H., Tao, H. W. & Zhang, L. I. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 58, 132–143 (2008).
Schinkel-Bielefeld, N., David, S. V., Shamma, S. A. & Butts, D. A. Inferring the role of inhibition in auditory processing of complex natural stimuli. J. Neurophysiol. 107, 3296–3307 (2012).
Liu, B. H., Wu, G. K., Arbuckle, R., Tao, H. W. & Zhang, L. I. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat. Neurosci. 10, 1594–1600 (2007).
Oswald, A. M., Schiff, M. L. & Reyes, A. D. Synaptic mechanisms underlying auditory processing. Curr. Opin. Neurobiol. 16, 371–376 (2006).
Wang, J., Caspary, D. & Salvi, R. J. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport 11, 1137–1140 (2000).
Chen, Q. C. & Jen, P. H. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear. Res. 150, 161–174 (2000).
Tan, A. Y. & Wehr, M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience 163, 1302–1315 (2009).
Lee, S. H. et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012).
Hamilton, L. S. et al. Optogenetic activation of an inhibitory network enhances feedforward functional connectivity in auditory cortex. Neuron 80, 1066–1076 (2013).
Aizenberg, M., Mwilambwe-Tshilobo, L., Briguglio, J. J., Natan, R. G. & Geffen, M. N. Bidirectional regulation of innate and learned behaviors that rely on frequency discrimination by cortical inhibitory neurons. PLoS Biol. 13, e1002308 (2015).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Rudy, B., Fishell, G., Lee, S. & Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61 (2011).
Spitzer, N. C. Neurotransmitter switching in the developing and adult brain. Annu. Rev. Neurosci. 40, 1–19 (2017).
Seybold, B. A., Phillips, E. A., Schreiner, C. E. & Hasenstaub, A. R. Inhibitory actions unified by network integration. Neuron. 87, 1181–1192 (2015).
Phillips, E. A. & Hasenstaub, A. R. Asymmetric effects of activating and inactivating cortical interneurons. Elife 5, e18383 (2016).
Moore, A. K. & Wehr, M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J. Neurosci. 33, 13713–13723 (2013).
Li, L. Y. et al. Differential receptive field properties of parvalbumin and somatostatin inhibitory neurons in mouse auditory cortex. Cereb. Cortex. 25, 1782–1791 (2015).
Yavorska, I. & Wehr, M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front Neural Circuits 10, 76 (2016).
Deneve, S. & Machens, C. K. Efficient codes and balanced networks. Nat. Neurosci. 19, 375–382 (2016).
Ulanovsky, N., Las, L. & Nelken, I. Processing of low-probability sounds by cortical neurons. Nat. Neurosci. 6, 391–398 (2003).
Natan, R. G. et al. Complementary control of sensory adaptation by two types of cortical interneurons. eLife 4, e09868 (2015).
Nelken, I. Stimulus-specific adaptation and deviance detection in the auditory system: experiments and models. Biol. Cybern. 108, 655–663 (2014).
Chen, I. W., Helmchen, F. & Lutcke, H. Specific early and late oddball-evoked responses in excitatory and inhibitory neurons of mouse auditory cortex. J. Neurosci. 35, 12560–12573 (2015).
Kato, H. K., Gillet, S. N. & Isaacson, J. S. Flexible sensory representations in auditory cortex driven by behavioral relevance. Neuron 88, 1027–1039 (2015).
Williamson, R. S., Ahrens, M. B., Linden, J. F. & Sahani, M. Input-specific gain modulation by local sensory context shapes cortical and thalamic responses to complex sounds. Neuron 91, 467–481 (2016).
Natan, R. G., Rao, W. & Geffen, M. N. Cortical interneurons differentially shape frequency tuning following adaptation. Cell Rep. 21, 878–890 (2017).
Phillips, E. A. K., Schreiner, C. E. & Hasenstaub, A. R. Cortical interneurons differentially regulate the effects of acoustic context. Cell Rep. 20, 771–778 (2017).
Nelson, A. et al. A circuit for motor cortical modulation of auditory cortical activity. J. Neurosci. 33, 14342–14353 (2013).
Schneider, D. M., Nelson, A. & Mooney, R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014).
Rock, C. & Apicella, A. J. Callosal projections drive neuronal-specific responses in the mouse auditory cortex. J. Neurosci. 35, 6703–6713 (2015).
Marlin, B. J., Mitre, M., D’Amour, J. A., Chao, M. V. & Froemke, R. C. Oxytocin enables maternal behavior by balancing cortical inhibition. Nature 520, 499–504 (2015).
Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011).
Zhang, S. et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014).
Lee, S., Kruglikov, I., Huang, Z. J., Fishell, G. & Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670 (2013).
