Abstract
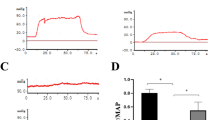
We previously showed that castration of rats reduced erectile function over time; when testosterone replacement therapy was started 4 weeks after castration, erectile function improved. In this study, we examined the mechanism of improvement in erectile function following testosterone replacement therapy in rats. Thirty 12-week-old rats were divided into castrated (Cast), castrated with subcutaneous administration of testosterone (Cast + T), and sham (Sham) groups. Erectile function and mRNA and protein expression were evaluated in the rats by using standard methods. To assess erectile function, we measured the intracavernosal pressure, mean arterial pressure, mRNA expression of endothelial growth factors, and protein expression of endothelial nitric oxide synthase (eNOS). The intracavernosal pressure/mean arterial pressure ratio was significantly lower in the Cast group, and testosterone administration significantly improved (P = 0.017). Compared to the Cast group, the Cast+T group exhibited significantly increased mRNA expressions of vascular endothelial growth factor A (VEGF-A), intercellular adhesion molecule 1 (ICAM-1), transforming growth factor-β (TGF-β), nerve growth factor (NGF), α-smooth muscle actin (α-SMA), caveolae associated protein 1 (Cavin-1), Cavin-2, Cavin-3, sirtuin 1 (Sirt-1), sphingosine-1-phosphate 1 (S1P1), S1P2, and S1P3 and eNOS protein expression. Testosterone replacement therapy improved erectile function in castrated rats by increasing growth factors and eNOS protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling–a clinical research center study. J Clin Endocrinol Metab. 1995;80:3025–31.
Bandyk MG, Sawczuk IS, Olsson CA, Katz AE, Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990;143:407–13.
Kavoussi PK, Smith RP, Oliver JL, Costabile RA, Steers WD, Brown-Steinke K, et al. S-nitrosylation of endothelial nitric oxide synthase impacts erectile function. Int J Impot Res. 2019;31:31–8.
Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl. 2005;26:242–8.
Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98:116–24.
Shen ZJ, Zhou XL, Lu YL, Chen ZD. Effect of androgen deprivation on penile ultrastructure. Asian J Androl. 2003;5:33–6.
Petrone L, Mannucci E, Corona G, Bartolini M, Forti G, Giommi R, et al. Structured interview on erectile dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res. 2003;15:210–20.
Corona G, Torres LO, Maggi M. Testosterone therapy: what we have learned From trials. J Sex Med. 2020;17:447–60.
Hwang I, Lee HS, Yu HS, Kim ME, Lee JS, Park K. Testosterone modulates endothelial progenitor cells in rat corpus cavernosum. BJU Int. 2016;117:976–81.
Li R, Meng X, Zhang Y, Wang T, Yang J, Niu Y, et al. Testosterone improves erectile function through inhibition of reactive oxygen species generation in castrated rats. PeerJ 2016;4:e2000.
Kataoka T, Hotta Y, Maeda Y, Kimura K. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14:1540–8.
Kataoka T, Hotta Y, Yamamoto Y, Fukamoto A, Takeuchi M, Maeda Y, et al. Effect of late androgen replacement therapy on erectile function Through structural changes in castrated rats. Sex Med. 2021;9:100348.
Kataoka T, Mori T, Suzuki J, Kawaki Y, Kito Y, Hotta Y, et al. Oxaliplatin, an anticancer agent, causes erectile dysfunction in rats due to endothelial dysfunction. J Sex Med. 2021;18:1337–45.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242–8.
Gho YS, Kleinman HK, Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999;59:5128–32.
Joseph IB, Nelson JB, Denmeade SR, Isaacs JT. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507–11.
Häggström S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161:1620–5.
Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995;376:517–9.
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–20.
Shvets E, Ludwig A, Nichols BJ. News from the caves: update on the structure and function of caveolae. Curr Opin Cell Biol. 2014;29:99–106.
Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–98.
Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–70.
Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–7.
Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–14.
McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–15.
Zhu B, Swärd K, Ekman M, Uvelius B, Rippe C. Cavin-3 (PRKCDBP) deficiency reduces the density of caveolae in smooth muscle. Cell Tissue Res. 2017;368:591–602.
Suades R, Cosentino F. Sirtuin 1/soluble guanylyl cyclase: a nitric oxide-independent pathway to rescue ageing-induced vascular dysfunction. Cardiovasc Res. 2019;115:485–7.
Kilic U, Gok O, Elibol-Can B, Uysal O, Bacaksiz A. Efficacy of statins on sirtuin 1 and endothelial nitric oxide synthase expression: the role of sirtuin 1 gene variants in human coronary atherosclerosis. Clin Exp Pharm Physiol. 2015;42:321–30.
Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–5.
Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–81.
Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006;114:1403–9.
Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999;99:301–12.
Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:10664–9.
Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, Marini M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47:409–16.
Acknowledgements
We acknowledge the assistance of the Research Equipment Sharing Center at the Nagoya City University.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI, 20K09563) from the Japan Society for the Promotion of Science (JSPS) and the 24th General Assembly of the Japanese Association of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization, TK and KK; Data curation, TK, IH, TM, YH, and YM; Formal analysis, TK and IH.; Funding acquisition, TK, YH, and KK; Investigation, TK, IH, and TM; Methodology, TK and YH; Project administration, TK; Supervision, YH, AS, YM, YFH, and KK; Writing-original draft, TK and KK; Writing-review & editing TK and KK.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kataoka, T., Ito, H., Mori, T. et al. Testosterone improved erectile function by upregulating transcriptional expression of growth factors in late androgen replacement therapy model rats. Int J Impot Res (2022). https://doi.org/10.1038/s41443-022-00627-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-022-00627-8