Poorthuis, R. B., Enke, L. & Letzkus, J. J. Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour. J. Physiol. 592, 4155–4164 (2014).
Fu, Y. et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014).
Koukouli, F., Rooy, M., Changeux, J. P. & Maskos, U. Nicotinic receptors in mouse prefrontal cortex modulate ultraslow fluctuations related to conscious processing. Proc. Natl Acad. Sci. USA 113, 14823–14828 (2016).
Kuchibhotla, K. V. et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat. Neurosci. 20, 62–71 (2017).
Pi, H. J. et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013).
Mesik, L. et al. Functional response properties of VIP-expressing inhibitory neurons in mouse visual and auditory cortex. Front Neural Circuits 9, 22 (2015).
Ibrahim, L. A. et al. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron 89, 1031–1045 (2016).
McGarry, L. M. et al. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits 4, 12 (2010).
DeFelipe, J. et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 14, 202–216 (2013).
Fino, E., Packer, A. M. & Yuste, R. The logic of inhibitory connectivity in the neocortex. Neuroscientist 19, 228–237 (2013).
Naka, A. & Adesnik, H. Inhibitory circuits in cortical layer 5. Front Neural Circuits 10, 35 (2016).
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Tsodyks, M. V., Skaggs, W. E., Sejnowski, T. J. & McNaughton, B. L. Paradoxical effects of external modulation of inhibitory interneurons. J. Neurosci. 17, 4382–4388 (1997).
Latham, P. E. & Nirenberg, S. Computing and stability in cortical networks. Neural. Comput. 16, 1385–1412 (2004).
Ozeki, H., Finn, I. M., Schaffer, E. S., Miller, K. D. & Ferster, D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron 62, 578–592 (2009).
Sussillo, D. Neural circuits as computational dynamical systems. Curr. Opin. Neurobiol. 25, 156–163 (2014).
van Vreeswijk, C. & Sompolinsky, H. Chaotic balanced state in a model of cortical circuits. Neural. Comput. 10, 1321–1371 (1998).
Rubin, D. B., Van Hooser, S. D. & Miller, K. D. The stabilized supralinear network: a unifying circuit motif underlying multi-input integration in sensory cortex. Neuron 85, 402–417 (2015).
Litwin-Kumar, A., Rosenbaum, R. & Doiron, B. Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. J. Neurophysiol. 115, 1399–1409 (2016).
Avermann, M., Tomm, C., Mateo, C., Gerstner, W. & Petersen, C. C. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J. Neurophysiol. 107, 3116–3134 (2012).
Tomm, C., Avermann, M., Petersen, C., Gerstner, W. & Vogels, T. P. Connection-type-specific biases make uniform random network models consistent with cortical recordings. J. Neurophysiol. 112, 1801–1814 (2014).
Vierling-Claassen, D., Cardin, J. A., Moore, C. I. & Jones, S. R. Computational modeling of distinct neocortical oscillations driven by cell-type selective optogenetic drive: separable resonant circuits controlled by low-threshold spiking and fast-spiking interneurons. Front. Hum. Neurosci. 4, 198 (2010).
Merolla, P. A. et al. Artificial brains. A million spiking-neuron integrated circuit with a scalable communication network and interface. Science 345, 668–673 (2014).
Markram, H. et al. Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492 (2015).
Wang, Y. et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 561, 65–90 (2004).
Hopfield, J. J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl Acad. Sci. USA 79, 2554–2558 (1982).
Guo, W. et al. Hearing the light: neural and perceptual encoding of optogenetic stimulation in the central auditory pathway. Sci. Rep. 5, 10319 (2015).
Loewenstein, Y., Yanover, U. & Rumpel, S. Predicting the dynamics of network connectivity in the neocortex. J. Neurosci. 35, 12535–12544 (2015).
Bandyopadhyay, S., Shamma, S. A. & Kanold, P. O. Dichotomy of functional organization in the mouse auditory cortex. Nat. Neurosci. 13, 361–368 (2010).
Rothschild, G., Nelken, I. & Mizrahi, A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat. Neurosci. 13, 353–360 (2010).
Chambers, B. & MacLean, J. N. Multineuronal activity patterns identify selective synaptic connections under realistic experimental constraints. J. Neurophysiol. 114, 1837–1849 (2015).
Rajan, K., Harvey, C. D. & Tank, D. W. Recurrent network models of sequence generation and memory. Neuron 90, 128–142 (2016).
Landau, I. D., Egger, R., Dercksen, V. J., Oberlaender, M. & Sompolinsky, H. The impact of structural heterogeneity on excitation-inhibition balance in cortical networks. Neuron 92, 1106–1121 (2016).
Aljadeff, J., Stern, M. & Sharpee, T. Transition to chaos in random networks with cell-type-specific connectivity. Phys. Rev. Lett. 114, 088101 (2015).
Yatsenko, D. et al. Improved estimation and interpretation of correlations in neural circuits. PLoS Comput. Biol. 11, e1004083 (2015).
Ko, H. et al. The emergence of functional microcircuits in visual cortex. Nature 496, 96–100 (2013).
Timme, N. M. et al. High-degree neurons feed cortical computations. PLoS Comput. Biol. 12, e1004858 (2016).
Ocker, G. K., Litwin-Kumar, A. & Doiron, B. Self-organization of microcircuits in networks of spiking neurons with plastic synapses. PLoS Comput. Biol. 11, e1004458 (2015).
Bassett, D. S. et al. Dynamic reconfiguration of human brain networks during learning. Proc. Natl Acad. Sci. USA 108, 7641–7646 (2011).
Bassett, D. S. et al. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 28, 9239–9248 (2008).
Harris, K. D. et al. How do neurons work together? Lessons from auditory cortex. Hear. Res. 271, 37–53 (2011).
Luczak, A., Bartho, P. & Harris, K. D. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62, 413–425 (2009).
Pachitariu, M., Lyamzin, D. R., Sahani, M. & Lesica, N. A. State-dependent population coding in primary auditory cortex. J. Neurosci. 35, 2058–2073 (2015).
Lin, I. C., Okun, M., Carandini, M. & Harris, K. D. The nature of shared cortical variability. Neuron 87, 644–656 (2015).
Okun, M. et al. Diverse coupling of neurons to populations in sensory cortex. Nature 521, 511–515 (2015).
Sadovsky, A. J. & MacLean, J. N. Scaling of topologically similar functional modules defines mouse primary auditory and somatosensory microcircuitry. J. Neurosci. 33, 14048–14060, 14060a (2013).
Gururangan, S. S., Sadovsky, A. J. & MacLean, J. N. Analysis of graph invariants in functional neocortical circuitry reveals generalized features common to three areas of sensory cortex. PLoS Comput. Biol. 10, e1003710 (2014).
Deneux, T., Kempf, A., Daret, A., Ponsot, E. & Bathellier, B. Temporal asymmetries in auditory coding and perception reflect multi-layered nonlinearities. Nat. Commun. 7, 12682 (2016).
Bathellier, B., Ushakova, L. & Rumpel, S. Discrete neocortical dynamics predict behavioral categorization of sounds. Neuron 76, 435–449 (2012).
Kuczala, A. & Sharpee, T. O. Eigenvalue spectra of large correlated random matrices. Phys. Rev. E 94, 050101 (2016).
Geffen, M. N., Gervain, J., Werker, J. F. & Magnasco, M. O. Auditory perception of self-similarity in water sounds. Front. Integr. Neurosci. 5, 15 (2011).
Gervain, J., Werker, J. F. & Geffen, M. N. Category-specific processing of scale-invariant sounds in infancy. PLoS ONE 9, e96278 (2014).
McDermott, J. H. & Simoncelli, E. P. Sound texture perception via statistics of the auditory periphery: evidence from sound synthesis. Neuron 71, 926–940 (2011).
Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci. 20, 353–364 (2017).
Acknowledgements
This work was supported by National Institutes of Health (Grant numbers NIH R03DC013660, NIH R01DC014700, NIH R01DC015527), Klingenstein Award in Neuroscience, Human Frontier in Science Foundation Young Investigator Award and the Pennsylvania Lions Club Hearing Research Fellowship to MGN. M.N.G. is the recipient of the Burroughs Wellcome Award at the Scientific Interface. J.F.B. was supported by National Institutes of Health (Grant number NIH NIMH T32MH017168). We thank the members of the Geffen laboratory and the Hearing Research Center at the University of Pennsylvania, including Dr Steve Eliades, Dr Yale Cohen, Dr. Mark Aizenberg, Dr Katherine Wood, and Christopher Angeloni for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blackwell, J.M., Geffen, M.N. Progress and challenges for understanding the function of cortical microcircuits in auditory processing. Nat Commun 8, 2165 (2017). https://doi.org/10.1038/s41467-017-01755-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-01755-2
This article is cited by
-
Parvalbumin neurons enhance temporal coding and reduce cortical noise in complex auditory scenes
Communications Biology (2023)
-
Neuronal activity in sensory cortex predicts the specificity of learning in mice
Nature Communications (2022)
-
Hearing loss and brain plasticity: the hyperactivity phenomenon
Brain Structure and Function (2021)
-
Development of Auditory Cortex Circuits
Journal of the Association for Research in Otolaryngology (2021)
-
Neural Responses and Perceptual Sensitivity to Sound Depend on Sound-Level Statistics
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.